Table of Contents
The Bureau of Mines research programs are partly directed to developing safety guidelines for the reduction of fires and explosions in industries that produce, transport, or utilize mineral fuels and their products. As a result of a request by the Department of Transportation, the Bureau is evaluating test methods for classifying hazardous materials and developing new methods, where necessary, for use in government transportation regulations. A report on methods for the classification of flammable liquids was recently prepared under this work. The present report is on flammable solids for which no classification test method is given in the transportation regulations. According to these regulations, a flammable solid is defined as any solid material, other than an explosive, which can be readily ignited or which can cause or contribute significantly to fire under the conditions encountered during transportation. Thus, to classify such materials, it is necessary to consider both their ignitability and flame spread behavior. Since existing test methods were not considered adequate for this purpose, new methods were developed which are described in this report. The methods are designed to evaluate (1) flammable solids that require high temperatures or an external energy source for ignition, and (2) extremely flammable solids that can self-ignite at normal ambient temperatures.
Proposed Method for Flammable Solids
Selection of a test method for classifying flammable solids is complicated by the fact that the ignitability or flammability hazard can vary greatly with the ignition stimulus, as well as with the physical form and size of the material. Minimum spark-ignition energies are frequently used to partially define the ignitability hazard of finely divided materials such as flammable dusts. However, such determinations are much less useful for coarse or massive solids since their spark-ignition energies tend to be extremely large. For example, the values for magnesium dust clouds can increase from 20 to at least 1,900 millijoules when the particle size is varied from about 50 to 200 microns. Minimum ignition temperatures, such as those determined in heated vessels, also are not considered suitable for this application. The ignition temperatures of most flammable solids, excluding the extremely flammable, are over 500° F and are primarily applicable to situations where the combustible and air are heated uniformly and under quiescent conditions to such elevated temperatures. Accordingly, a method was developed in which flammable solids could be compared by measuring their relative ease of ignition and rate of flame spread when exposed to flame in air. This simulates a typical ignition condition that could result from the inadvertent use of flame devices or from a fire produced in a cargo accident. A method for evaluating extremely flammable solids is given in a subsequent section of this report.
