Table of Contents
A jig tailing with gold content ranging from 0.33 to 0.43 oz/ton was leached in weak cyanide solution with extractions of up to 97 pct in 24 hours. By countercurrent batch leaching studies and laboratory pilot plant operation, minus 10- plus 20-mesh and minus 6- plus 16-mesh coconut carbon were loaded to 700 oz/ton of Au. Minor silver in the ore reported in the carbon in amounts of up to 100 oz/ton. Gold adsorption rate data and an equilibrium adsorption curve for minus 10- plus 20-mesh carbon at ambient temperatures were developed and used in the successful application of a cascade design theory. The work was performed as a part of the overall Bureau of Mines research effort to help maintain an adequate domestic supply of metals by making improvements in mineral-processing technology.
A medium-grade gold ore was jigged by company personnel to remove about one-half of the gold in a highly upgraded product. Samples of the jig tailing were provided to the Bureau of Mines for research on the application of the carbon-in-pulp process to higher grade, gold-bearing materials as a part of the overall Bureau of Mines research effort to improve mineral-processing technology. The process comprises treatment by cyanide leaching and subsequent adsorption of gold from leach slurries by carbon-in-pulp methods.
The jig tailing was about 90 pct minus 100 mesh; the samples submitted contained from 0.33 to 0.42 oz/ton Au and a minor but valuable amount of silver. Microscopic examination of the jig tailing showed that the sample consisted principally of limonite and quartz with small amounts of muscovite and tourmaline. The magnetic fraction of the jig tailing contained gold of up to about 25 um in diameter completely enclosed in limonite. The nonmagnetic fraction contained gold as free, irregularly shaped grains of up to about 150 mesh with partial coatings of iron oxides.
Batch Adsorption Tests with Minus 10- Plus 20-Mesh Carbon
Carbon-in-pulp batch adsorption tests with minus 10- plus 20-mesh activated coconut carbon were made to estimate the equilibrium adsorption curve and to determine adsorption rates for gold in cyanide leach slurries of the ore. This step was followed by countercurrent, batch adsorption tests prior to pilot testing.
The first batch of ore was leached with 0.1-pct NaCN solution at 50 pet solids and a pH of 10.9 attained by adding 1 pound of lime per ton of ore. Leaching extractions at 24 and 69 hours were 90 and 95 pct, respectively. The final solution contained 0.36 oz/ton Au.
Batch Testing for Gold Adsorption Rates
Four carbon-in-pulp batch adsorption tests were made to obtain rate data over the range of gold concentration in solution and carbon expected for a four-stage, carbon-in-pulp process. The expected strength of the pregnant solution and previous knowledge of gold loading on carbon were used as guides. The batch tests were made in containers ranging in size from a 4-liter beaker to a 40-liter plastic container. Agitation was by mixer-impellers. The test conditions used in these tests are given in table 1.
Samples of slurry were removed periodically for solution analysis. At the end of the test, the final slurry percent solids was determined, and water losses were calculated on a pro rata basis for the successive sampling time intervals. Sampling was taken to be uniform, and gold loading on carbon was calculated from the equations
qj = (Co j – Cj) (W/M)j……………………………………..(1)
and Co j = Co (ro/rj)………………………………………(2)
where Cj = oz/ton Au, solution,
qj = oz/ton Au for carbon in solution of concentration Cj,
C0 = initial solution concentration,
r = weight ratio of solution to ore solids,
and W/M = weight ratio of solution to carbon.
By use of equation 1, the effect of water evaporation is accounted for in the calculation of gold loading on carbon.
