Table of Contents
As a continuing endeavor, the Bureau of Mines is developing columbium and tantalum alloys for high-temperature applications. This investigation was undertaken to improve the high-temperature properties displayed by the more common columbium alloys that are commercially available.
In a previous investigation beneficial improvements were realized by alloying columbium with elements such as hafnium, tungsten, titanium, and zirconium,, Alloy Cb-15Hf-5W-2Zr had the best high-temperature strength, but alloy Cb-15Hf-5W-10Ti was the most resistant to oxidation. Although these and many of the other currently available alloys rely on solid solution strengthening for attainment of their desirable high-temperature properties, much research is being conducted on solid solution plus dispersed phase strengthening to achieve further improvements.
No detailed precipitation study was made in this investigation; however, further improvements may be expected for the alloys by precipitation-hardening effects of carbon, aluminum, and chromium.
Twenty-eight bar-shaped ingots, each weighing about 150 grams, were prepared by nonconsumable-electrode arc-melting of columbium-base alloys to which additions of carbon, aluminum, and chromium had been made; furthermore, tests were conducted to explore the effects of the additions on the workability, recrystallization, oxidation resistance, and mechanical properties of these alloys.
Although the required properties of metals for satisfactory performance at high temperatures can be developed by alloying, the better alloys so developed are usually not sufficiently workable to enable one to fabricate them into desired forms. A large amount of work has been done to improve columbium alloys. Some of this development work is being referred to in the text of this report.
Experimental Procedure and Results
Various complex base compositions are being used in this investigation; therefore, for better clarity, the following designations will be used for the base compositions (in atomic percent) throughout this report:
[Y] = Cb-15Hf-5W
[Z] = Cb-10Ta-10Hf-5W-5Mo-10Ti-2Zr
[V] = Cb-10Ta-10Hf-5W-5Mo-10Ti-2Zr-2V
None of the above alloys are commercially available. However, [Y] was selected as a base composition, having favorable properties as discovered in a previous investigation. The remainder of the compositions were selected on the basis of utilizing the strengthening mechanisms referred to in the abstract.
High-purity columbium prepared by the electron-beam-melting process was used as base material. Supplier’s analyses are given as follows:
Carbon – 40 to 50 ppm
Nitrogen – 20 to 30 ppm
Oxygen – 40 to 50 ppm
The alloying additions were made using elements of the highest purity available. All the metals were cleaned with various acid solutions followed by a rinse with water and then acetone. Columbium and tantalum were used in the form of cold-rolled sheet. Hafnium and zirconium were in the form of iodide-process crystal bars. Titanium and chromium were small chips; aluminum, molybdenum, and vanadium were used as a sheet. The carbon was added as columbium carbide from a mixture analyzed as columbium-13.5 weight-percent carbon. Tungsten was added as columbium-tungsten from an alloy analyzed as columbium-30 weight-percent tungsten. Alloy compositions were proportioned in atomic-percent.
All alloys were melted into 150-gram bar-shaped ingots in an arc-melting furnace with a nonconsumable tungsten electrode. The melting was done on a water-cooled copper hearth in a helium atmosphere using about 150 to 300 amperes and 24 volts.
After melting, the ingots were machined to improve the surface for rolling or forging and to provide a flat surface for hardness measurements. Two sections were sawed from each ingot; one section was used for metallographic examination, and the other was used for determination of fabrication procedures. The remainder of the ingot was prepared for fabrication into samples for recrystallization, tensile, and oxidation studies.
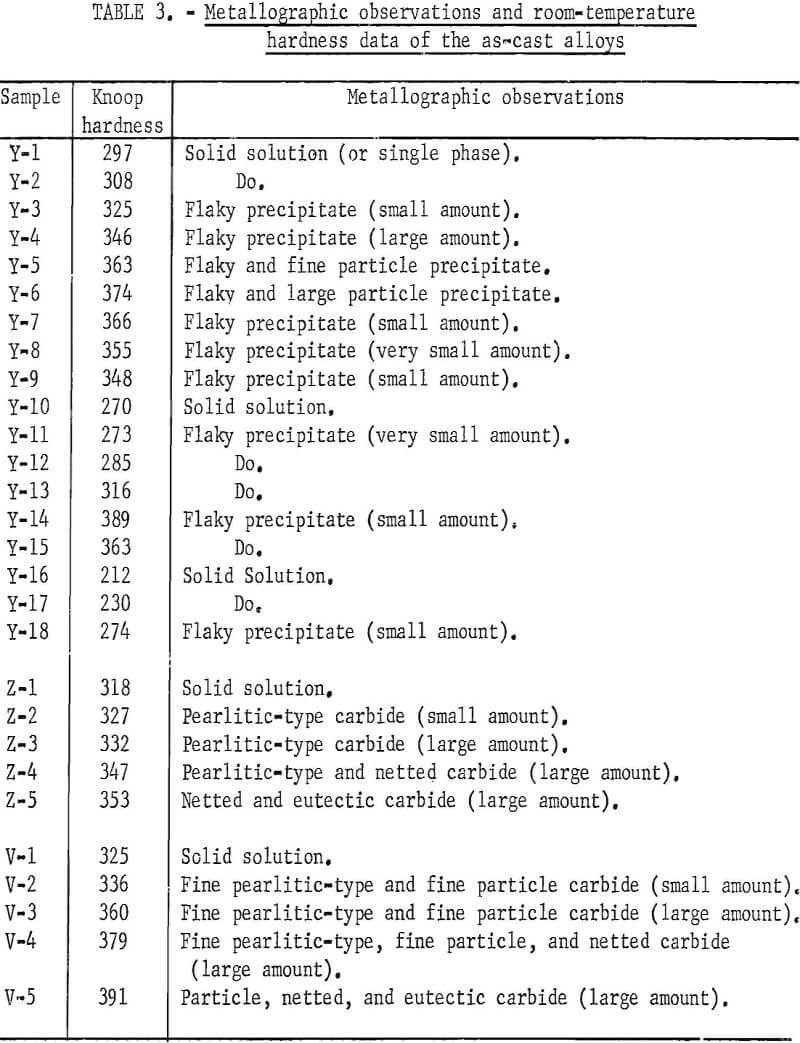
Compositions of the ingots are shown in table 1. Samples Y-1, Y-2, Y-10, Z-1, and V-1 are base compositions to which further additions were made. Y-3 to Y-6, Y-14, Y-15, and Y-18 are alloys containing aluminum; Y-7 to Y-9 and Y-11 to Y-13 are alloys containing chromium; Y-16 and Y-17 are high-titanium alloys, and Z-2 to Z-5 and V-2 to V-5 are alloys containing carbon.