Figure 1 shows a sketch of the apparatus proposed for determining the ease of ignition of flammable solids. The apparatus consists essentially of a rotating disk with a variable slot near the outer circumference through which a pencillike flame is allowed to pass and impinge on the sample. The slot is ¼ inch wide and is fitted with an adjustable cover to vary the length of the opening. A butane torch capable of producing a uniform jet, approximately ¼ inch in diameter and 1-¼ to 1-½ inches long, is employed as the flame source. A propane torch would give essentially the same results. These tests were made with a commercially available torch (Turner burner with pencilpoint burner head, model No. 603) at a gas pressure of 7±0.5 psig. The minimum time for ignition (sustained flame) is obtained by varying the speed of the rotating disk and the length of the slot opening; disk speeds between 0.4 and
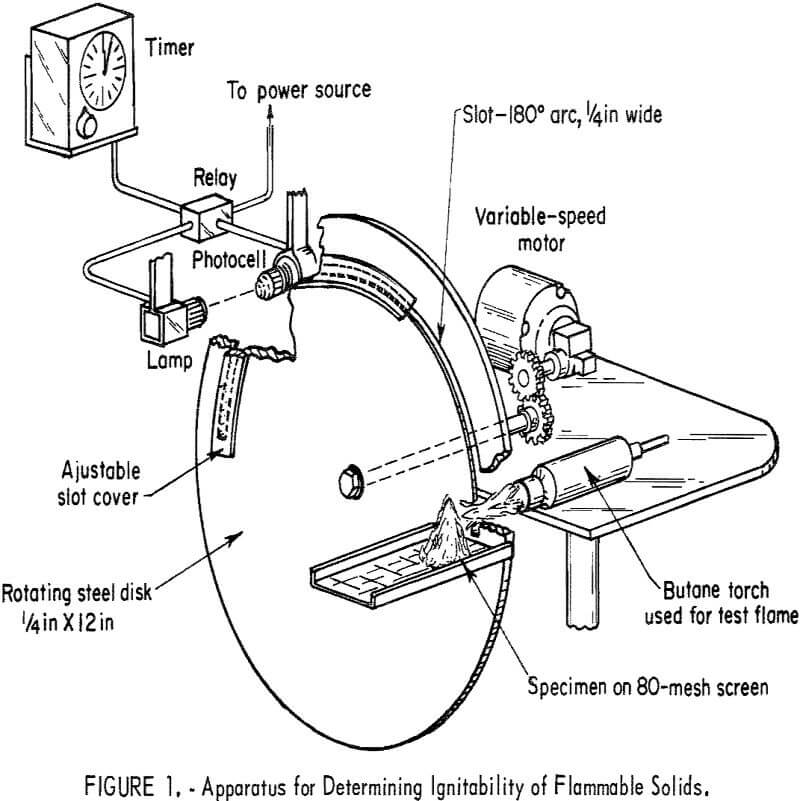
8.5 rpm were used in the present work. Duration of the flame in the slot opening is determined by means of a photoelectric cell and timer, as shown in figure 1. This apparatus is similar in principle to one developed earlier by the Bureau for evaluating the ignitability of potentially unstable substances like organic peroxides. The latter method used a more severe heat source (oxygen-hydrogen flame) with the sample confined to a small cup, as compared to a totally unconfined sample in the present method. German investigators have proposed the use of a propane or manufactured coal gas flame from a Bunsen burner, as well as various other heat sources, for comparing the ignitability of flammable solids. However, since ignition occurs more readily when the flame is applied to the exposed surfaces of a sample bed, that is, from above rather than from below the bed, the use of a Bunsen flame is greatly limited because of convection and buoyancy effects.
In the proposed method, the flame from the butane torch is applied near the base of the sample bed; which is cone shaped for powders or granular materials and which is supported by an 80-mesh stainless steel screen to permit vertical circulation of air through the bed. A cone-shaped bed, at least 1 inch in height, was required to obtain the lowest and most reproducible ignition times; smaller size beds yielded inconsistent results for the coarser or less ignitable materials. In addition, the distance between the sample and burner was fixed at approximately 1-¼ inches, beyond which the ignition times can be expected to increase. With sheet materials, small strips are supported in a vertical position to provide favorable conditions for ignition; the width of the strips is varied, depending upon the thickness of the sample. Generally, the shortest ignition times occur when the flame impinges edgewise on the strips.
