Table of Contents
Recycled titanium scrap is mixed with titanium sponge for ingot production and, therefore, plays an important role in the production of mill products. Clean and segregated scrap is recycled fay producers that regard their scrap-recovery procedures as proprietary. Unless a rigorous procedure is observed for segragating titanium scrap by alloy content, the scrap is not used in ingot production.
The steel industry consumes some of the titanium scrap as an additive in steel; however, tin-containing alloys such as the Ti-5 Al-2.5 Sn alloy are less desirable for steel production, and the market price is low compared with the price for other scrap such as Ti-6 Al-4 V alloy. Satisfactory segregation of scrap is difficult to maintain. Machine cuttings from chromium-bearing stainless steels are contaminants that are difficult to remove from titanium cuttings with a magnet. Removal of nonmagnetic metallic contaminants has been accomplished by the anodic solution treatment in the scrap-cleaning process described in this report. Stainless steels are also removable by the hydride-crush-dehydride method of preparing titanium for powder metallurgy.
Characteristics of Scrap Titanium Alloy Chips
Each type of titanium alloy has its own machining characteristics; some are more difficult to machine than are steels of the same hardness level.
Titanium’s low thermal conductivity, combined with the low tool-chip contact area, results in high tool-tip temperatures; for example, at the same cutting speeds, a temperature of 1,000° F developed at tool edge when turning steel, but in the titanium alloy, the temperature reached 1,500° F. The high tool-tip temperatures and the galling effect of titanium result in rapid tool wear. Some breaking of the carbide cutter leaves pieces of the cutting tool in the chips.
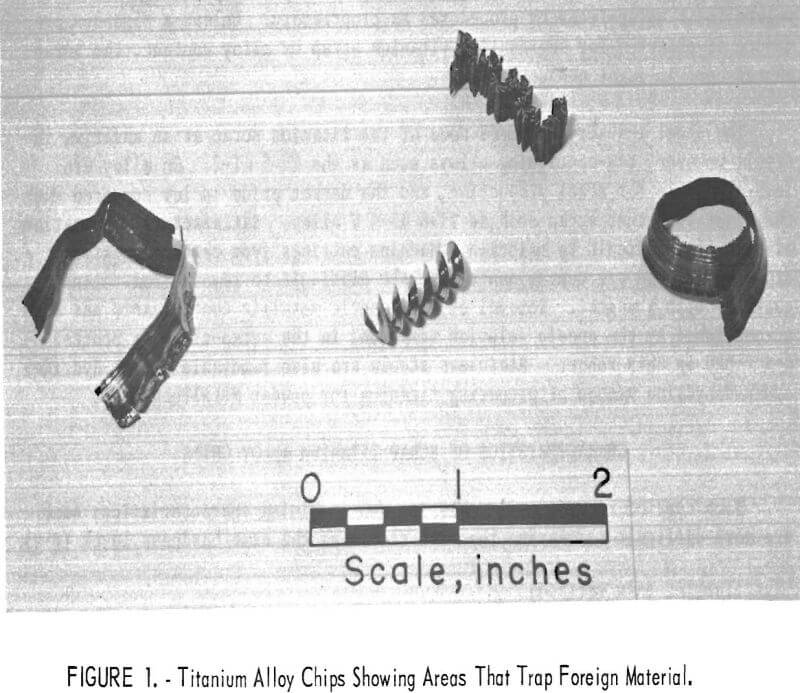
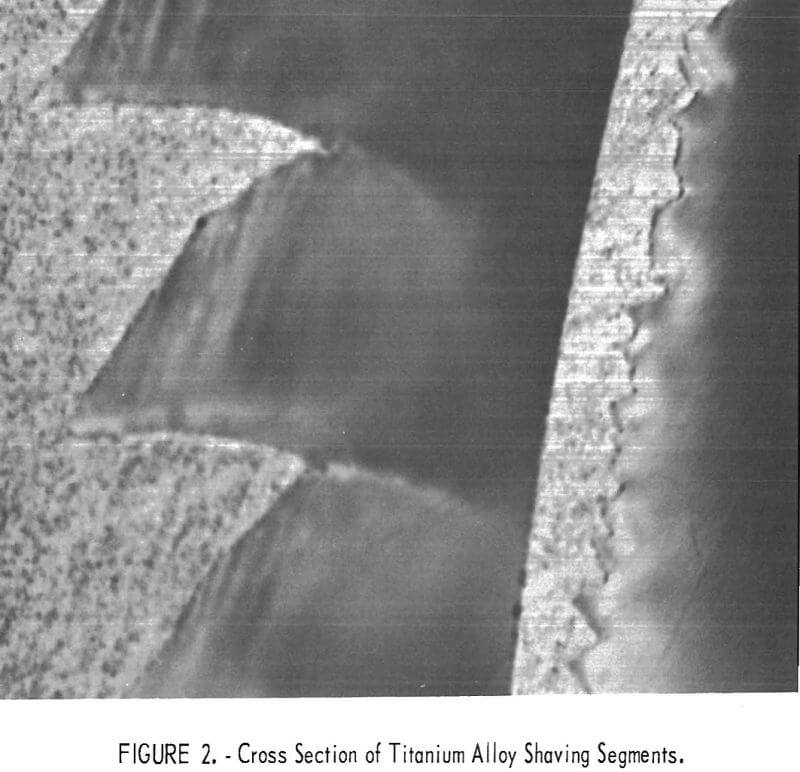
Typical titanium alloy chips are shown in figure 1. The titanium chips form a continuous band at the shaving surface that is in contact with the tool; on the opposite surface, segments of metal that resemble saw teeth are formed, as shown by a cross section of the shaving in figure 2. These segments form an excellent trap for oils, shop dirt, and other extraneous contaminants. A microscopic examination of the smooth surface shows contaminants of varied sizes, which are thought to be mainly tungsten carbide (WC) particles left from the cutting tool. No proof could be obtained. The WC particles were visible through a 30-power binocular microscope and would probably go into solution when the chips were melted.