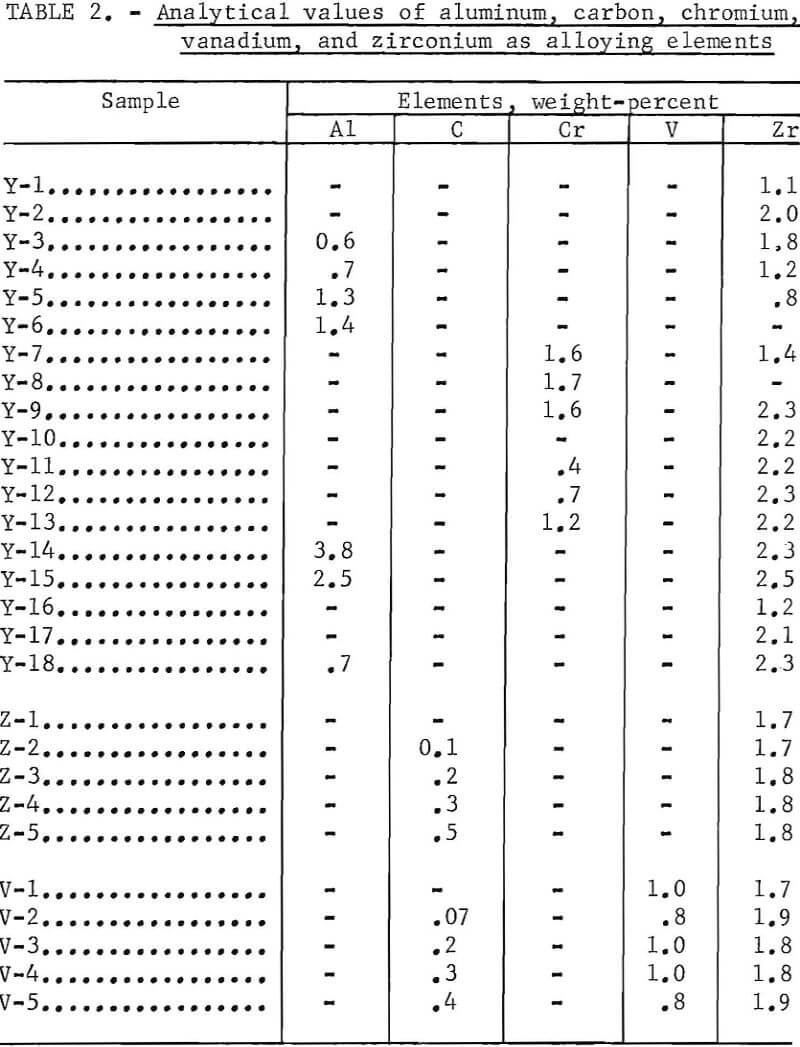
Table 2 shows the analytical values for aluminum, carbon, chromium, vanadium, and zirconium as alloying elements. Generally, the results show good agreement (for the elements analyzed) between the nominal and analytical values for all the columbium alloys investigated. Y-14 and Y-18 show an increase in aluminum. All the [Y] designated alloys appear to be high in zirconium content. The difference may be due either to inhomogeneity or to the wide variety of melting points in the materials used. Sampling was made from lathe turnings from top and bottom of the ingots.
Microstructure
Figures 1 through 16 show typical as-cast structures of representative alloys. Standard mounting and polishing procedures were used with final polishing on wheels impregnated with ¼-micron diamond compound. An etching solution consisting of 10 ml of H2SO4, 10 ml HF, 10 ml H2O, and five drops HNO3 was used to reveal the structures for the microstructure analysis.