Since the ignitability of finely divided solids can vary with particle size, the test samples should be at least as fine as the materials may be during their shipment. Fine powders can be evaluated primarily in their “as-received” condition. However, in the case of coarse materials, samples as fine as about 50 to 150 mesh (Tyler screen series) should also be evaluated, by pulverizing and/or screening the “as-received” materials, insofar as is possible.
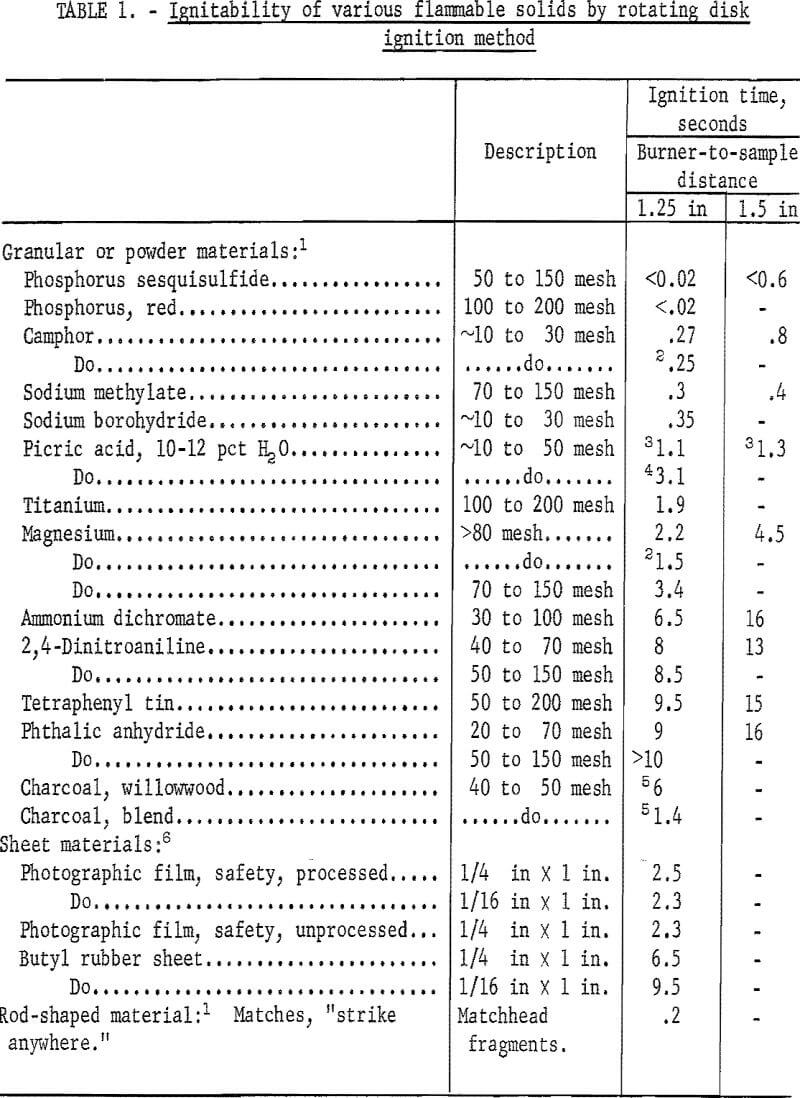
Table 1 summarizes the ignition times that were obtained with the rotating disk apparatus for various representative flammable solids. Most of the data are average values of at least two trials. Reproducibility is indicated by the following average ignition times and deviations for replicate trials with three of the finely divided solids. Burner-to-sample distance was 1-¼ inches.
Camphor……………………………………………………………….0.27±0.2 second (4 trials)
Ammonium dichromate………………………………………….6.5+0.3 seconds (4 trials)
Tetraphenyl tin……………………………………………………….9.4±0.6 seconds (3 trials)
With-the 1-¼-inch burner-to-simple distance, the ignition times ranged from less than 0.02 second for powders such as phosphorus sesquisulfide and red phosphorus to at least 9 seconds for phthalic anhydride and tetraphenyl tin; corresponding times with a 1-½-inch burner-to-sample distance ranged from less than 0.6 second to at least 15 seconds. Metal powders like titanium and magnesium, as well as water-wet picric acid (10-12 percent H2O), displayed a greater ignitability hazard than ammonium dichrornate, but less than that of sodium methylate, sodium borohydride, or camphor, each of which ranked just below the most highly ignitable solids. Note that a new stock sample of picric acid was more difficult to ignite than an old stock sample whose water content was apparently lower. For coarse materials whose particle size was varied in these tests, the ignitability hazard generally did not increase when samples as fine as approximately 50 to 150 mesh were used. Because of the nature of such solids as camphor, sodium borohydride, and the water-wet picric acid, their evaluation was necessarily limited to representative samples of the rather coarse “as-received” materials. As expected, wood charcoal samples did not produce normal ignition by this test method, although sustained incandescence was obtained. In addition, none of the sheet-type samples of safety photographic film and butyl rubber displayed a high ignitability hazard.