The physical properties of thin chips from finished products differed considerably from the thick chips from ingots and mill products. Thin chips had about one-half of the bulk density of the thick chips, were approximately 5 mils thick, and formed into tight, elongated curls (with the segmented surface on the inside of the curls). The segments were much smaller than those of the thick chips, the thickness of which ranged from 10 to 20 mils. Some
chips had a heavy coating of cutting oils but very little shop dirt and no visible oxide.
Exploratory Tests
Tungsten Carbide Additions
Results of tests made to determine the particle size of WC that could be identified after melting into an alloy ingot are shown in table 1. Ingots were prepared in the same manner as were arc-melted buttons used for hardness testing. A mixture of sized WC particles and solvent-degreased Ti-6 Al-4 V chips was compacted in a hydraulic press to form a disk. Compacts were arc-melted in inert gas then turned over and melted a second and third time. Bottom surfaces of test buttons were ground lightly and then polished to reveal the WC particles. Tungsten carbide particles shown in figure 3 are minus 20- plus 30-mesh size.
Tungsten carbide particles finer than 100 mesh (0.006 inch) could not be detected after melting, although buttons were cut in sections to expose all areas. Apparently, these WC particles go into solution with Ti-6 Al-4 V. This was verified by the hardness of these buttons, which increased from 40 Rockwell C obtained on buttons containing plus 100-mesh WC particles, to 51 Rockwell C.
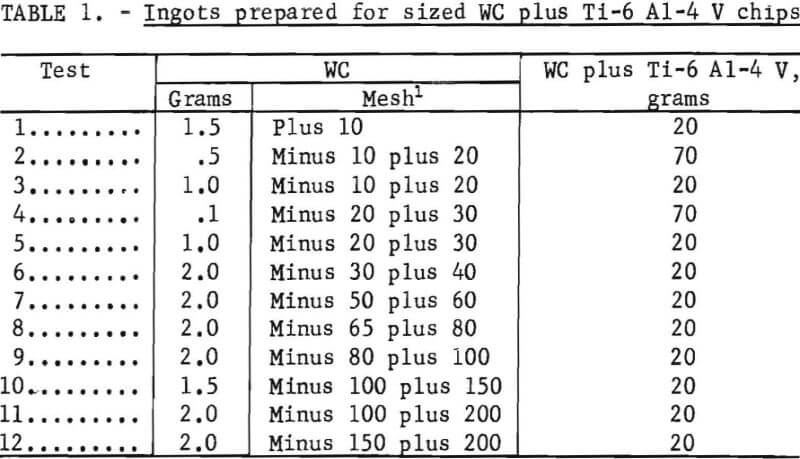
Note.-WC was on bottom in tests 1-9 but not discernible in tests 10-12.

Hydride-Crush-Dehydride
Hydride-crush-dehydride tests were made on solvent-degreased Ti-6 Al-4 V chips to determine the feasibility of this procedure for removing WC, stainless steels, nickel alloys, and other metal contaminants from the chips. Hydriding is one method for preparing titanium powder for the powder metallurgical (P/M) fabriation of titanium and titanium alloys, Titanium production parts are made by the P/M method.
One to five grams of plus 10-mesh stainless steel particles were added to a 10- to 30-gram charge of chips. A charged hydriding tube was evacuated, purged twice with inert gas, and then heated to 200° C under vacuum to drive off volatiles. Hydrogen was fed in; absorption began at 350° C and increased rapidly, attaining a maximum at 700° to 800° C, and then ended quickly. The charge was cooled to room temperature under a static pressure of hydrogen. Hydrogen content of the product was 2.5 to 3 percent. It was brittle and easily pulverized with a mortar and pestle. Hydriding of the stainless steel particles was insufficient to alter their physical properties noticeably; they remained unaffected when the hydride was crushed. An almost complete separation was made by screening the crushed hydride through a 20-mesh or finer screen.
Tungsten carbide bits fractured when the titanium hydride was ground in a steel mortar. Scriver has compiled a list of the types of cutting tools currently used on titanium, which includes high-speed steels and carbide-type tools. Fragments of cutting tools found in the chips and turnings were mainly cobalt-tungsten grades that were magnetic because of the cobalt binder. Tungsten carbide particles were virtually 100 percent recoverable magnetically when added to hydride chips and crushed. It was hoped that the WC could be removed by screening after the titanium hydride was crushed. To test this, 1 gram of plus 200-mesh-size magnetic WC was added to 9 grams of hydride chips and crushed. Recovery of WC from different screen sizes by use of a magnet showed that 4 to 20 percent of the WC passed through a 325-mesh screen by the time 98 percent of the titanium hydride was pulverized to minus 325 mesh. This proved that only 80 to 96 percent of the WC was removed by grinding and screening titanium hydride.
Dehydriding was accomplished by heating the crushed hydride in a vacuum-tube furnace after evacuating and purging the tube twice with helium gas. Degassing of the hydride was started with the vacuum pump. Degassing for 15 hours at 750° to 850° C resulted in a stable residual pressure of 0.001 torr. The hydrogen content was less than 0.019 percent after dehydriding.
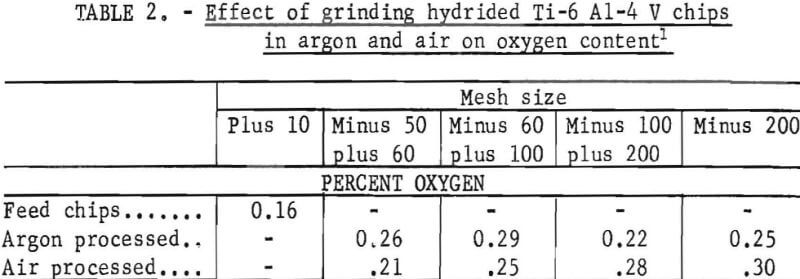
The effect on oxygen content of hydride-crush-dehydride chips ground in an inert atmospheric chamber and in air is shown in table 2. The oxygen increased for all mesh sizes in both the inert-gas atmosphere and in the air processing. This oxygen increase is inherent in a process in which small-particle titanium is produced. An increase in oxygen is undesirable, so this approach was abandoned. The oxygen values in table 2 may be low because they were analyzed on a vacuum fusion apparatus in which aluminum tends to prevent oxygen escape from the molten platinum flux used in the analysis.