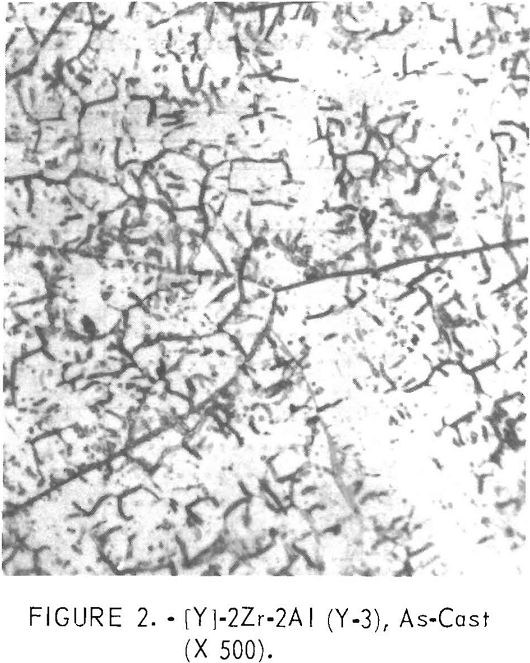
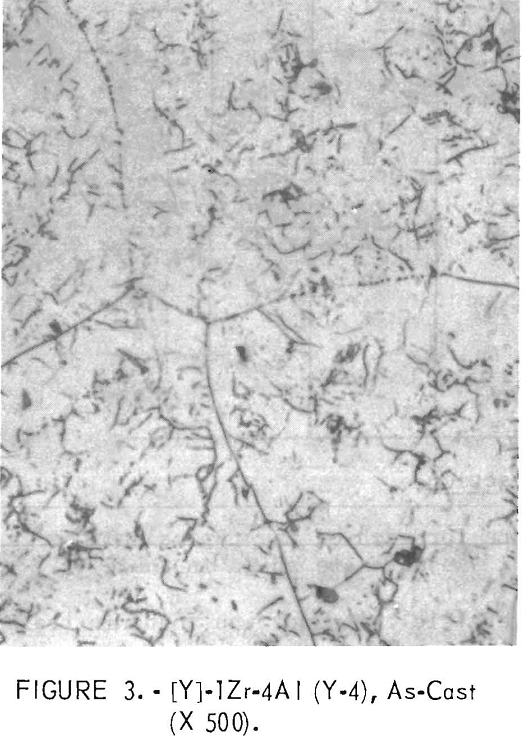
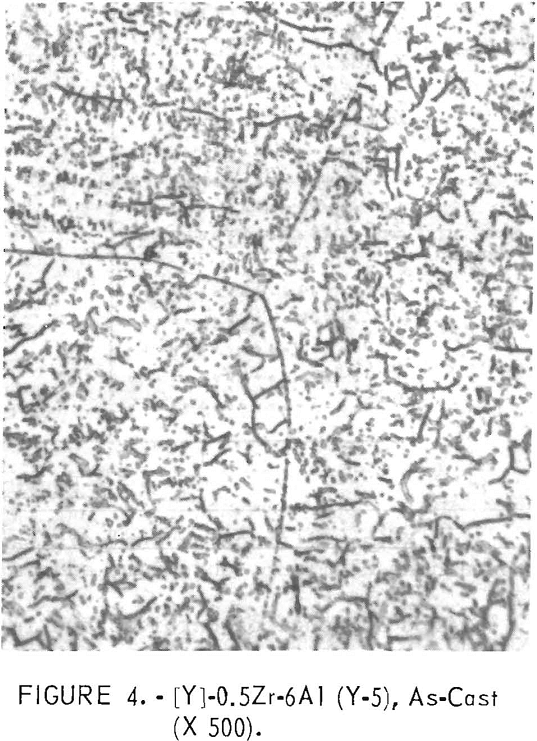
Figures 1 through 5 show a change in structure with increasing aluminum content. Figure 1 shows a single-phase, solid-solution structure of the base alloy. Figure 2 shows that an alloy which replaces the large amount of titanium with a small amount of aluminum is mostly a flaky precipitate. This flaky precipitate is also illustrated in figure 3, with an increase in aluminum content. Figures 4 and 5 follow, illustrating that, as the amount of the aluminum content increases further, the flaky precipitate and small particles of precipitate mix.

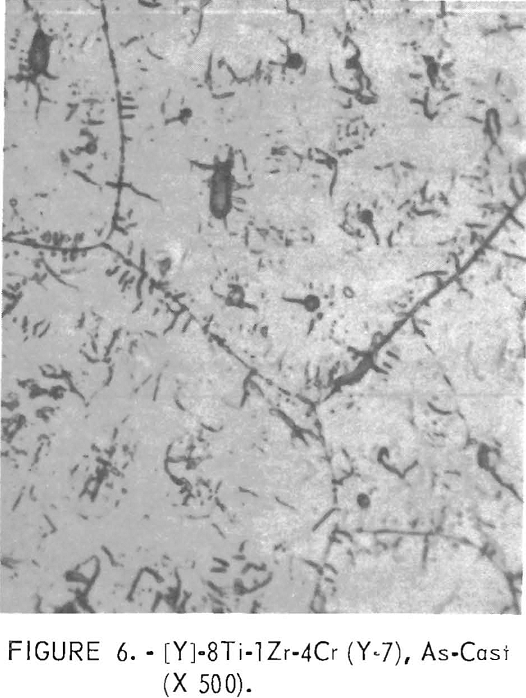
Figure 6 shows an alloy structure containing chromium. The amount of flaky precipitate appears to be greater with the addition of chromium than for those without chromium.
