Table of Contents
Because of its poisonous qualities, the arsenic which is sometimes present in coal and in the atmospheric dust resulting from coal combustion can present a threat to health. As part of its continuing program to reduce health hazards in the mineral industries and to combat air pollution, the Bureau of Mines has surveyed methods for determining the presence of arsenic in coal. In a spectrographs survey of trace elements in coal ash, arsenic, reported as As was detected in the ash in 67 percent of the eastern coals tested, in 41 percent of the samples from the Central States, and in 16 percent of those from the Western States. The limit of detection by the spectrographs method is 0.005 percent of the ash. The survey shows that some ash samples contain as much as 0.2 percent, but the ashes in which it is detected usually contain less than 0.03 percent arsenic.
A more rapid chemical method of determining small quantities of arsenic in coal is desirable to supplement the spectrographic method and ISO Recommendation No. 601. According to the ISO method, the organic matter in the coal is destroyed by wet oxidation with boiling H2SO4 and HNO3, and the arsenic is obtained in solution. The arsenic is separated as arsine by the action of zinc in H2SO4 and absorbed in dilute iodine solution. The arsenic collected is determined colorimetrically by the molybdenum-blue reaction. The colorimetric procedure developed by Crawford, Palmer, and Wood, was reported by Crook and Wald to give better duplication of results than had been obtained with the former paper stain methods of estimating arsenic.
As the wet oxidation process with boiling H2SO4 and HNO3 is somewhat tedious, Crook and Wald also used a more rapid dry oxidation method in which the coal sample mixed with potassium permanganate and MgO was ignited in a stream of oxygen. They determined the arsenic by the colorimetric method in a H2SO4 solution of the ash residue. Kunstmann and Bodenstein added MgO to the coal sample and ashed the mixture at 650° to 700° C. After treating the ash with H2SO4 the arsenic was determined according to the ISO method. They mentioned that Archbutt and Jackson extracted arsenic from coke with hot concentrated HNO3 without ashing.
In this investigation arsenic is determined by the ISO method, using wet oxidation and by two modifications. In the first modification, the coal sample is mixed with MgO and ashed at 650° C. The ash residue is treated with dilute H2SO4 and the determination completed according to the ISO method. In the second modification, the arsenic is extracted from the coal sample with hot dilute HNO3 by a procedure similar to that used for determining pyritic sulfur. H2SO4 is added to the filtrate containing the HNO3 extract, and the solution is evaporated to fumes of SO3 to remove HNO3. Arsenic in the H2SO4 solution is determined by the ISO method.
This report gives results for arsenic obtained by the ISO method and by the two modifications for 16 coals ranging in arsenic content from 0.0003 to 0.011 percent. Results are included for arsenic determinations in float- and sink-fractions of three coals, and for arsenic retained after high-temperature ashing tests at 750° to 1,300° C.
Procedures
ISO Recommendation No. 601
Reagents
Zinc, granular, 20 mesh.
Hydrazine sulfate solution, 0.15 percent. Dissolve 0.15 gram in 100 ml of water.
Stannous chloride solution, 40 percent. Dissolve 20 grams of SnCl2·2H2O in concentrated HCl and dilute to 50 ml with concentrated HCl.
Potassium iodide solution, 15 percent. Dissolve 7.5 grams of KI in 50 ml of water. Prepare fresh daily.
Lead acetate solution, saturated. Prepare fresh daily.
Sulfuric acid solution, approximately 7 N. Add 200 ml of concentrated H2SO4, cautiously, to about 700 ml of water, cool and dilute to 1,000 ml.
Sulfuric acid solution, 5 N. Add 140 ml of concentrated H2SO4, cautiously, to about 500 ml of water, cool and dilute to 1,000 ml. Standardize against sodium carbonate using methyl orange as indicator and adjust to 5.0 N.