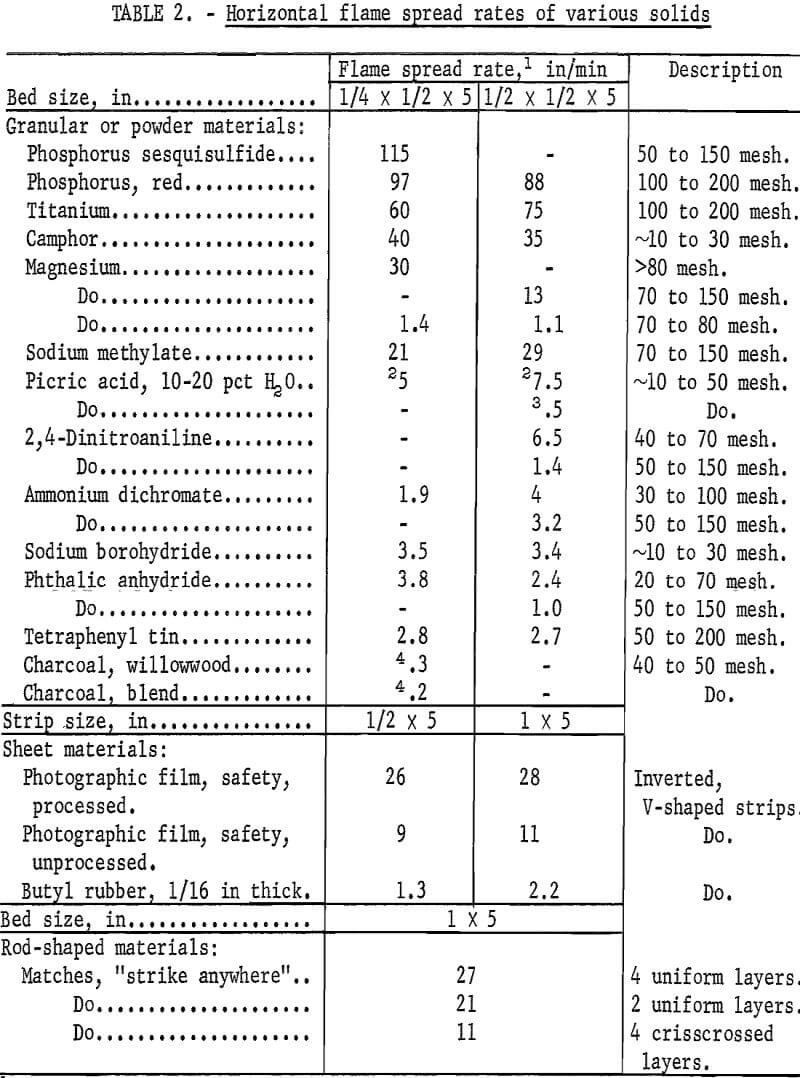
To classify flammable solids, a test method is also proposed for determining their horizontal flame spread rates. Although the rates are generally higher with upward burning, depending upon the angle, they are not normally determined in this manner with finely divided solids because of the problem of holding the samples in place. As in the ignition experiments, the samples are supported by a stainless steel screen (80 mesh) on a rectangular burning rack and ignited at one end with a butane torch or a similar flame source. Samples having essentially the same particle size ranges as in the ignition experiments are also used here. The sample bed should be at least ¼ inch high and ½ inch wide with all sides of the bed exposed to air. The rates are determined by measuring the time required for the flame to travel over a 5-inch sample bed, although longer beds can be used to increase the accuracy of the measurements. A total bed length of 7 inches was used here and the rates were measured over the final 5 inches of burning. To facilitate making these measurements, the burning rack is equipped with two fuse wires (0.5 amp) spaced 5 inches apart and connected to appropriate relays and an electric timer; a stopwatch can also be used for slow-burning solids. The flame spread rates for sheet-type samples are determined by using ½-inch or 1-inch by 5-inch strips that are folded to form an inverted V-shaped channel and which are supported on the burning rack by a few fine wires (0.01 inch). Rod- or bar-shaped samples are arranged in a crisscross pattern or other pattern that is most favorable for burning. In the case of materials such as matches, the layers should be arranged to permit measurement of the rate over the surfaces of the matchheads, that is, over the most flammable part.
Similar burning tests are proposed by the Department of Health, Education, and Welfare (HEW) for determining the flammability of hazardous household substances. However, the method proposed in the HEW regulation for rigid and pliable solids does not require the use of folded strips, which is a necessary condition for sustained horizontal burning of some flammable solids, including photographic safety films. Also, the method described in the above regulation for powders and granular solids can give lower burning or flame spread rates than expected because of large heat losses and limited air circulation due to the type of sample container used in that method, a flat, rectangular, aluminum foil boat. Furthermore, the proposed visual determination of burning time in the latter method is not reliable for fast-burning substances.