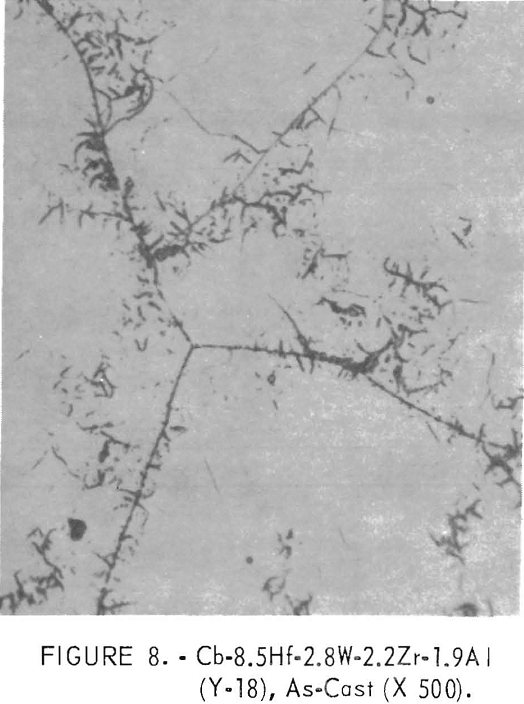
Figure 7 shows the single-phase structure of a high-titanium alloy. The microstructure of an alloy which replaces titanium with a small amount of aluminum has a small amount of flaky precipitate, as shown in figure 8.
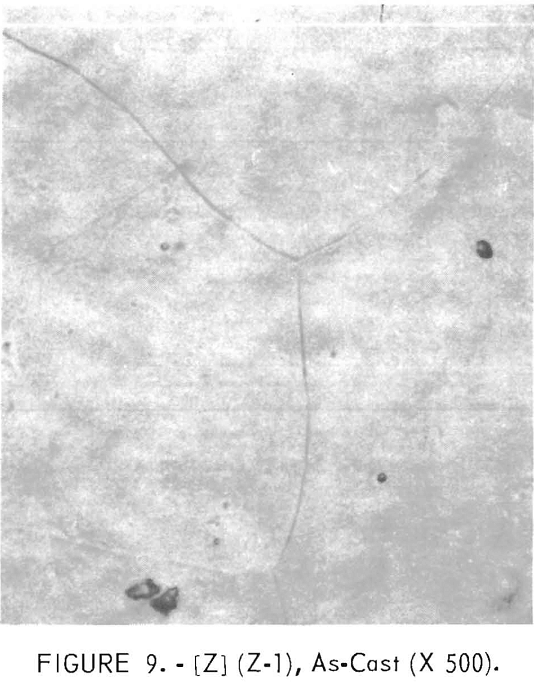
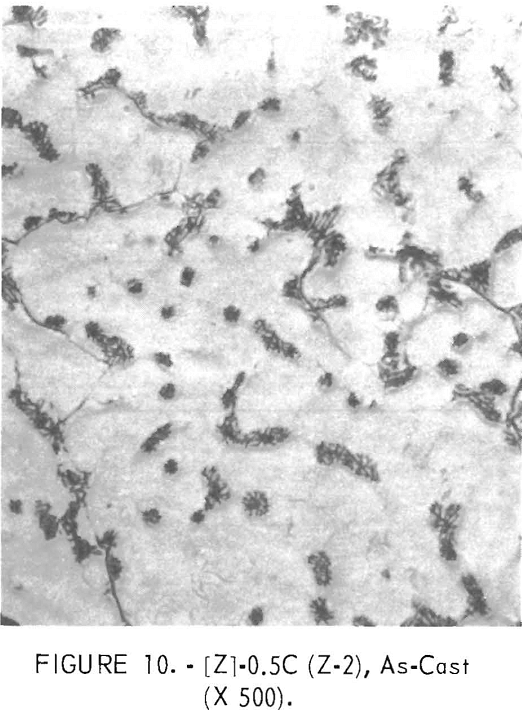
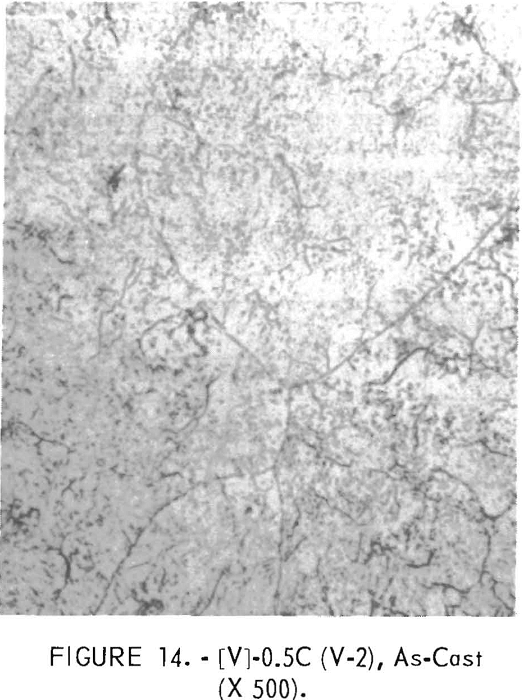
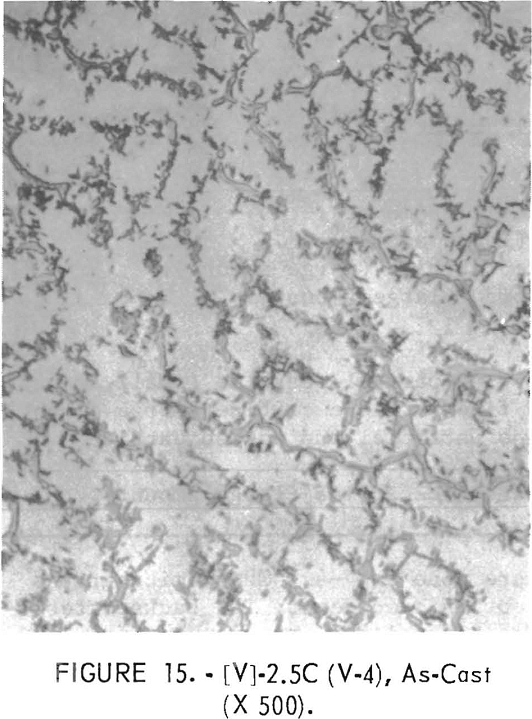
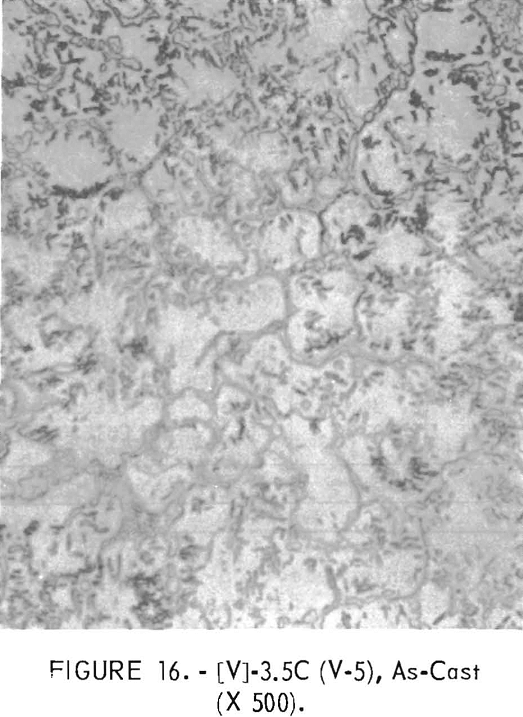
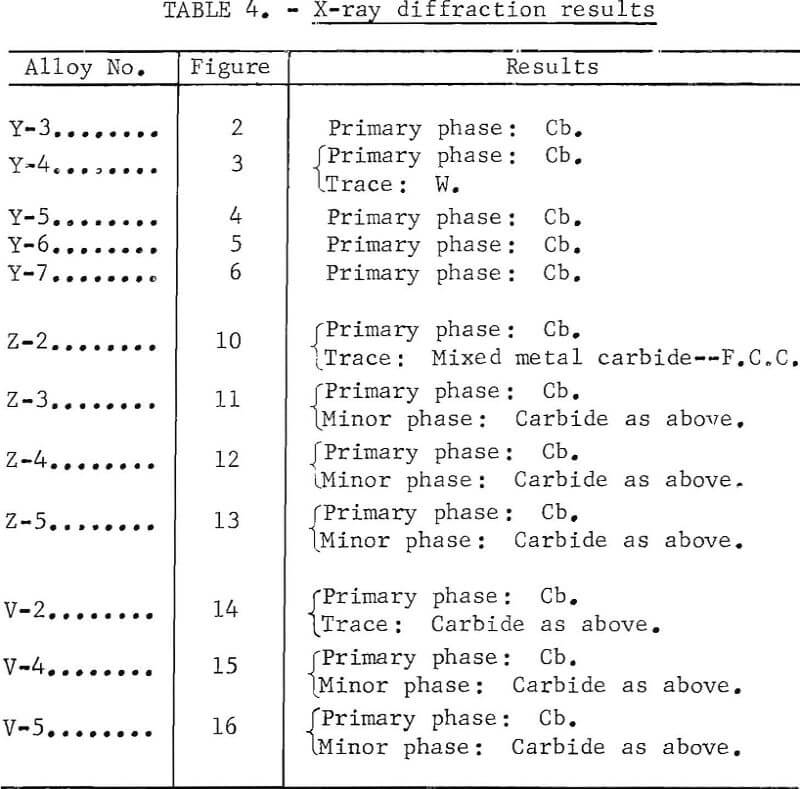
Figures 9 through 16 show the change of structure with increasing carbon content in the [Z] and [V] alloys. The base alloy containing no carbon has a single-phase structure as seen in figure 9. With the addition of small amounts of carbon, the microstructure of figure 9 changes into that of figure 10, The pearlitic-type dark parts are areas of precipitation and contain columbium carbide. With increasing carbon content, as seen in figures 11 through 13, the pearlitic-type structure changes into a netted and eutectic structure on the grain boundary. Figures 14 through 16 show changes in structure of alloys containing vanadium and carbon. As seen by comparing figures 14 with 10, 15 with 12, and 16 with 13 the alloys containing vanadium always have a finer and a more uniformly-dispersed precipitate. The results of these metallographic observations are summarized in table 3.
X-Ray Diffraction
An X-ray diffraction analysis was done for each of the typical alloys containing aluminum, chromium, and carbon. Results are shown in table 4.