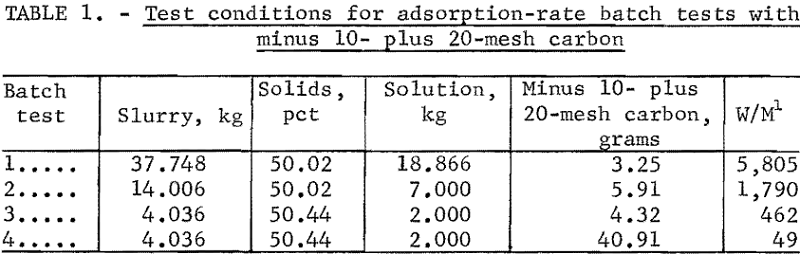
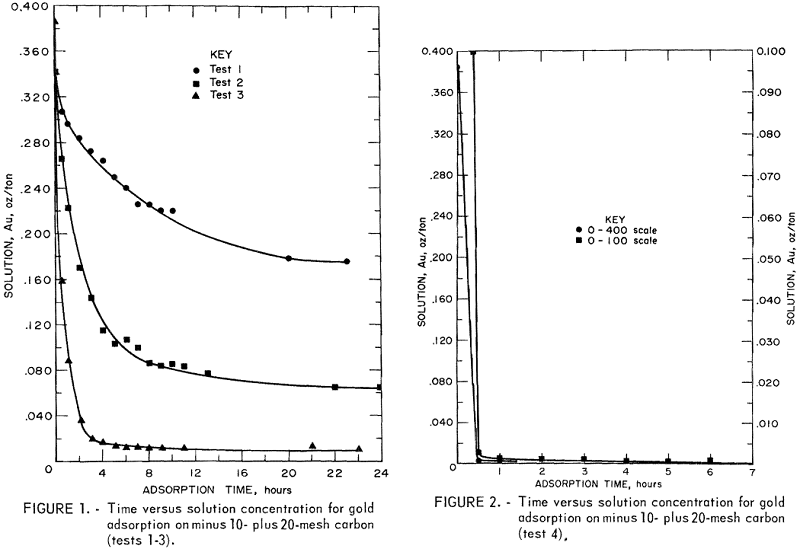
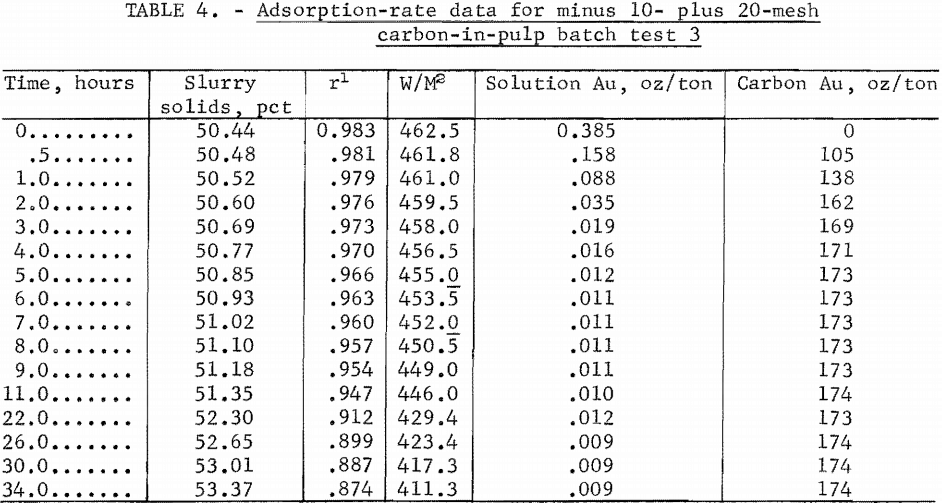
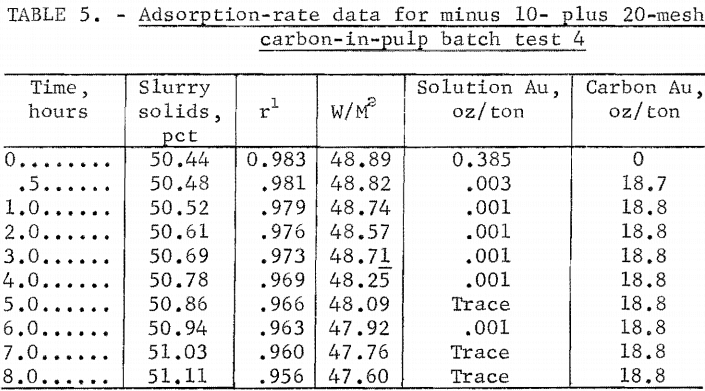
The adsorption rate data for the four tests are shown in tables 2 through 5. Time versus solution concentration for tests 1 through 3 are shown graphically in figure 1 and for test 4, in figure 2. The data show that in 0.5 hour, test 4 reached a solution concentration change of 0.0029 oz/ton/hr. Tests 3,
2, and 1 reached the same rate in about 4, 10, and 20 hours, respectively, with 90 pct of the adsorption previous to this rate occurring in about 1.6, 5, and 14 hours, respectively. The time differences emphasize the necessity of obtaining rate data in the vicinity of projected concentration changes in dynamic systems. A maximum carbon loading of 1,010 oz/ton Au was obtained.
The gold-silver content of the pregnant and intermediate solutions was determined by atomic adsorption analyses. The barren solutions were assayed in 30 to 70 assay ton portions by the Chiddey method for cyanide solution analyses. The loaded carbon was ashed and fire assayed.