Table 2 summarizes the flame spread rates obtained for various flammable solids by the Bureau’s proposed method. The rates were reproducible to within ±10 to ±20 percent, depending upon their magnitude, and did not vary greatly when the height of the sample bed was increased from ¼ to ½ inch. (Lower rates usually occur when smaller sample beds are employed.) As noted, the rates of flame spread for the granular or powder materials varied from about 90 in/min or more for phosphorus sesquisulfide and red phosphorus to less than 4 in/min for materials such as phthalic anhydride and tetraphenyl tin. The wood charcoal samples again displayed the least hazard and did not propagate flame after ignition. In comparing tables 1 and 2, it is evident that the ignition data for some materials do not necessarily give the same order of hazard ranking that is indicated by the flame spread data. For example, sodium borohydride is easier to ignite than titanium and magnesium but the latter solids have much higher flame spread rates than that of sodium borohydride. Note also that the rates of metal powders such as magnesium depend greatly upon particle size. However, in the case of nonmetal powders, the flame spread rates are not necessarily increased with a decrease in particle size, particularly if the powders tend to agglomerate when forming the sample bed. Thus, although fine samples (~50 to 150 mesh) of the coarse materials should be evaluated where possible, their flame spread hazard with coarser or “as-received” samples must be given equal consideration. Generally, most of the flammable solids that displayed a high ignitability hazard also had high flame spread rates >10 in/min.
Proposed Method for Extremely Flammable Solids
No standard method is available for evaluating extremely flammable solids, such as pyrophoric powders, which can ignite spontaneously on exposure to air at ambient temperature. A test developed by the Bureau of Explosives (American Association of Railroads) for pyrophoric liquids is considered by a United Nations working group to be adaptable to some solids but not to powderlike substances. This method requires rather large quantities of hazardous materials and involves the use of a sawdust reacting medium, although the particle size, moisture content, and grade of sawdust are not specified. Also, the maximum relative humidity that is specified, 75 percent, may not be high enough for evaluating the pyrophoricity of some materials.
A method is proposed here for determining the ease of ignition of pyrophoric-type substances using small samples at ambient temperatures of 90° or 130° F and at various humidity conditions. The higher temperature is the maximum specified by the Department of Transportation for classification regulations. This method requires an environmental chamber in which the relative humidity can be varied from 50 to 90 percent and controlled to within ±5 percent. The sample is placed in the center of a 4-inch-diameter by 6-inch-long glass reaction tube that is mounted vertically in the environmental chamber and packed with glass wool to minimize heat losses. The reaction tube is open at both ends to permit circulation of air. The extent of reaction is determined by visual observations and by measuring the temperature rise near the top of the sample bed using a 30-gage iron-constantan thermocouple; output of the thermocouple is fed to a continuous-pen recorder. Because of their high reactivity, the samples must be handled in a nitrogen “dry box” and stored in gastight containers.