Good evidence of carbides and intermetallic compounds was found in these samples by X-ray diffraction. However, when the precipitated phase in each sample was examined with the microprobe, no carbides or intermetallics as such were found. It is concluded that either their size is considerably smaller than 1 micron or they were removed from the surface or covered during



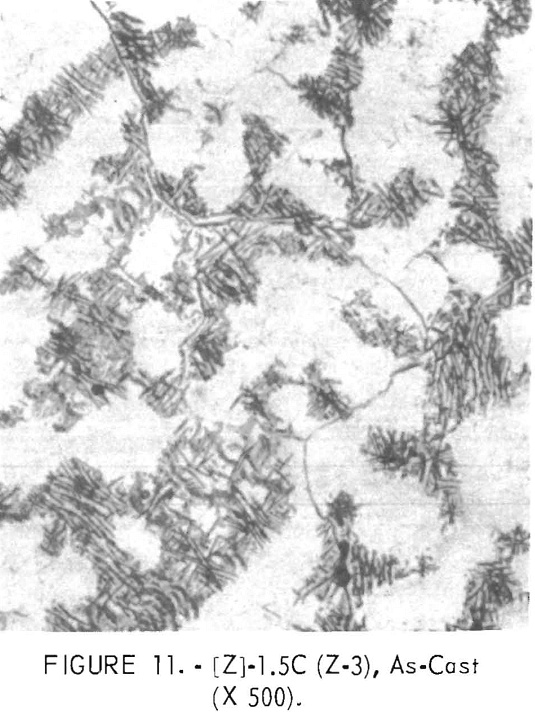
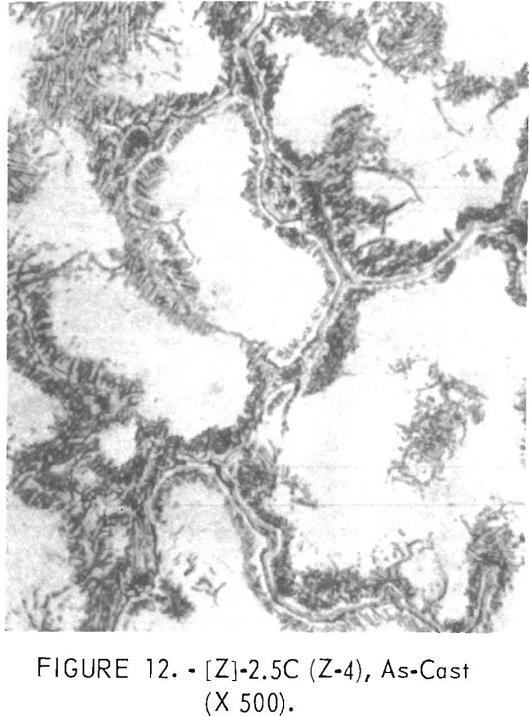
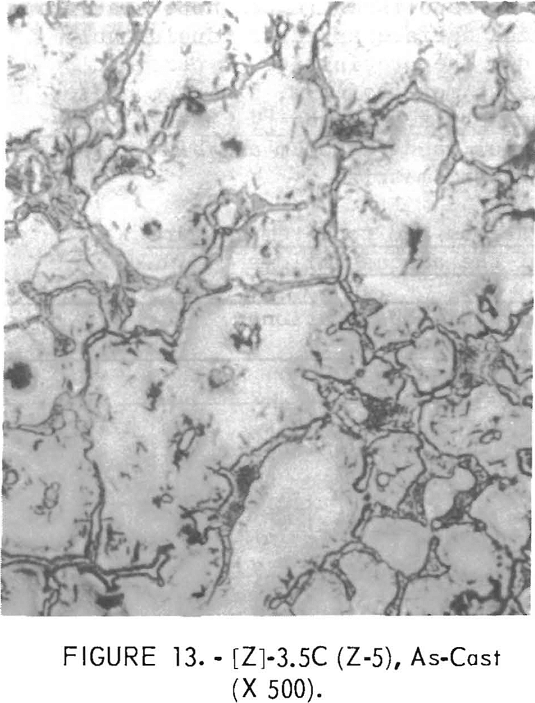
polishing and etching. One sample, Y-4, was repolished in the hope that the intermetallic phase would appear. While the surface appeared considerably different, no intermetallics were revealed. The precipitate or portion remaining in [Y] composition samples contains more hafnium, aluminum and/or titanium, chromium, and zirconium than the matrix. In the [V] and [Z] composition samples hafnium, zirconium, titanium, and vanadium are higher in the present surface of the precipitates than in the matrix.
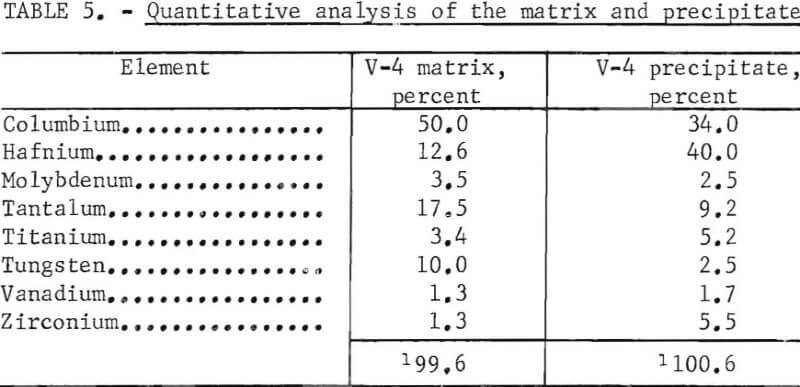
Scanning-electron-beam micrographs were done on V-4. These micrographs are typical of what was observed with the other specimens. The backscattered-electron image shows that there is considerable relief. No regions with low atomic number elements are seen in the inverse specimen current photo# Higher hafnium but lower tungsten and tantalum concentrations in the precipitate region are shown in the X-ray intensity displays. Quantitative analysis of the matrix and precipitate add up to about 100 percent with metallic elements alone further suggesting that no carbide is present at the surface.. Results are shown in table 5.
If the elusive phases are very small, the electron microscope would be useful in their examination. However, if the grains are one or two microns across and are being removed during polishing, special techniques would be necessary to retain them for confirmation by the microprobe.