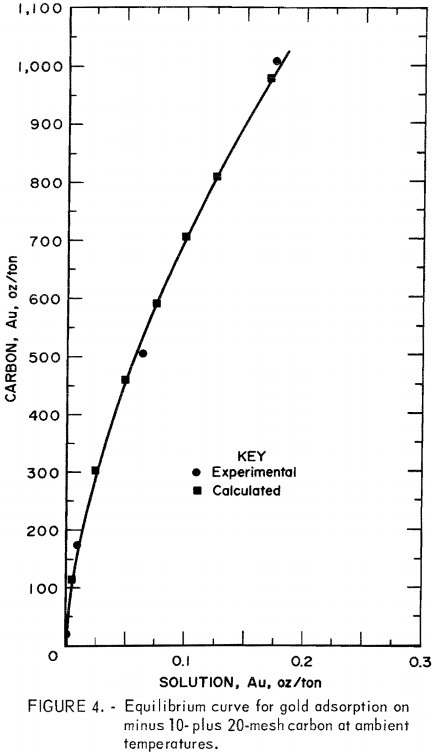
An Equilibrium Adsorption Curve for Gold Adsorption on Minus 10- Plus 20-Mesh Carbon
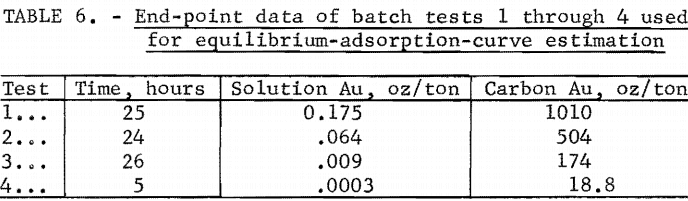
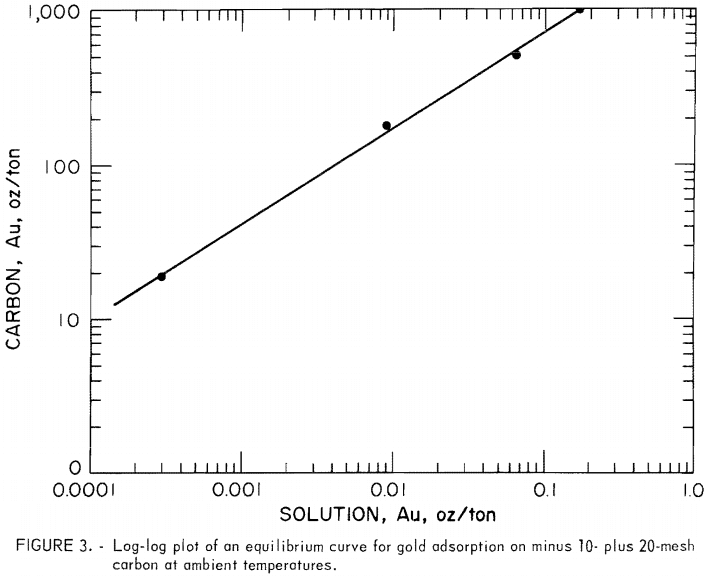
For the purposes of cascade design in countercurrent, carbon-in-pulp systems, the solution and carbon gold concentrations close to equilibrium in tests 1 through 4 were used to estimate an equilibrium curve for gold adsorption on minus 10- plus 20-mesh coconut carbon at ambient temperatures. The selected end-point data of these tests from tables 2 through 5 are shown in table 6. The data were correlated by the least-squares method, and the resulting straight line was drawn as shown in figure 3. The equations of the line are, in units of ounces per ton,

Equation 4 is typical of the Freundlich equation obtained over small ranges of concentration in dilute solution. Equation 3 was used to calculate points on the adsorption curve, which were plotted as shown in figure 4.
Countercurrent, Carbon-in-Pulp Batch Testing
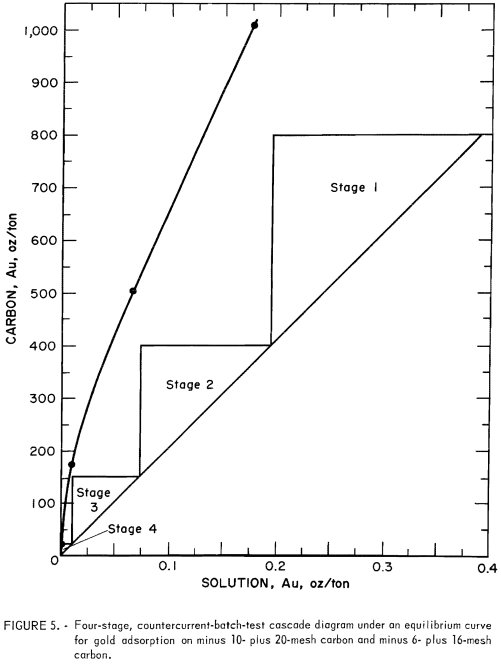
Prior to laboratory pilot plant testing of gold adsorption and recovery by the carbon-in-pulp method, a four-stage, countercurrent batch test was made with minus 10- plus 20-mesh coconut carbon. That the carbon could be loaded to a satisfactory level was apparent from previous batch tests. The counter-current batch tests were made to evaluate carbon-concentration requirements and to test a theory of cascade design under development. The scale of the batch testing was limited to 4-liter beakers containing 2 liters of leach solution in a slurry of about 50 pct solids.
 Cascade Design for Countercurrent Batch Test
Cascade Design for Countercurrent Batch Test
The estimated gold-adsorption equilibrium curve for minus 10- plus 20-mesh carbon as shown in figure 4 serves as a boundary line beneath which the carbon-in-pulp system must operate. The adsorption curve provides guidance for the number of stages to be used under specified conditions. The leach slurry was 50.46 pct solids with a pregnant solution containing 0.391 oz/ton Au and 0.04 pct NaCN at pH 10.7. The target carbon loading was set at 800 oz/ton Au and the target barren solution, at 0.0005 oz/ton Au. Therefore, the operating line slope was
W/M = m= 800/(0.391 – 0.0005) = 2,049……………………………….(5)
The target gold loadings on carbon for four test stages were set at 800, 400, 150, and 18.8 oz Au/ton; the four stages of the countercurrent batch test are shown in figure 5 as four steps on the operating line.