Solvent Cleaning
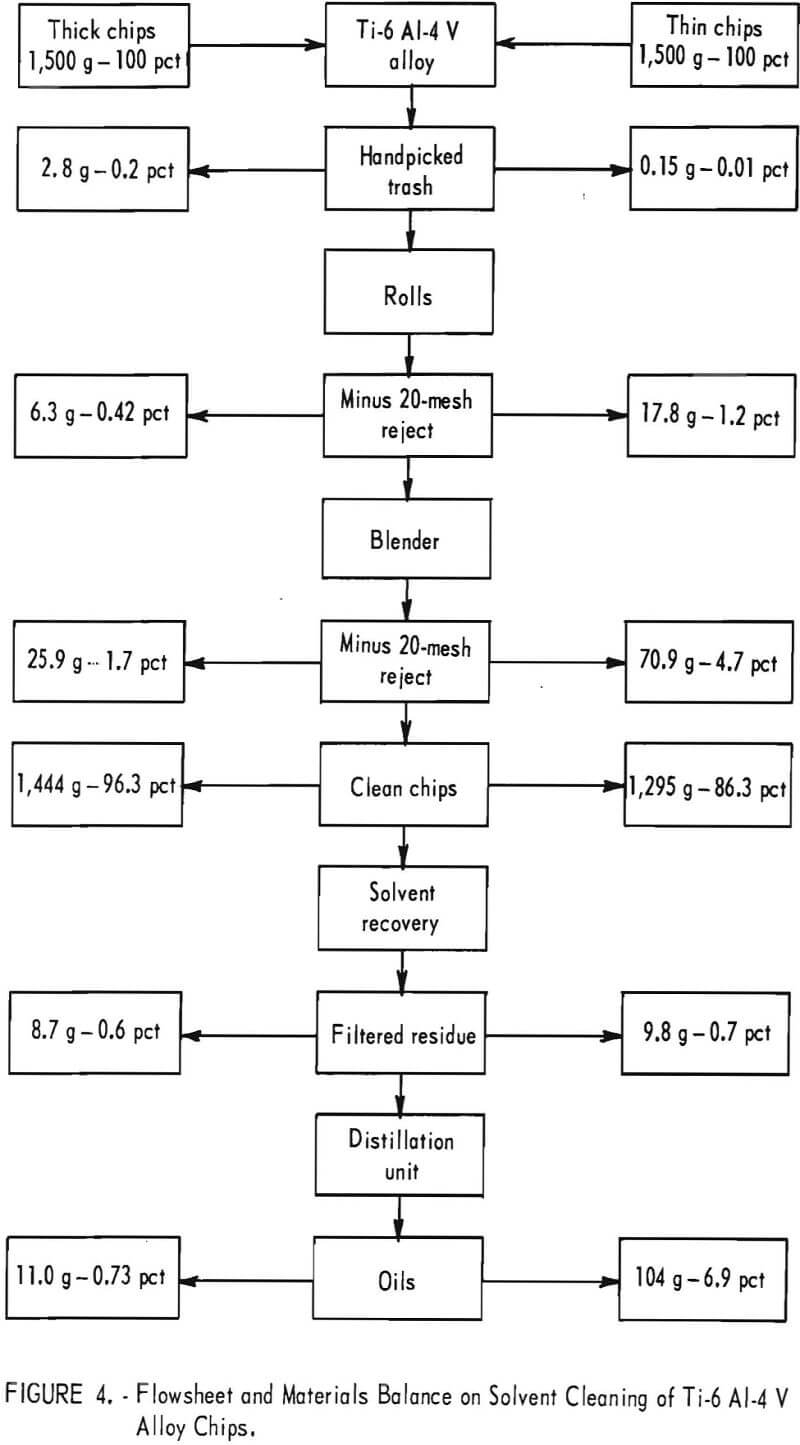
Cleaning tests were made on Ti-6 Al-4 V alloy chips, using a 1-gallon-capacity blender for mixing. Figure 4 shows a flowsheet and materials balance for solvent cleaning of alloy chips. Using ethylene dichloride as the solvent, chips were processed in 300- to 400-gram batches by mixing for 1 minute in three separate solvent additions. The fresh solvent added to the third mix was used for the second mix and then used as the starting solution for the first mix. Used solvent was recovered by distillation.