Ammonium molybdate solution, 1 percent in 5 N H2SO4. Dissolve 1 gram of (NH4)6Mo7O24·4H2O in about 80 ml of 5 N H2SO4 and dilute to 100 ml with the same acid.
Stock iodine solution, approximately 0.02 N. Dissolve 2.54 grams of iodine in 25 ml of water containing 8 grams of KI. Dilute to 1,000 ml and store in a dark glass bottle.
Working iodine solution, approximately 0.001 N. Dilute 5 ml of the stock iodine solution to 100 ml. Prepare fresh daily.
Stock arsenic solution (1 ml equals 1 mg As). Weigh 0.1320 gram of arsenious oxide, previously dried at 110° C for 2 hours, and dissolve in 50 ml of water containing 0.5 ml of 70 percent NaOH. Add 2 ml of the 5 N H2SO4 and dilute to 100 ml.
Working arsenic solution (1 ml equals 10 micrograms As). Dilute 1 ml of the stock arsenic solution to 100 ml.
Wet Oxidation Procedure
Transfer a 1-gram sample of coal crushed to pass a No. 60 sieve, to a 300 ml Kjeldahl flask and mix with 7.0 ml of H2SO4. Then add 3.5 ml of HNO3 slowly through a dropping funnel to avoid excessive frothing; after the initial reaction subsides, heat the mixture gently over a gas burner. When white fumes of SO3 only are being evolved, add 0.2 to 0.4 ml of HNO3 by drops into the flask. Brown fumes will be evolved for several minutes. Continue these additions of HNO3 until all visible carbonaceous matter has been oxidized. This will require about 1-½ hours. After cooling, wash the walls of the flask with about 10 ml of water, add a few drops of HNO3, and heat the solution again to complete the oxidation of organic matter. Wash the walls of the flask with water and heat the solution to fumes of SO3, repeating the addition of water and heating to fumes of SO3 at least once to remove the last traces of HNO3; after the last addition of water transfer the solution and any residue to an evolution flask keeping the final volume about 35 ml.
Colorimetric Determination
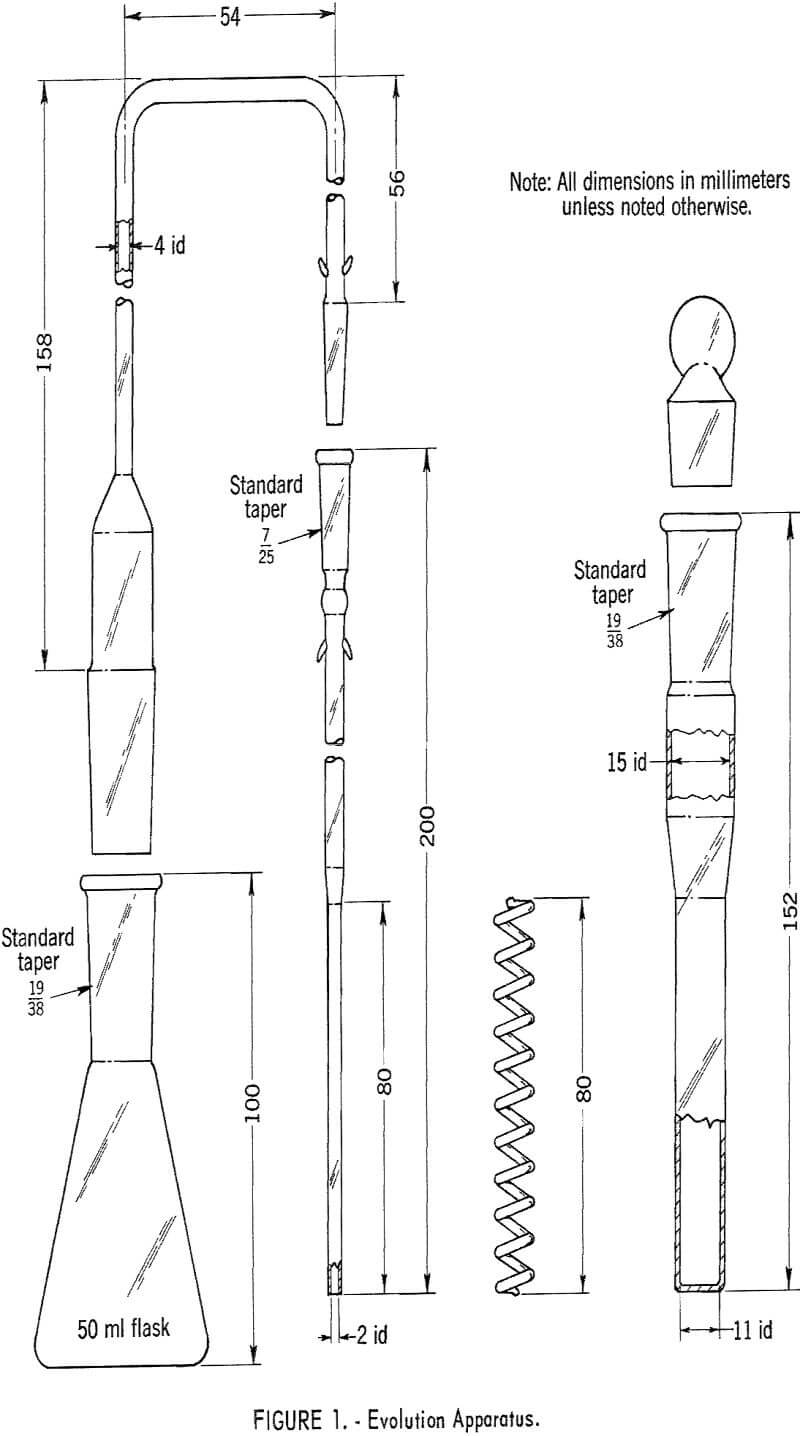