Theoretical Aspects of Cascade Design
When the stages of a cascade have been set as shown in figure 5, the calculation of the carbon concentration in the slurry of each stage requires the determination of the carbon-retention time in each stage. For the i’th stage of a cascade, let (ci , qi) be the solution and carbon gold concentration values, respectively, at discharge. Let (c1-1 , q1+1) be the solution and carbon gold concentration values,
respectively, at stage entry. The carbon-retention time, in hours, for the i’th stage is,
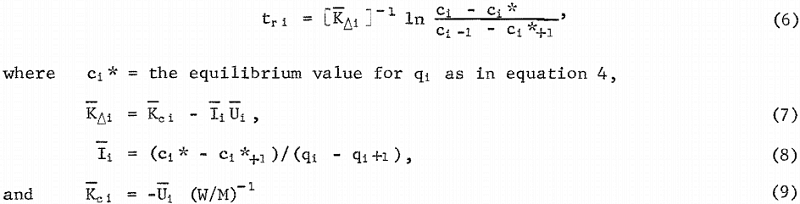
In equations 6 and 7, Ki is the mean driving force rate coefficient for the i’th stage. In equations 7 and 9, Kc i is_the mean solution overall mass-transfer coefficient for the i’th stage, and Ui is the mean carbon overall mass-transfer coefficient with a solution driving force for the i’th stage. The solution driving force at any point (cj , qj) is (ci – Ci). The units of K and Kc, are
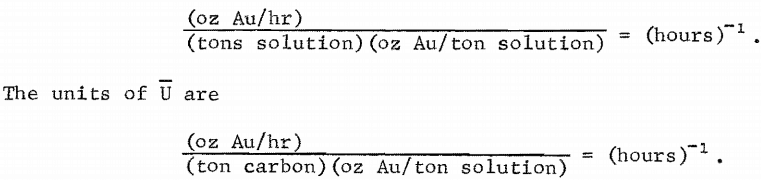
In equation 9, Ui is estimated from batch-test data from solution and carbon gold-concentration values as close as possible to the i’th stage. If the batch-test data are (cj , qj) at time tj and (ck, qk) at tk, tj < tk, and tjk = (tk – tj) , then U is estimated by
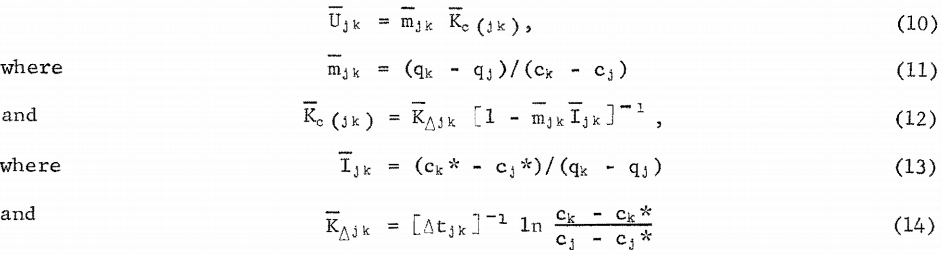
Estimation of Batch Carbon Retention Times
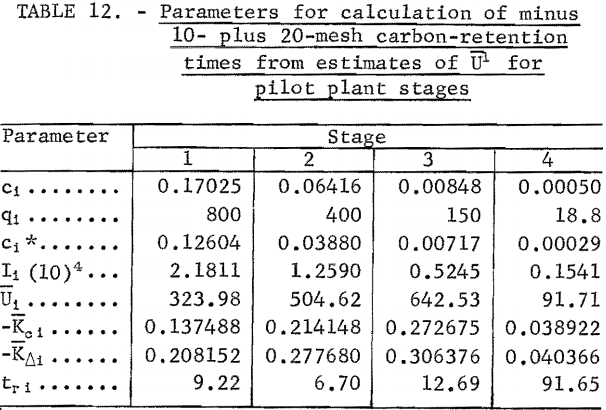
The equations of the previous section are used for the calculation of carbon-retention times at each stage. The target gold concentration of the solution discharge from each stage was calculated from target gold loading on carbon in each stage and the operating line slope of equation 5. The target discharge concentrations are listed as the (ci, qi) in table The calculation of the carbon-retention times requires the estimation of U for each stage.