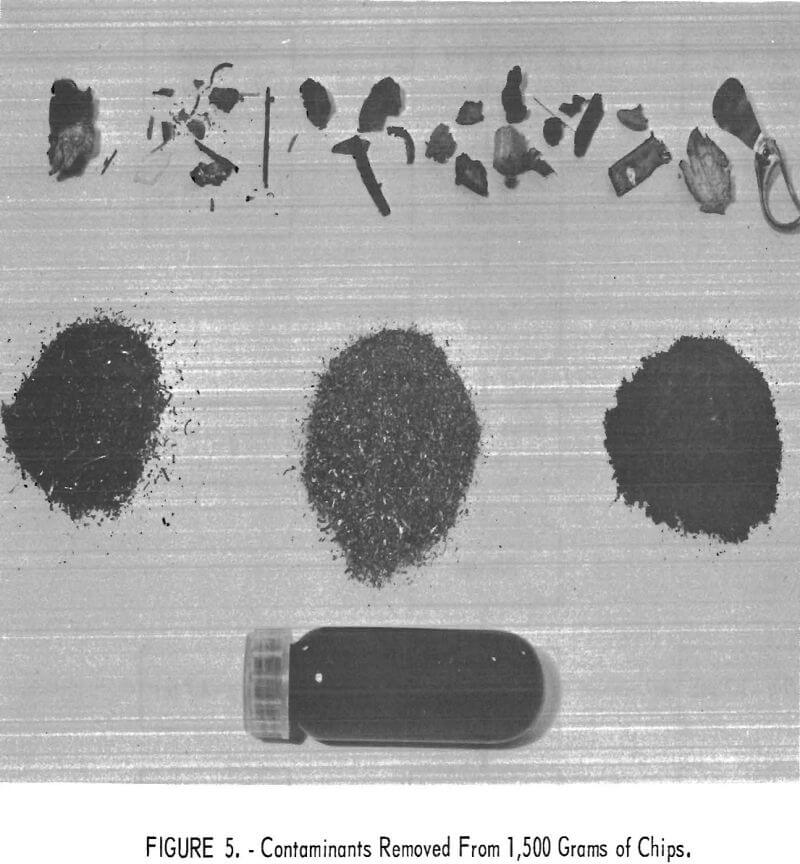
The contaminants removed from 1,500 grams of thick chips are shown in figure 5. Handpicked trash is at the top minus 20-mesh reject after rolls reduction and screening is at the bottom left corner; minus 20-mesh reject after blender mixing is at center; filtered residue from used solvent is at right corner. The test tube contains oils recovered from distilled solvent.
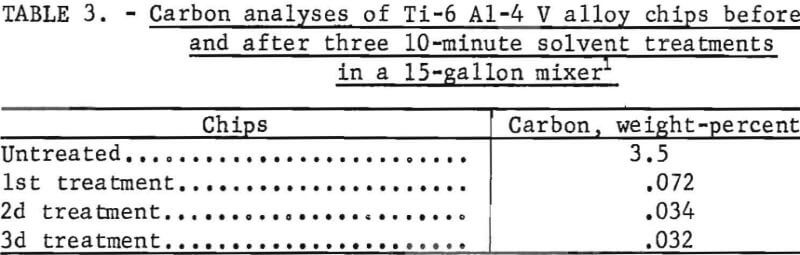
These tests showed that chlorinated hydrocarbon solvent would remove the oils from alloy chips. Solvent losses were larger than desired; therefore, the ethylene dichloride was replaced by perchloroethylene. Tests were conducted with a static basket vapor degreaser and a 15-gallon mixer (using perchloroethylene) to determine if the blender could be replaced. A carbon analysis of the chips determined the effectiveness of oil removal. Carbon analysis of one batch of thin chips as received ranged from 2.7 to 3.6 percent. After treatment in the vapor degreaser, the carbon content was 1.1 to 1.8 percent, whereas the carbon content of the chips treated in the blender was 0.03 to 0.04 percent. From the results shown in table 3 of carbon analyses of alloy chips cleaned in a 15-gallon mixer using perchloroethylene as a solvent two treatments gave satisfactory degreasing.
Detergent Cleaning
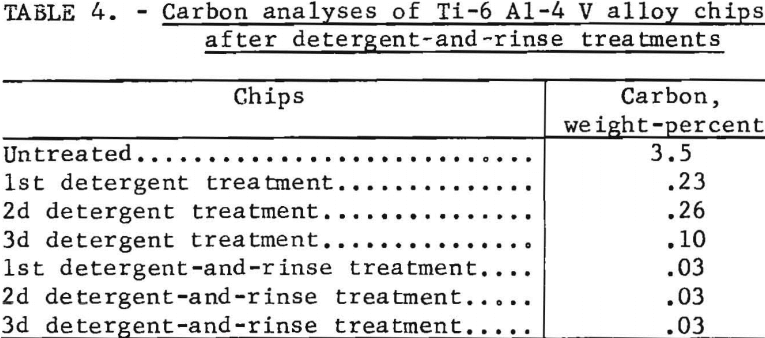
The effectiveness of a detergent to clean the turnings was tested by using a solution of 1 pound of a commercial alkaline cleaner dissolved in 7 gallons of 40° C water. Three and one-half pounds of turnings were placed in the solution and treated for 10 minutes in a 15-gallon mixer. Turnings were treated a second and third time in fresh detergent solutions. A portion of the turnings from each treatment was given a water rinse treatment. Carbon analyses in table 4 show that practically all of the oils that can be removed by detergent are removed during the first treatment. The residual coating left from the detergent solution was removed by a water rinse treatment. Detergent cleaning was effective; however, neither an efficient method of recovering oils from solution nor a satisfactory recovery of detergent solution for nonpolluting disposal was obtained. Detergent cleaning requires more study but could probably replace the solvent degreasing method and lower the costs of the process.
Anodic Solution Tests
Anodic solution tests were made using specimens of stainless steel 316 and 321, Hastelloy C, Inconel, molybdenum, and WC tool bits. All of these materials dissolved anodically in the 10 weight-percent sulfuric acid. Flakes of black, white, and yellow precipitates floated continuously off the pieces of WC tool bits and settled on the bottom. X-ray analysis showed that the precipitates were tungstic oxides, which dissolved readily in a 10-percent NaOH solution. No soluble tungsten remained in the H2SO4 electrolyte.
Other exploratory tests were made in liter beakers, using a direct-current source, a titanium strip for the cathode, and an anode basket of perforated titanium. With an electrolyte of 10 weight-percent sulfuric acid and a 5-volt potential, the titanium anode basket attained passivity within seconds; at 10 volts, a slow decomposition of electrolyte continued at a fraction amperage flow. In initial tests, iron, copper, or molybdenum was mixed with titanium alloy chips and electrolyzed in 10 percent H2SO4 at 5 volts. In all tests, the cell amperage decreased as the additive metal dissolved.