Before the evolution of arsine, add 2.0 ml of the KI solution and 0.5 ml of the SnCl2·2H2O solution to the sample in the evolution flask, mixing after each addition. Allow the solution to stand for 15 minutes. During this time set up the evolution apparatus shown in figure 1. Add 5 ml of 0.001 N iodine solution from a pipet to the absorption tube containing the helix. Place cotton plugs in both cones of the delivery tube, moistening the larger one with lead acetate solution, and weigh out 5 grams of zinc. Add the zinc to the evolution flask and assemble the apparatus.
Note: Some tests may give excessive evolution of hydrogen and frothing that carries over into the absorption tube. If this occurs, repeat the experiment adding about half of the zinc at first. After about 20 minutes when gas evolution has nearly ceased, add the remainder of the zinc and complete the test.
After evolution has taken place for 1 hour, disconnect the absorption tube and remove the helix. Add 0.5 ml of ammonium molybdate solution and 0.2 ml of hydrazine sulfate solution to the absorption tube from burets, closing the tube with a No. 19 glass stopper and mixing after each addition. Suspend the tube In boiling water for 10 minutes, then cool to room temperature. Transfer the blue-colored solution to a 1-cm cell and measure absorbance at 835 millimicrons, with water as reference. Carry a blank determination through the procedure.
Prepare a calibration curve using 0.0 to 1.6 ml of the standard working arsenic solution and 35 ml of 7 N H2SO4, carrying each test, through the evolution and colorimetric procedures. Use the absorbance reading for the 0.0 arsenic solution for a blank correction. The calibration curve is nearly a straight line with the absorbance ranging from 0 to 0.87 for 0 to 16 micrograms of arsenic.
Percent arsenic = (a – b) x 0.0001/w
where w = weight of sample in grams,
a = micrograms arsenic equivalent to absorbance of sample, and
b = micrograms arsenic equivalent to absorbance of blank.