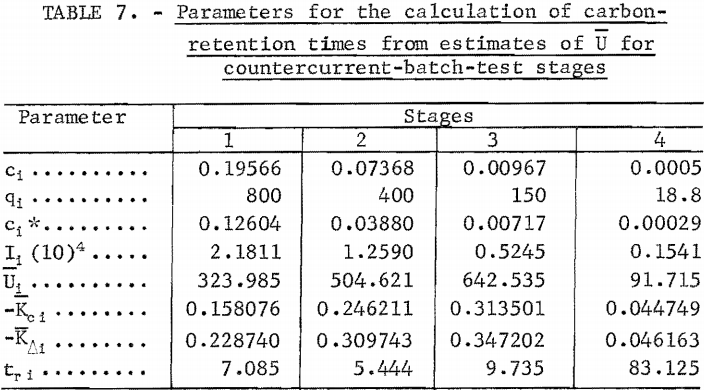
The gold-adsorption-rate data of batch tests 1 through 4 (tables 2 through 5) were used for the estimation of U for stages 1 through 4, respectively. The batch-test data and parameters for the estimation of U are summarized in table 8. The batch-test gold loadings, qj and qk , shown in table 8, were selected at the same values as the target stage loadings. This is ideal but not always practical in estimation work. The slopes, mjk, were approximated by the mean slope from initial and final gold concentrations in solution and in carbon. The solution concentrations , cj, and ck , were calculated from qj , qk , and mj k. The values of tj and tk were obtained from the time and concentration graphs of figures 1 and 2 at corresponding values of cj and ck , or from the original data where available.
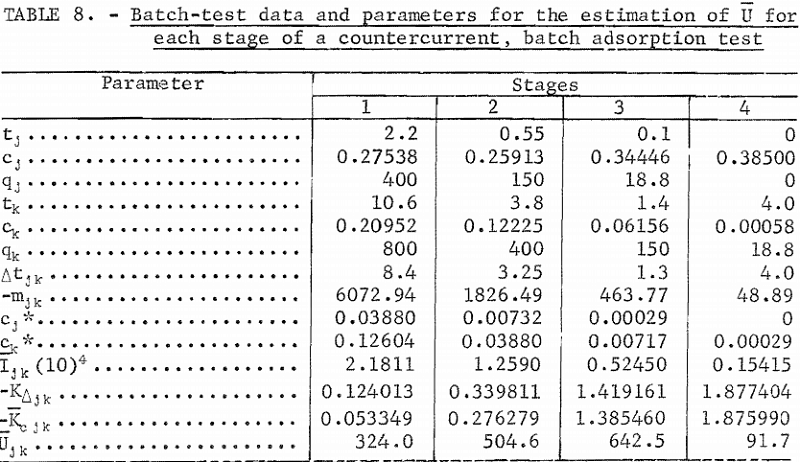
In determining the cj, and ck , sectional variations of the gold adsorption curve of figure 4 and equation 4 were used. For stages 1 and 2, an estimate based on end-point data of batch tests 1, 2, and 3 was developed using the equation
![]()
For stages 3 and 4, an estimate based on end-point data of tests 3 and 4 was developed using the equation
![]()
The values of Ijk, Kjk. Kcj k, and Ujk in table 8 were calculated by equations 13, 14, 12, and 10, respectively. The values of are the estimates of Ui given in table 7. The values of Kci, Ki, and tr i in table 7 were calculated by equations 9, 7, and 6, respectively. It should be noted that carbon-residence time for stage 4 is much larger than for the first three stages because the mass-transfer coefficient is so much smaller in the solution and carbon gold concentration region of stage 4.
Calculation of Batch Carbon Concentrations
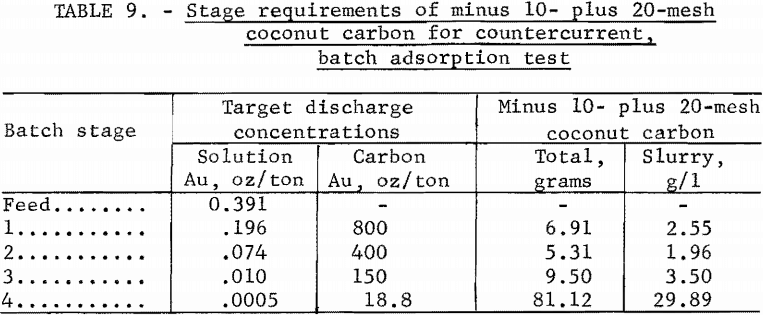
Batch stage carbon concentrations were calculated on the basis of a downstream movement of slurry each hour equal to an individual batch slurry volume containing 2,000 grams of solution. The corresponding upstream movement of carbon was calculated from equation 5 to be M = 0.98 g/hr. Let Mi be the total carbon required in each stage slurry and
Mi = Mt ri……………………………(17)
Carbon requirements based on equation 17 are shown in table 9 with carbon concentrations based on 2.714 leters of slurry at 50 pct solids.
Countercurrent Batch Test Operation
Each batch stage was a 4-liter beaker with a mechanical stirrer. After loading the proper amounts of slurry and carbon for each stage the slurries were agitated for 1 hour. The carbon then was screened out on a 28-mesh screen and retained for use in the next cycle. The carbon was washed, and wash water with solid residues was discarded. The undiluted slurries were moved to the next stage. The beakers were not washed, and carbon sticking to the walls of the beakers was left in place. Thus, at the end of each cycle, batch slurry movement was from stage i to stage i + 1. A fresh batch of slurry (about 2.714 liters) was poured into the beaker at stage 1. Slurry from stage 4 was sampled for solution gold analysis.