The exploratory tests proved that an anodic solution technique was suitable for removing metallic contaminants from titanium alloy chips. This procedure was tested on a larger scale by mixing 1,000 grams of titanium chips with 20 grams of selected contaminants. The mixture was placed in an 8- by 12-inch polypropylene plating barrel having 3/32-inch perforations and containing two titanium-tipped anode leads (fig. 6). The plating barrel was connected anodically and rotated in a tank containing 90 liters of 10 weight-percent H2SO4 electrolyte using a titanium cathode. All tests were conducted at room temperature and at a constant voltage of 5 volts. The tests were stopped when the current decreased to or below that established for pure titanium chips.
In figure 7 some of the data was plotted as current versus time for titanium alloy chips and chips containing other metal impurities. One test
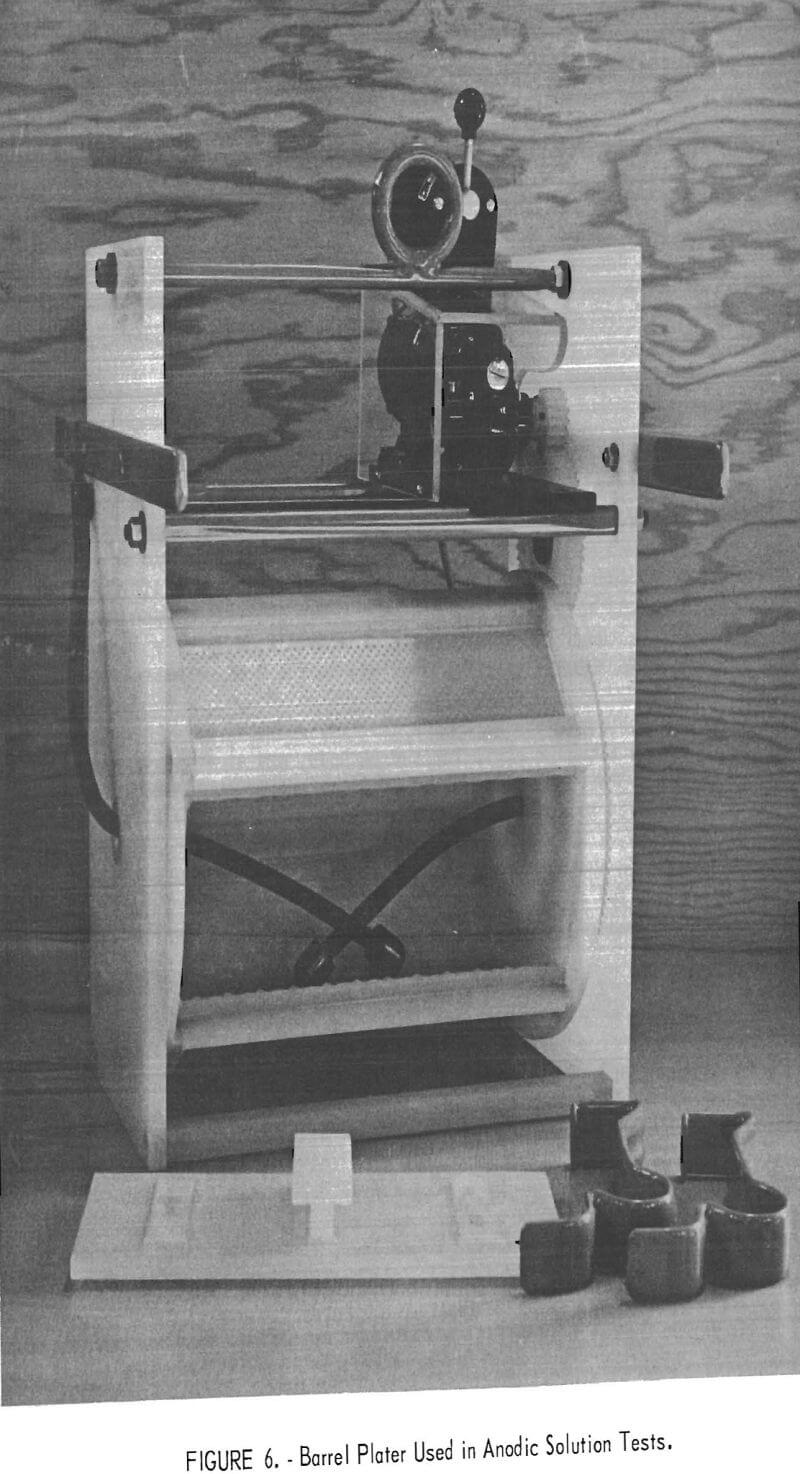
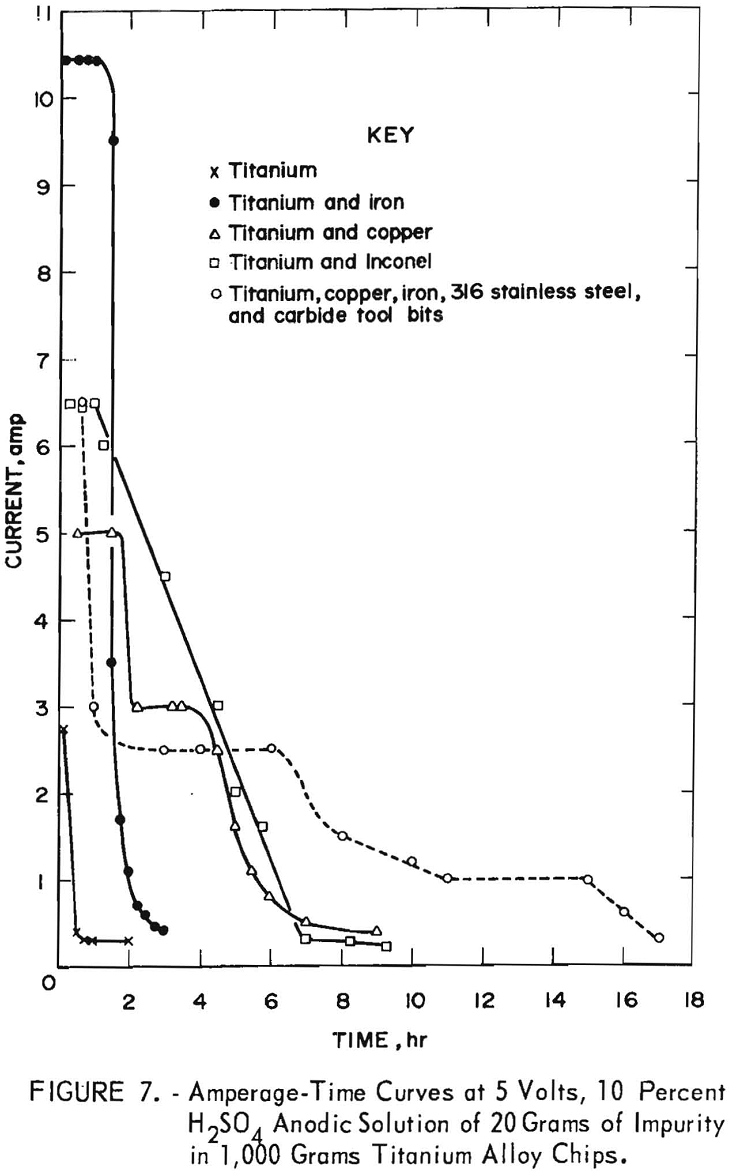
contained 5 grams each of the four impurities (fig. 7).