Note: If a coal contains more than 0.0016 percent arsenic, take an aliquot of the sample solution for the determination so that the amount of arsenic present is within the range of the method. When a coal with high arsenic content is found, make another determination and dilute the sample solution to 50 ml in a volumetric flask. Transfer an aliquot containing less than 16 micrograms of arsenic to an evolution flask, add 7 N H2SO4 to make the volume about 35 ml, and complete the determination.
MgO Ashing
Mix a 1-gram sample of coal in a porcelain crucible with 1 gram of MgO and moisten with 2 to 3 ml of limewater. Heat the crucible and contents slowly to 650° C in a ventilated muffle furnace and maintain this temperature for 1 hour.
After cooling, add 15 ml of 7 N H2SO4 to the contents of the crucible. Transfer the solution and any residue to an evolution flask, using about 20 ml of 7 N H2SO4. Determine arsenic by the colorimetric method.
Nitric Acid Extraction
Mix a 1-gram sample of coal in a 250-ml beaker with 50 ml of 1:7 HNO3. Cover the beaker with a watch glass and boil the contents gently for 30 minutes. Filter the coal and acid mixture on a 9-cm filter paper and wash six times with the diluted HNO3, collecting the filtrate in a 200-ml Erlenmeyer flask.
Add 14 ml of 1:1 H2SO4 to the filtrate and evaporate to fumes of SO3. Finish the fuming by swirling the flask over the flame of a burner. After cooling, wash the walls of the flask with about 10 ml of water and evaporate to fumes again. Repeat this operation at least once to insure the removal of all the HNO3.
Finally transfer the solution to an evolution flask, dilute with water to about 35 ml, and determine arsenic by the colorimetric method.
Results