The proper weight of carbon (M) was moved countercurrent to slurry flow at the end of each cycle by volumetric measurement on the basis of carbon bulk density at 0.45 g/ml. Thus, the carbon movement was from stage i to stage i – 1. M grams of fresh carbon was put into the new batch of slurry at stage 4. The M grams of carbon removed from stage 1 was fire assayed for gold and silver. After the proper amount of carbon had been advanced through the stages, agitation was started for the next cycle.
Before the countercurrent test was started, the initial carbon was pre-loaded from slurries of the ore to about the gold concentrations shown in table 9. Slurry solutions likewise were adjusted approximately. The system was operated for 10 cycles; resulting feed and carbon data are shown in table 10.
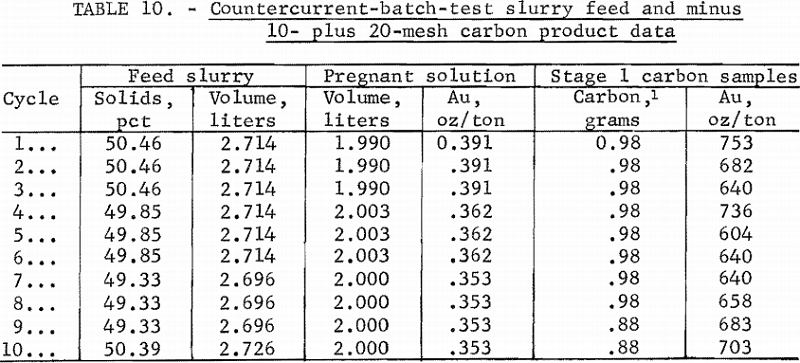
In the last two cycles, the carbon movement was dropped from 0.98 to 0.88 g/hr as a compensation for lower gold values of the pregnant solution.
A total of 27.10 liters of slurry averaging 49.93 pct solids was processed. The pregnant solution contained a mean of 0.367 oz/ton Au with 0.02 pct NaCN at pH 10.5. The mean barren solution content from the fourth stage was <0.0003 oz/ton Au» The stage-1 carbon samples ranged from 604 to 753 oz/ton Au with an average of 674 oz/ton; this compares with a theoretical carbon product of 763 oz/ton Au, based upon mean operating data with W/M = 2080.8.
There are several potential sources of error in the batch-test procedure. There is some attrition of carbon, and carbon samples, measured volumetrically, are not precise in weight. Because of ore solids trapped in the carbon, dry-weighted samples give lower unit gold analyses than those based upon 1 ton of pure carbon. There are also solution losses in the sampling procedures. However, the cyclic, batch evaluation procedure provides reliable guidance for pilot work.
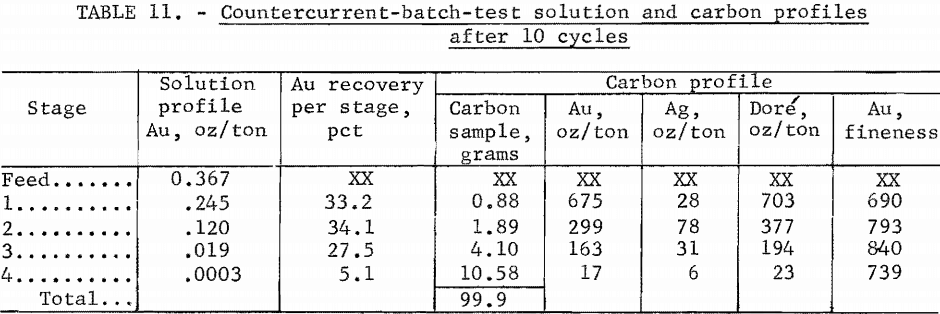
At the end of cycle 10, the carbon and solution in each of the stages were sampled, and analyses are shown in table 11.
Laboratory Pilot Test with Minus 6- Plus 16-Mesh Carbon
A countercurrent, carbon-in-pulp, pilot plant test was made for the adsorption and recovery of gold on minus 6- plus 16-mesh coconut carbon from cyanide leach slurries of the jig tailing. The ore was in two lots containing 0.33 and 0.42 oz/ton Au and was leached in cyanide solutions containing 0.05 and 0.1 pet NaCN, respectively, at pH 11. Leaching at 50 pet solids for 24 hours gave gold extractions of 97 pct. Lime consumption was 1.5 lb/ton of ore.
Laboratory Pilot Plant Cascade Design
The gold adsorption equilibrium isotherm for minus 6- plus 16-mesh coconut carbon was estimated to be about the same as the isotherm for minus 10- plus 20-mesh coconut carbon shown in figure 4. The basis for this estimation was that previous work on silver adsorption had shown little difference between the silver isotherms at ambient temperatures for the two sizes of carbon. Leaching results indicated that a pregnant solution containing 0.34 oz/ton Au would be available for the test. Using this value, the target barren solution concentration was set at 0.0005 oz/ton Au, and the target carbon loading at 800 oz/ton Au. The operating line is shown in figure 5 with the isotherm of figure 4. The operating line slope was
W/M = m = 800/(0.34 – 0.0005) = 2356.41.