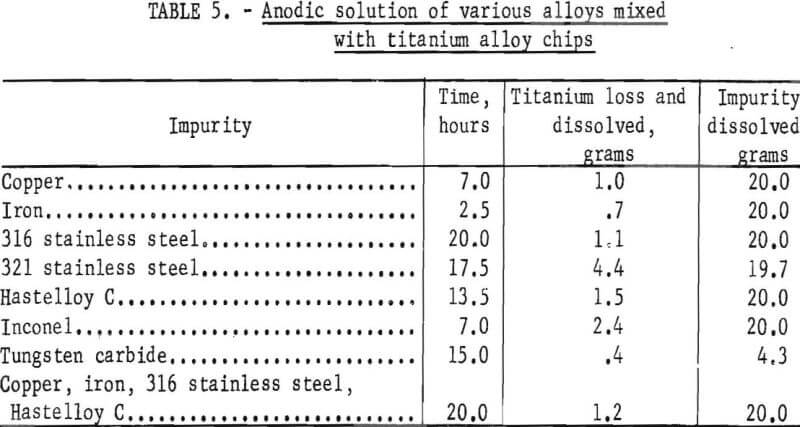
When each test was completed, the scrap was washed in tap water for 30 minutes and in distilled water for 15 minutes. It was then dried in an oven for 16 hours. A magnet was passed over the magnetic alloy scrap to remove any iron and tungsten carbide that remained undissolved; scrap containing nonmagnetic alloys and copper was visually inspected for undissolved material. The dried titanium alloy scrap and undissolved impurity scrap from each test were weighed, and the amounts dissolved were recorded in table 5.
The titanium losses were caused mainly by particulate material formed by attrition from tumbling, which then washed through the 3/32-inch perforation. This was substantiated by the 2.2 grams of dissolved titanium in the used electrolyte after the test series. Electrolyte loss was 18 liters after 105 hours of electrolysis.
The anodic solution times ranged from 2.5 hours for iron to 20 hours for type 316 stainless steel. Satisfactory anodic solution of tungsten carbide was not attained, partly owing to greater particle thickness, but mainly owing to the particle shape, which made it difficult to maintain electrical contact with the alloy. Magnetic separation was more satisfactory for removal of tungsten carbide.
Chemical Solutions Tests
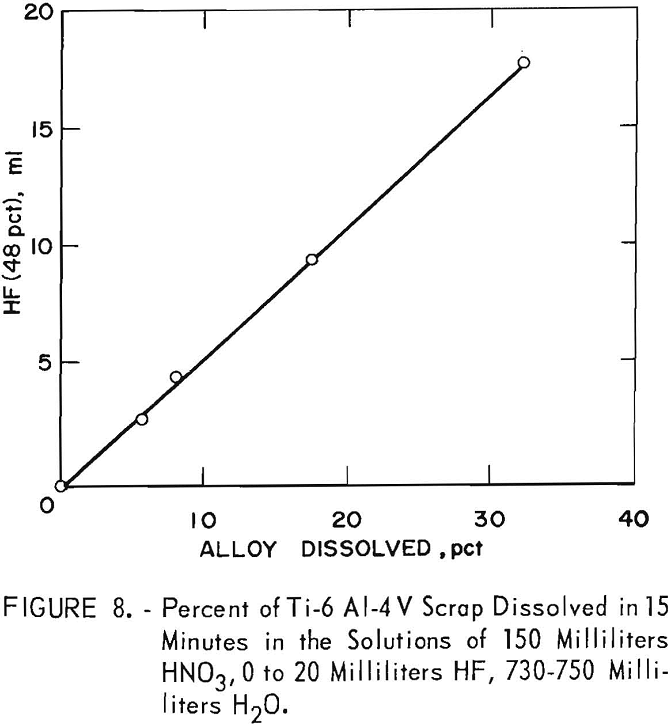
Composition of the etching solution was derived from tests using an acid solution of 150 milliliters HNO3, 0 to 20 milliliters HF, and 730 to 750 milliliters H2O. The solution rate on titanium scrap was directly proportional to the HF content (fig. 8). When the solution contained 50 milliliters HNO3, 20 milliliters HF, and 930 milliliters H2O, the dissolving titanium precipitated out; X-ray analysis showed the oven-dried precipitate to be TiO2 (anatase). This pickle is a special cleaning step that would not be necessary in most operations.