Hardness
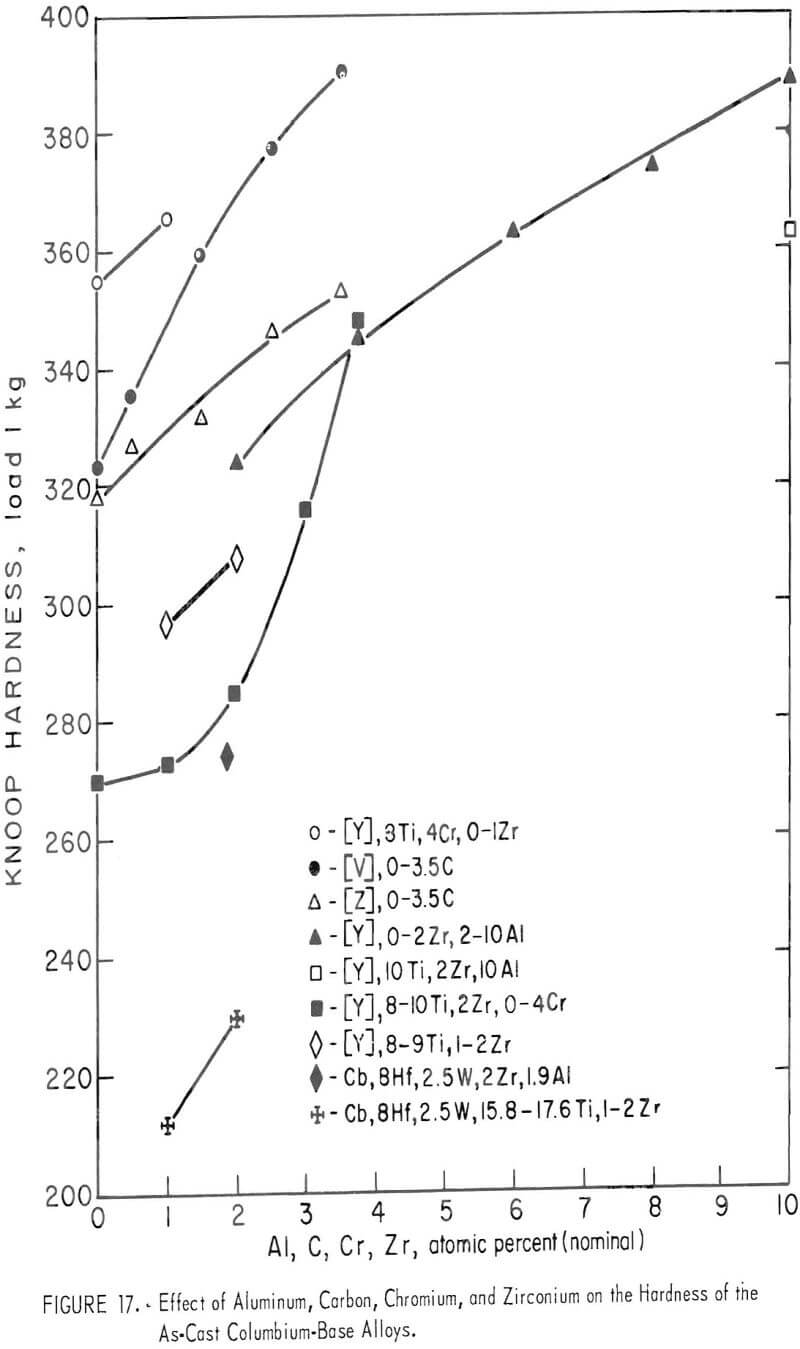
The machined ingots in the as-cast condition were subjected to hardness tests with the results shown in table 3 and figure 17. The hardness values of these alloys in general become higher with increasing amounts of aluminum, carbon, chromium, and zirconium. The hardening (because of the addition of chromium) becomes more marked when the chromium content is more than 2 atomic-percent. The rate of hardening with the addition of carbon is promoted when the alloy contains vanadium.