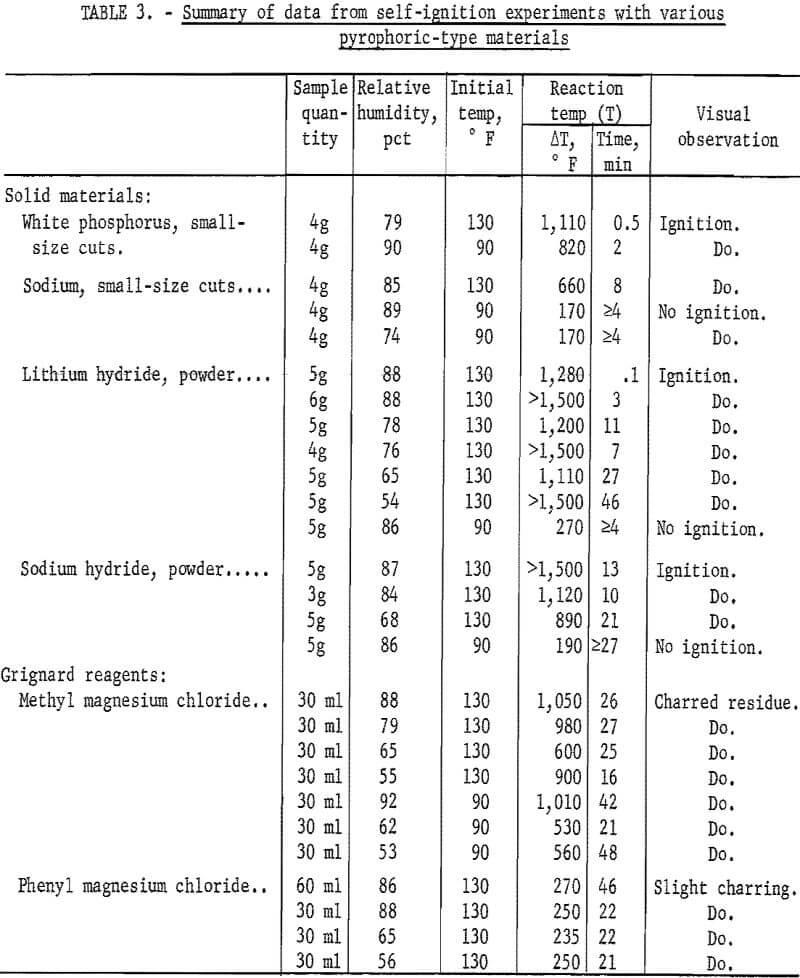
The present experiments were carried out in a 27-cubic-foot environmental chamber that was available, although a smaller chamber of only a few cubic feet capacity could be used. The chamber was equipped with heating controls and a 12-inch fan (1,500 rpm) to circulate the air through a closed-loop system. A steel shield was installed between the fan and the glass reaction tube to reduce the air flow around the reaction site; however, the results proved to be insensitive to such changes. Sample size and relative humidity were varied to obtain the optimum conditions for ignition; approximately 5 grams (0.16 oz) appeared to be adequate for the pyrophoric solids that were tested. With suspensions or solutions of pyrophoric materials, a sample of 30 ml or more was used; such samples were added to a small quantity of asbestos or glass wool before being placed in the center of the packed reaction tube.
Table 3 summarizes typical data from self-ignition experiments with several representative pyrophoric materials. The white phosphorus ignited at initial temperatures of 90° and 130° F, whereas the sodium, sodium hydride, and lithium hydride ignited only at the higher temperature over the range of relative humidities employed. Although data from all trials are not given, ignition probability tended to be greater at the higher relative humidities.
As noted by the 130° F data for the two hydrides, the times required for ignition generally decreased when the relative humidity was varied from 54 to 88 percent with lithium hydride and from 68 to 87 percent with sodium hydride. Since the measured temperature rises were dependent upon thermocouple location, the values shown in table 3 do not represent maximum values. Where ignitions are indicated, these were verified by visual observation of flame. Normal ignitions were not observed to occur with the two Grignard reagents at 90° or 130° F, although the temperature rises produced with the methyl magnesium chloride reagent (2.85 molar) were at least 1,000° F at the higher- humidity conditions. The phenyl magnesium chloride reagent (2.54 molar) produced only slight charring at 130° F and measured temperature rises were not over 270° F. Essentially the same results were found with this reagent when the sample quantity was increased from 30 to 60 ml or to 120 ml; also, the temperature rises were not any greater when the sample was mixed with a dry red oak sawdust rather than glass wool. Nevertheless, although the temperature rises were less for this material than for the others examined here, they are evidence of noticeable self-reaction which could conceivably lead to ignition under more ideal reaction conditions, such as those possible with large lots of the reactant materials. An increase in the concentration of the Grignard reagent may also increase the possibility of ignition.
Classification of Flammable Solids by Proposed Test Methods
The classification of most flammable solids should be possible by the test methods described in this report. Since the ease of ignition of a solid may give a different order of hazard ranking than indicated by its flame-spread behavior, both combustion properties must be considered to obtain a reliable classification. As outlined below, three classes of flammable solids are recommended for the transportation regulations. Where the classification of a particular solid is in doubt, the class reflecting the greater hazard should be assigned.