Processing Unit
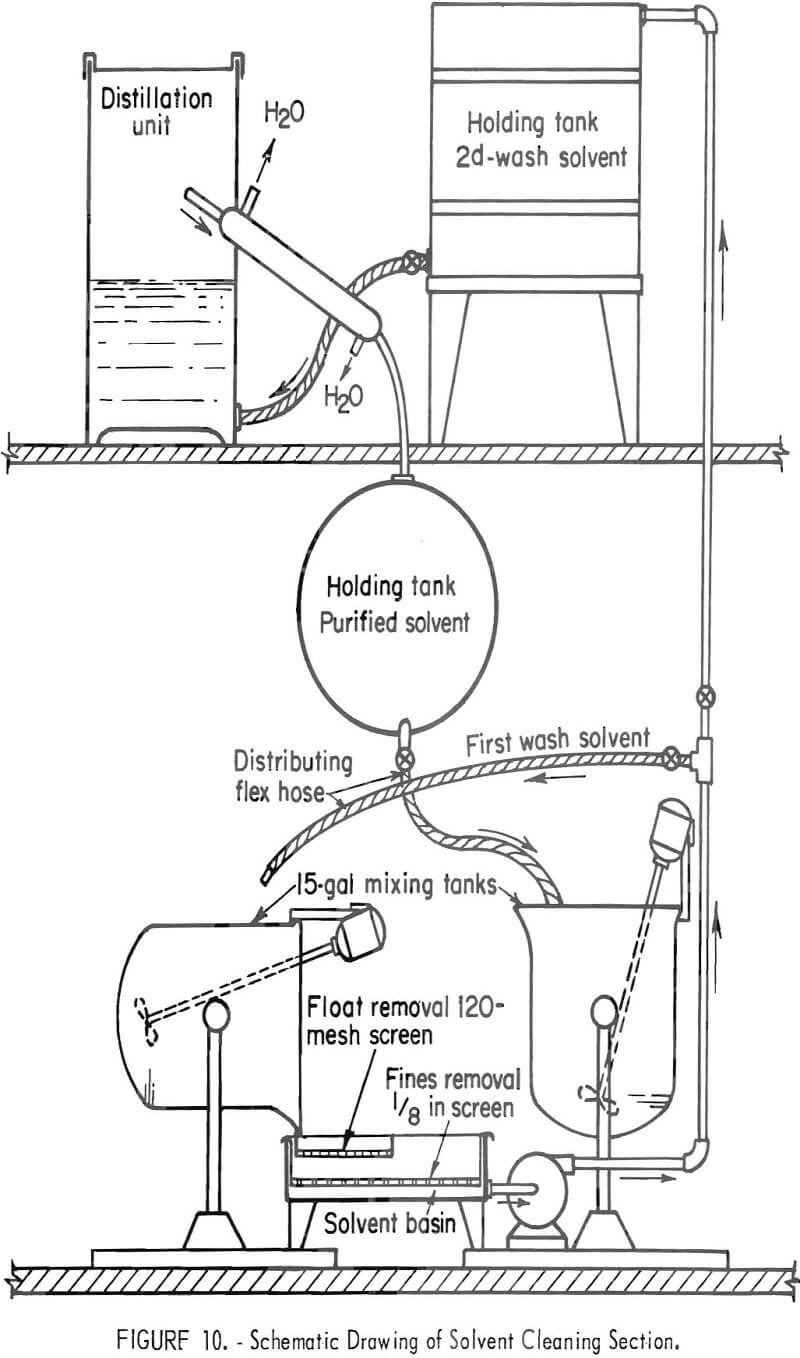
A complete processing unit was assembled to clean titanium alloy chips that would be vacuum-melted into ingots. A flowsheet is shown in figure 9. Large pieces of paper were hand-picked from the chips during the loading operation. Two 15-gallon mixers were each loaded with 6 pounds of thick titanium chips or 3.5 pounds of thin chips; 8 gallons of perchloroethylene was added to the first mixer. A schematic drawing of the solvent cleaning unit is shown in figure 10, and a photograph of the unit, in figure 11.
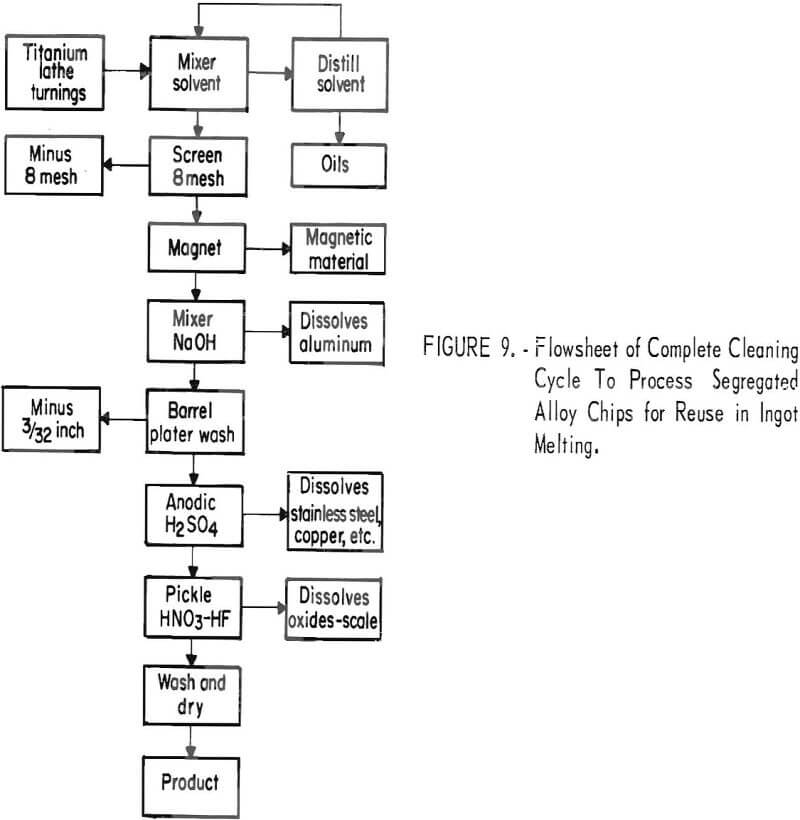
As the solvent was poured over a 120-mesh screen, the floating material (wood, paper, etc.) was caught. The solvent drained to a sump and was pumped into the holding tank. New perchloroethylene was added to the first mixer and agitated for 10 minutes as before. The solvent was again decanted through the 120-mesh screen to catch floating material and then pumped into the second mixer. In this way, each batch of solvent was used on two batches of chips before being pumped into the holding tank. From the holding tank, the solvent went to the distillation unit. The solvent-cleaned chips were then poured onto an 8-mesh screen to drain. They were drained, dried, and passed over an 8-mesh vibrating screen. The magnetic material was removed from the plus 8-mesh fraction. If visual examination revealed that the scrap contained large amounts of aluminum alloy turnings that needed to be removed, it was treated in the 15-gallon mixer with 25 weight-percent NaOH, then water-washed and loaded into a barrel plater with titanium-tipped contact (fig. 6). It was next treated anodically in 10 weight-percent H2SO4 until the current flow was reduced to approximately \ ampere. This dissolved the stainless steel, copper, etc. The barrel plater was moved to a water-wash tank and then into the pickle tank containing 4 gallons of concentrated HNO3, 1 quart of 48 percent HF, and 20 gallons of water. This pickle solution may also be eliminated if there is no visible oxide on the chips. The chips were water-washed, dumped from the barrel plater into drying pans, and oven-dried at 50° C.