The target gold loading on the carbon was set as follows: stage 1, 800; stage 2, 400; stage 3, 150; and stage 4, 18.8 oz/ton Au. The four stages for the pilot plant test are shown in figure 5 as steps on the operating line and are those used for the countercurrent batch test.
Estimation of Pilot-Stage Carbon-Retention Times
Adsorption-rate data for gold adsorption on minus 6- plus 16-mesh coconut carbon were estimated from rate data for minus 10- plus 20-mesh carbon by a factor developed from relative surface areas of the two sizes of carbon. The gold adsorption rates for the larger size carbon, therefore, were estimated to be 0.6 times the rates for minus 10- plus 20-mesh carbon.
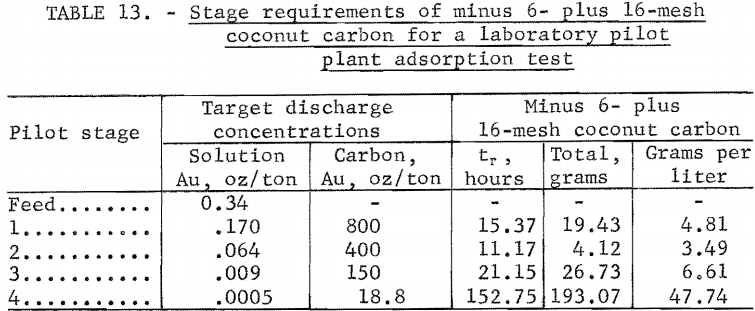
The target discharge gold concentrations in solution were calculated from the target gold loadings on carbon and the operating line slope of equation 18. The target discharge concentrations are listed as the (ci , qi) in table 12. Since target gold loadings are the same as in the countercurrent batch test, the estimates of Ui in table 7 were used in table 12. The values of Kci, Ki) and tr i in table 12 were calculated by equations 9, 7, and 6, respectively. The values of tr i in table 12 were divided by 0.6 to obtain carbon-retention times for minus 6- plus 16-mesh carbon which are shown in table 13.
Calculation of Pilot-Stage Carbon Concentrations
Pilot-stage carbon concentrations were calculated on the basis of a downstream slurry movement each hour equal to a stage cell volume of 4.044 l/hr containing 2,979 grams of water at 50 pct solids. The corresponding upstream movement of carbon was calculated from equation 18 to be M = 1.264 g/hr. Carbon requirements based on equation 17 are shown in table 13 with carbon concentrations based on cell volumes of 4.044 liters.
Laboratory Pilot Plant Operation
The pilot plant was a cascade of four tanks with 4-liter volumes at overflow through apertures covered with 20-mesh screen to retain the minus 6- plus 16-mesh carbon. Slurry agitation was mechanical with four-bladed impellers. Slurry was pumped from an elevated feed tank by a Cole-Parmer pump that also metered the feed to the first adsorption tank. Carbon was moved from the slurries with a small, 28-mesh-screen dipper. Samples were lightly washed with water and measured in a small pharmaceutical graduated cylinder, submerged in water, using a bulk-weight-to-volume ratio of 0.45 g/ml.
Target operating conditions were (1) 50 pct solids in the slurry with a flowrate of 4.044 l/hr, 67.4 ml/min, corresponding to a 1-hour slurry- retention time; (2) a solution flowrate of 2.979 l/hr with countercurrent carbon movement of 1.264 g/hr, (2.81 ml in bulk volume); and (3) minus 6- plus 16-mesh carbon concentrations as shown in table 13.
After putting the initial carbon in each tank according to table 13, the feed was metered to the plant for 26 hours without carbon movement to load the carbon with a total gold content close to what it would contain at equilibrium. Then carbon movement was started and continued for 36 hours, which is best summarized in three periods of countercurrent flow as shown in table 14.
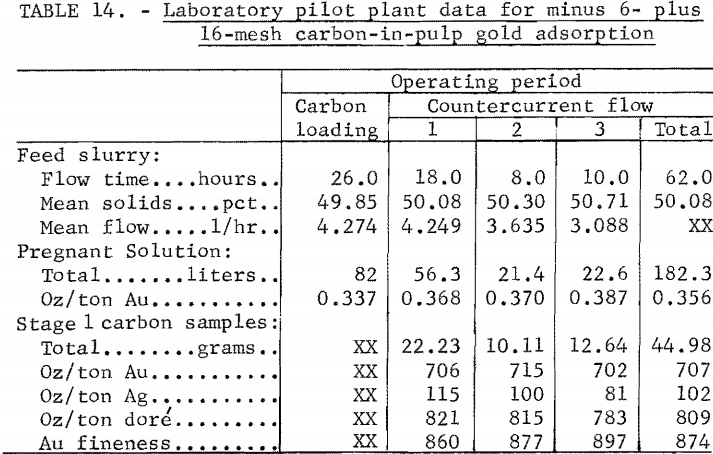
In the first period of countercurrent flow, the mean slurry flow rate was 4.25 l/hr compared with the target flow rate of 4.04 l/hr. After the initial setting of the feed pump each day, there was an upward trend in flow rate, which was ignored as long as the barren solution analysis was satisfactory. During the 18 hours of operation in this period, the mean barren analysis was 0.0009 oz/ton Au, and the barren analysis drifted upward from <0.0003 to 0.0015 oz/ton Au, compared with the target of 0.0005 oz/ton. The mean content of the carbon product in this period was 706 oz/ton Au and 115 oz/ton Ag.