Comparison of Three Methods
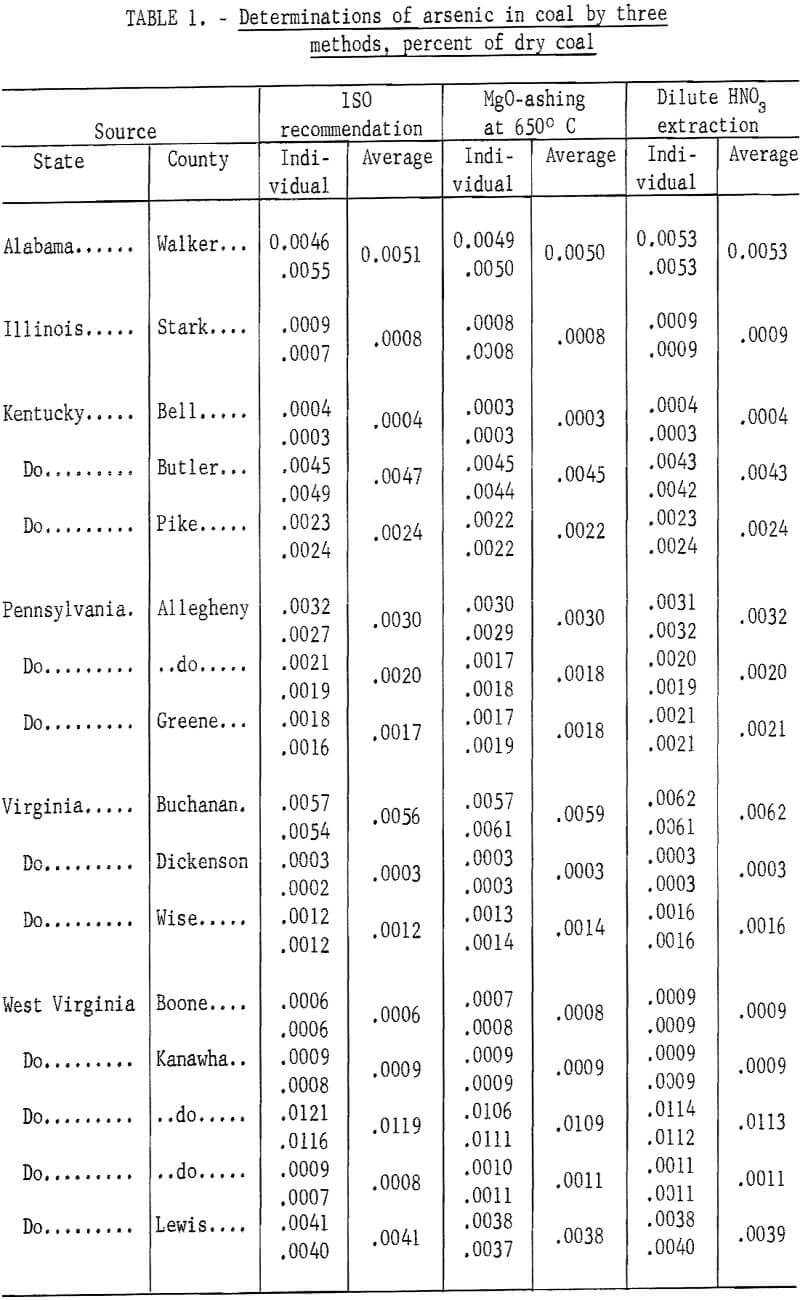
Results of arsenic determinations of 16 coals by the ISO method with wet oxidation of the coal sample are given in table 1. Arsenic determinations of the same coals by the modified methods using MgO-ashing and HNO3 extraction are included in the table for comparison. Average results by the ISO wet oxidation and the two modified methods agree within acceptable limits indicating that both the MgO-ashing and the HNO3 extraction methods can be applied to determine arsenic in coal.
To estimate completeness of the extraction of arsenic by HNO3 in these tests, the coal residue and filter paper were ashed in a porcelain crucible at 750° C and tested for arsenic. The ashes contained only small quantities of arsenic ranging from 0.1 to 3 micrograms. The proportion of these small amounts to the arsenic found in the acid extract indicated that the acid extraction method averaged 96 percent recovery of arsenic in the 16 coals tested.
Because several coal samples with unusually high arsenic content were used for these comparative tests, the results in table 1 are generally higher than the average arsenic content of coals from the sources shown.
Arsenic in Float- and Sink-Fractions
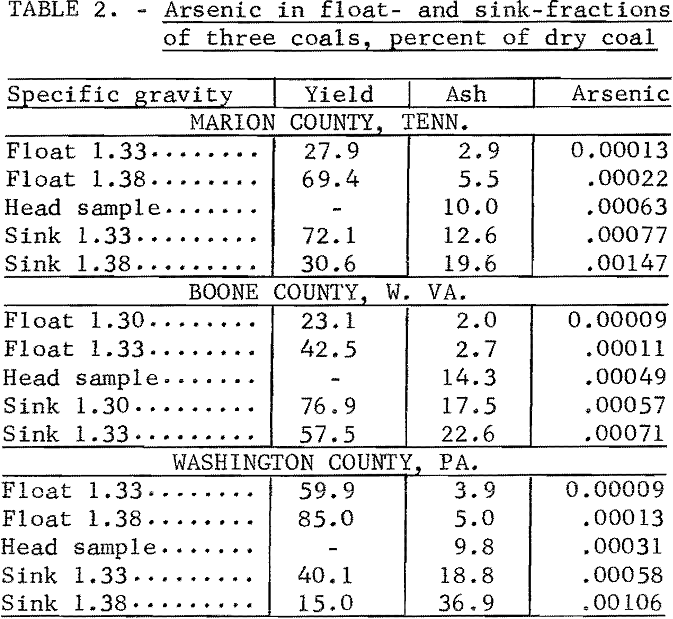
Mixtures of gasoline and carbon tetrachloride having specific gravities of 1.30 and 1.33, or 1.33 and 1.38, were used in float- and sink-tests of three coals. Each coal was separated into two float and two sink fractions. These fractions, with the head sample, gave five portions for each coal having a wide range of ash content. The coals were from Tennessee, West Virginia, and Pennsylvania. Arsenic was determined by the MgO-ashing method.