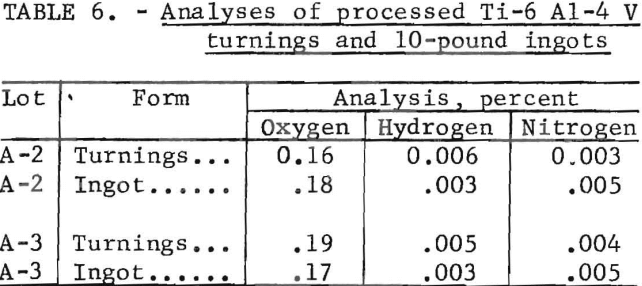
The Albany Metallurgy Research Center prepared 10-pound ingots from cleaned alloy chips for evaluation. Analyses of two batches of Ti-6 Al-4 V
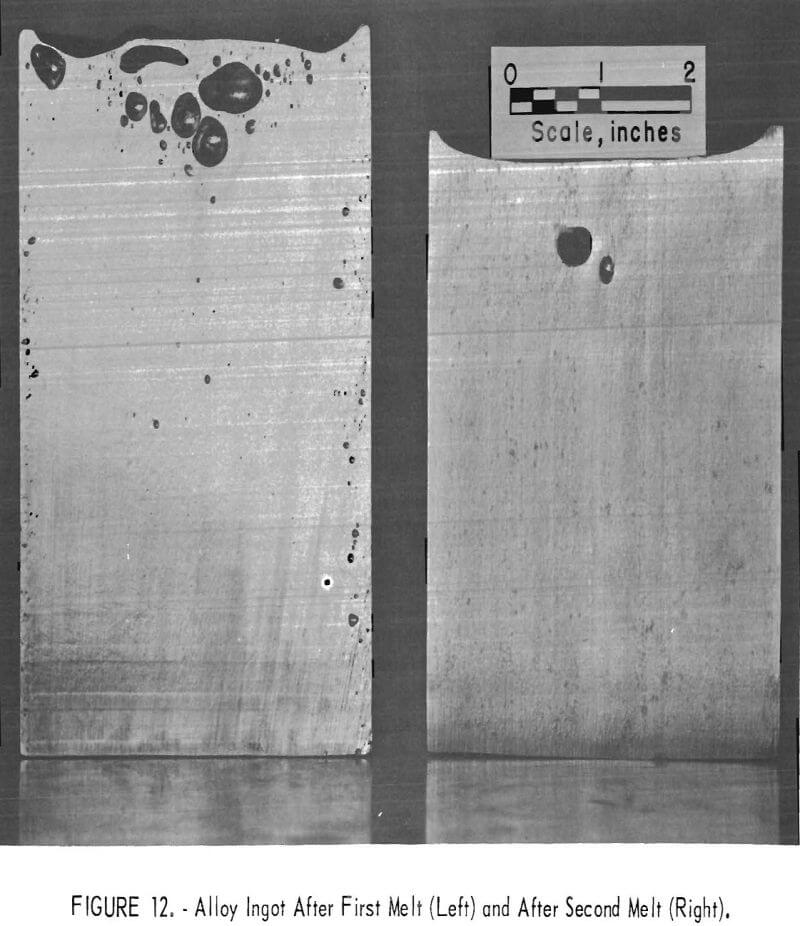
turnings and two ingots are shown in table 6. The process appears satisfactory for recycling titanium alloy chips. In experiments on ingot melting, all of the first melt ingots had gas holes near the outside edges. A second melt eliminated this condition, as shown by the cross section of ingots in figure 12 from both the first and second melts.
Summary
Associated laboratory tests on removal of impurities from segregated titanium shavings were described. The tests included the following: Impurity removal by the hydride-crush-dehydride method for powder metallurgy fabrication; evaluation of sized WC impurities in molten titanium alloy button ingots; and tests on solvent cleaning, chemical solution, and anodic solution.
The process that was developed and operated for complete cleaning of the segregated titanium alloy chips for reuse in ingot melting included the following steps;
- Solvent degreasing.
- Magnetic removal of iron and tungsten carbide.
- Aqueous hydroxide to remove aluminum alloys.
- Anodic treatment in sulfuric acid to remove stainless steels nickel alloys, etc.
- Pickling in nitric-hydrofluoric acid for surface oxide removal.
- Water washing and oven drying.
The ingots prepared by the Albany Metallurgy Research Center from the cleaned scrap showed practically no differences in interstitials content from those of ingot specifications. Gas holes that formed near the outside edges of the first melt ingots were eliminated by remelting.
Steps 3 and 5 could probably be eliminated for most uses, and shops using this procedure could economize by checking each step and using only those required for their operation.