Continued leaching action in the feed slurry slowly raised the gold content of the pregnant solution during the campaign. Therefore, the carbon movement was kept the same and the target slurry flow was recalculated to 3.705 l/hr for a pregnant solution of 0.37 oz/ton Au. Closer monitoring of the feed flow rate was also put into effect. During 8 hours of operation in the second period of carbon movement, the mean slurry feed rate was 3.64 l/hr, and the initial average barren analysis was 0.003 oz/ton Au, dropping to 0.002 oz/ton Au. The barren solution also contained an average 0.009 oz/ton Ag.
Although the pregnant solution ratio of silver to gold was about 1 to 4, the carbon loading ratio was about 1 to 6 and the barren silver content was satisfactory. To prevent the possible accumulation of silver in the system carbon, especially in the last two stages, the target slurry flow rate was adjusted to 3.05 l/hr. The basis of adjustment was a target carbon load of 800 ounces of Au plus Ag per ton from a pregnant solution containing 0.46 ounces of Au and Ag per ton. The carbon movement rate was not changed. During 10 hours of operation in the third period of carbon movement, the mean slurry feed rate was 3.09 l/hr and the initial average barren analysis was 0.001 oz/ton Au, dropping to 0.0006 oz/ton. The mean silver content of the barren solution was 0.008 oz/ton.
In 62 hours of pilot plant operation, a total of 403 pounds of jig tailings was processed in a cyanide leach slurry at an average 50.1 pct solids. The mean pregnant solution analysis was 0.356 oz/ton Au.
In 36 hours of carbon movement, the mean slurry-retention time was 0.93 hours, and the mean countercurrent weight ratio of solution to carbon was 2,228.28, or 0.449 grams of carbon per liter of flowing solution. The mean pregnant solution analysis was 0.372 oz/ton Au and the mean barren was 0.0006 oz/ton Au. The mean carbon product contained 707 oz/ton Au and 102 oz/ton Ag.
After the last carbon movement, final solution and carbon profiles were obtained as shown in table 15. In comparing carbon loading, the variable effects of entrained ore solids in the carbon should be kept in mind. The relative solution and carbon analyses are reasonable. The dore-fineness measure of the relative gold and silver content in the carbon declined from 952 in stage 1 to 438 in stage 4.
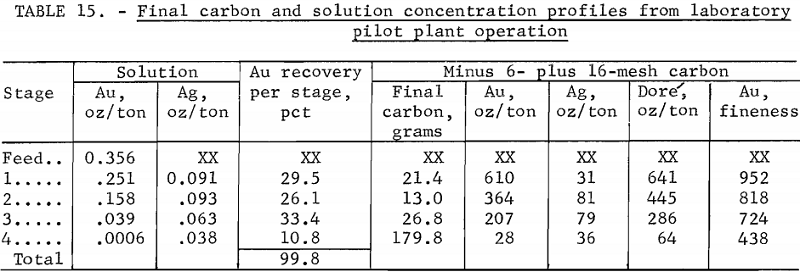
Gold balances checked within 5 pct, and carbon balances, within 4 pct. If the difference in initial and final carbon weights were attributed to attrition, the loss was 0.135 lb/ton of ore.
Conclusions
- A jig tailing with gold content ranging from 0.33 to 0.42 oz/ton was amenable to cyanide leaching with gold extraction of up to 97 pct in 24 hours at 50 pct solids. Minor silver content reported in the leach solutions at <0.1 oz/ton.
- In a 4-stage, 10-cycle, countercurrent, batch test, minus 10- plus 20-mesh coconut carbon was loaded to an average of 670 oz/ton Au from a pregnant solution containing 0.37 oz/ton Au, leaving a barren solution of <0.0003 oz/ton Au.
- In a 4-stage, 36-hour countercurrent, laboratory pilot plant test, minus 6- plus 16-mesh coconut carbon was loaded to an average of 710 oz/ton Au and 100 oz/ton Ag from a pregnant solution containing 0.37 oz/ton Au and <0.1 oz/ton Ag, leaving a barren solution of 0.0006 oz/ton Au.
- Batch tests on the cyanide leach slurries of the jig tailings provided valuable rate data and a gold adsorption equilibrium curve at ambient temperatures for minus 10- plus 20-mesh coconut carbon. A theory of cascade design, using the developed data, was successfully applied to the countercurrent batch test and the laboratory pilot plant test.