Yields of the float and sink fractions, and the ash and arsenic contents are given in table 2. The arsenic content decreased as the ash decreased, showing that arsenic in these coals was associated with the mineral matter. Sensitivity of the colorimetric method for determining small quantities of arsenic was demonstrated by the tests, because arsenic was not detected spectrographically in ash from the three head samples.
High-Temperature Ashing Tests
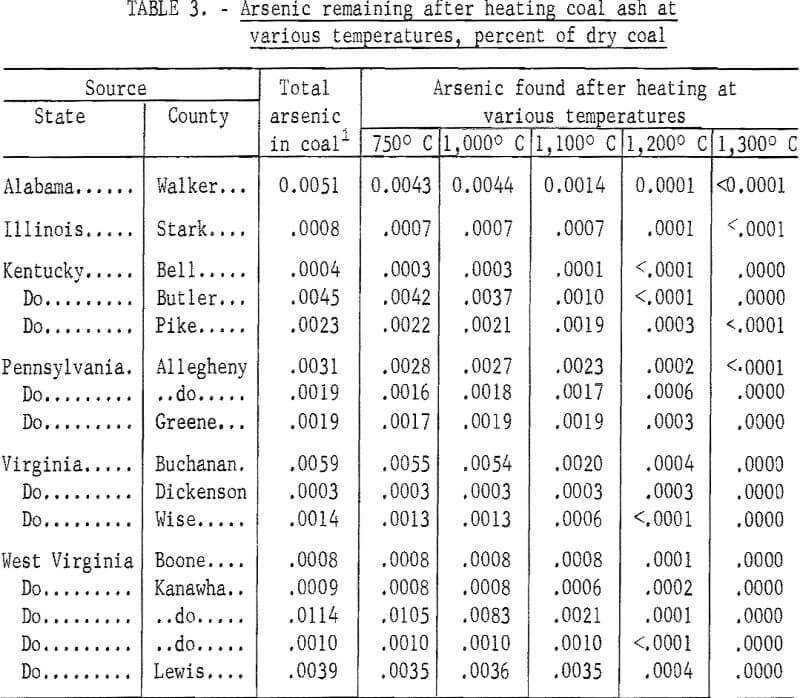
The ISO colorimetric method was used to determine the loss of arsenic when ashes are heated to temperatures corresponding to those attained during combustion of coal in a boiler furnace. One-gram samples of coal were ashed at 750° C in porcelain capsules according to the ASTM method for determining ash content. The ashes were treated with 7 N H2SO4 and arsenic was determined by the ISO colorimetric method. Tests were continued at higher temperatures, first preparing an ash sample at 750° C, then transferring it to a platinum capsule and heating it in a ventilated platinum wound electric furnace for 1 hour at 1,000° C, Arsenic content of the heated ash was determined; similar tests of ash samples from each coal were made at 1,100°, 1,200°, and 1,300° C.
Average results for 16 coals given in table 3 show that only a small quantity of arsenic is lost below 1,000° C. At higher temperatures an appreciable amount of it is volatilized; virtually all of it is removed at 1,300° C. Although the tests indicate that most of the arsenic in coal is volatilized during combustion, it is probable that, because of the high temperature required, some arsenic may remain in the cooler parts of the furnace and not escape with the flue gas.
Conclusions
- ISO Recommendation No. 601 for determination of arsenic in coal gives satisfactory results.
- A modification, using furnace ashing of the coal sample mixed with MgO, requires less manipulation and gives results similar to those obtained by the ISO wet oxidation method.
- A second modification, in which the arsenic is extracted from the coal sample with hot dilute HNO3 without ashing, also gives satisfactory results for arsenic.
- Determinations of arsenic in float- and sink-fractions of coal indicate that the arsenic is associated mainly with mineral matter in the three coals tested.
- High-temperature ashing tests show that very little arsenic is lost up to 1,000° C, but virtually all of it is removed from the ash at 1,300° C.