Class 1: Flammable solids which may ignite when exposed to flame such as a butane torch, but which propagate flame horizontally at rates less than 10 in/min by the proposed method.
Class 2: Flammable solids which are rated highly flammable either because of their great ease of ignition when exposed to flame such as a butane torch, or because of their ability to propagate flame at rates greater than 10 in/min. (Solids which ignite in less than 1 second by the proposed flame exposure test would be included in this class.)
Class 3: Extremely flammable solids which may ignite spontaneously in dry or moist air at ambient temperatures equal to or less than 130° F. (Solids which react to produce flame or temperature rises over 500° F by the proposed pyrophoricity test would be included in this class.)
One group of flammable solids that is not included in the above classification is the spontaneous-heating type which may ignite at ambient temperature from slow oxidation. The reaction time for such solids is usually several hours or more. This group includes such solids as granulated charcoal, animal fibers/and particularly, substances which have been contaminated with vegetable oils. To evaluate their maximum spontaneous heating hazard, an adiabatic tester similar to the type developed by the National Bureau of Standards is necessary; however, the design of this type of tester is relatively complex. Simpler designs such as the one developed by Factory Mutual Laboratories simulate adiabatic conditions rather roughly and are not necessarily intended for evaluating solids. Although solids capable of spontaneous heating can pose a serious ignition hazard, they are far less hazardous than pyrophoric materials. Therefore, they should normally be assigned to class 2 of the proposed classification.
On the basis of the present experimental data, the flammable substances that were investigated were classified according to the proposed scheme. Table 4 shows the classification ratings for the various materials by this method. This table also compares the ratings or classes that are given by the Inter-Governmental Maritime Consultative Organization (IMCO) and the National Fire Protection Association (NFPA) for the same materials and for a few others. The IMCO classification designates flammable solids as class 4 with the following subclasses:
Class 4.1: Flammable solids that are easily ignited by external heat sources.
Class 4.2: Spontaneously combustible solids or liquids.
Class 4.3: Substances that emit flammable gases when wet and which may ignite spontaneously in some cases.
The NFPA classes of flammability are defined as follows:
Class 1: Materials that must be preheated to ignite.
Class 2: Materials that must be exposed to relatively high ambient temperatures to ignite and solids that readily give off flammable vapors.
Class 3: Materials that can ignite under almost all ambient temperatures. Solids that can create flash fires and burn rapidly.
Class 4: Materials which vaporize at normal ambient temperatures or are easily dispersed, and which can readily form explosive mixtures in air.
In the NFPA classification, water-reactive materials are included under a separate reactivity hazard category.
Since the classification systems employ different numerical ratings or classes for hazard identification, it is difficult to make a direct comparison of the listings given in table 4. Nevertheless, some significant differences which are evident are worth noting. Under the Bureau classification, white phosphorus and the alkali metals and hydrides that were tested are assigned to class 3, which is identified as the most hazardous class on the basis of pyrophoricity in dry or moist air. Except for white phosphorus, such solids are also included in the most hazardous class by the IMCO classification because of their high reactivity with moisture or water. In comparison, the NFPA flammability classification gives a very low flammability hazard rating (class 1) for some of these same materials (for example, sodium), even though they may ignite spontaneously in moist air at near ambient temperatures. On the other hand, these materials are also listed as highly water-reactive substances by the NFPA. In the case of Grignard reagents, these should tend to fall in the pyrophoric class or a lower class, depending upon their composition and concentration. Substances which display an equally high flammability and water-reactivity hazard should be assigned to both hazard categories in the Department of Transportation classification regulations; a classification test method for evaluating water-reactive substances is currently being developed by the Bureau.
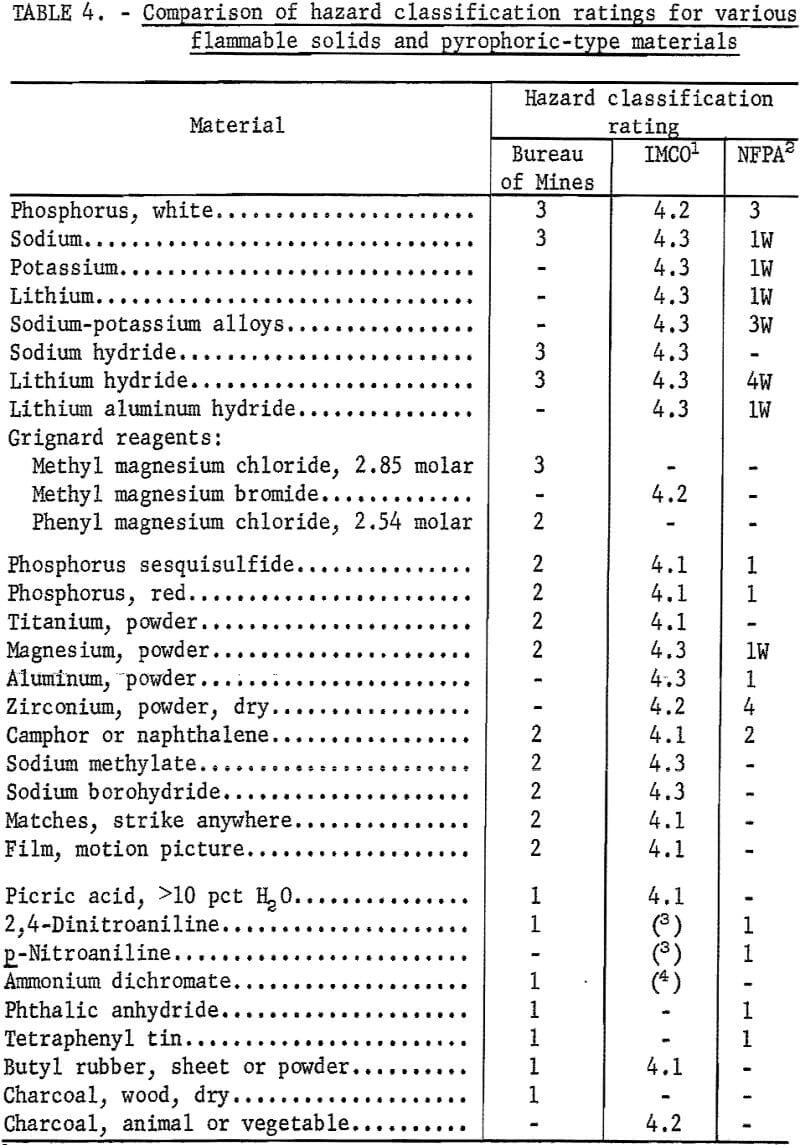
With flammable solids like phosphorus sesquisulfide and red phosphorus, the hazard rating (class 2) assigned by the Bureau classification is more severe than that given by the NFPA code (class 1). The lowest IMCO class, which includes most flammable solids, is assigned to the above powders and to materials such as titanium, camphor, motion picture film, and “strike anywhere” matches. All of these are in the intermediate hazard class by the Bureau scheme. Other finely divided solids in this grouping include magnesium, sodium methylate, and sodium borohydride. Although these are not extremely flammable when dry, they are potentially water-reactive, as indicated by the IMCO classification.
The least hazardous flammable solids that were evaluated in this work are included in the last group of substances listed in table 4. This group includes picric acid (>10 pct H2O), ammonium dichromate, tetraphenyl tin, and other materials having a low ignitability and flammability hazard by the proposed test methods. Under the IMCO classification, some of the materials having a low burning hazard are assigned to other hazard categories instead. For example, ammonium dichromate is classified as an oxidizing substance and dinitroaniline is classified as a poisonous (toxic) substance. Although most charcoals will tend to display a low burning hazard by the proposed flame spread test, they should be assigned to class 2 instead of class 1 under the Bureau classification system because of the spontaneous heating hazard that reportedly can be encountered with such materials. In all cases, the hazard classification should reflect the maximum hazard that the flammable solid may present under transportation conditions likely to be encountered.