Fabrication
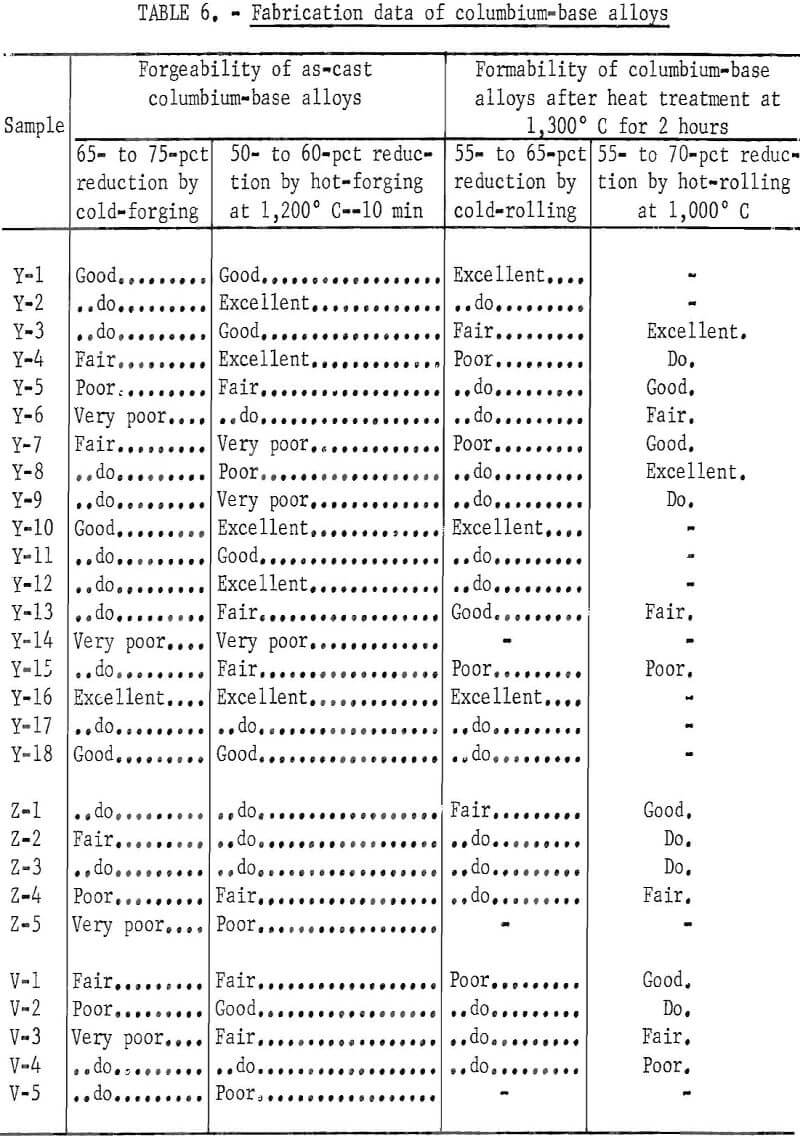
A series of tests was made to establish the fabrication procedure for either rolling or hammer forging and to determine if the metal was sufficiently ductile for making sheets for further testing. The degree of formability or forgeability was expressed as follows: excellent for no cracks; good for slight-edge cracks; fair for deep edge cracks; poor for cracks from edge to center; very poor for breakdown.
The samples were hammer-forged, annealed, and then rolled. The alloys were forged at room temperature and 1,200° C. For the 1,200° C forging, the samples were coated with glass of the following composition: 100 grams frit (200 mesh), 5 grams bentonite, 1 gram gum tragacanth, one-half gram carboxy-methyl cellulose, and 80 to 90 grams water. The specimens were dipped into the glass slurry, air-dried for 24 hours, and preheated for 10 minutes at 1,200° C before forging. The alloys that were forged at 1,200° C were annealed at 1,300° C in vacuum for 2 hours and furnace-cooled. All the alloys were completely recrystallized by this annealing treatment. Some of the alloys annealed at 1,300° C were reduced in thickness 55 to 65 percent by cold-rolling. The alloys for which cold-rolling was difficult were hot-rolled by repeated heating before each pass at 1,000° C in air. The thickness after these rollings was about 0.04 inch (1.0 mm).
Fabrication data are presented in table 6. All the base alloys containing vanadium and high titanium have good fabricability. Forgeability and formability decrease gradually with increasing aluminum, carbon, or chromium content; but they are easy to fabricate by cold working, provided the alloys contain only 4 percent aluminum or 2 percent chromium. Alloys containing 8 percent aluminum 2.5 percent carbon, or 4 percent chromium can be fabricated by rolling at 1,000° C. No improvement is noted with the addition of 1 or 2 percent zirconium. The alloys containing vanadium have poor fabricability.
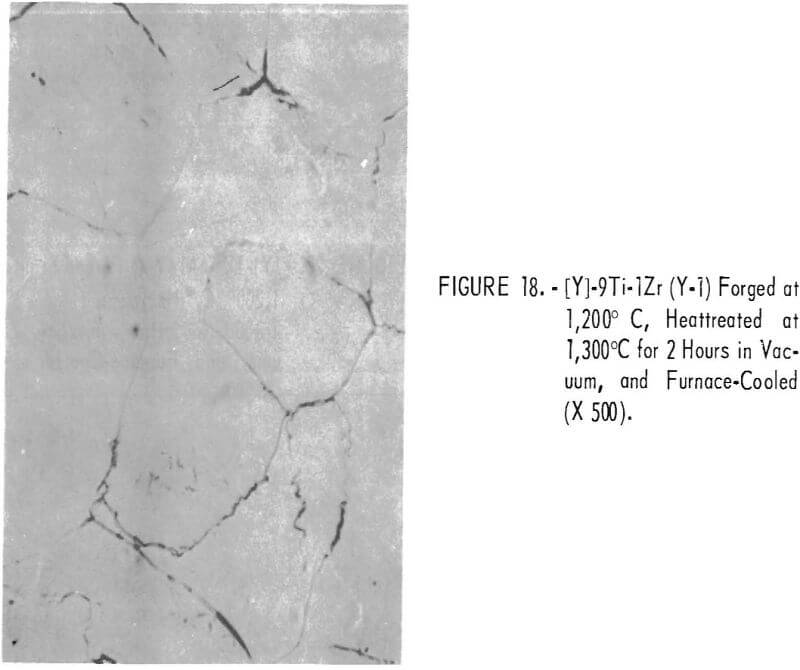
Figures 18 through 27 show typical structures of alloys forged at 1,200° C and then heat-treated at 1,300° C for 2 hours. When comparing figures 1 to 16 with figures 18 to 27, one can see, in general that the grain size of the alloys was refined and homogeneity improved. The flaky precipitate in the alloys containing small amounts of aluminum became fine particles, but the flaky precipitate in alloys containing chromium changed hardly at all.
The pearlitic-type and eutectic carbide in alloys containing carbon became fine particles and globular carbide by hot-forging and heat-treating. The results of these metallographic observations are summarized in table 7.
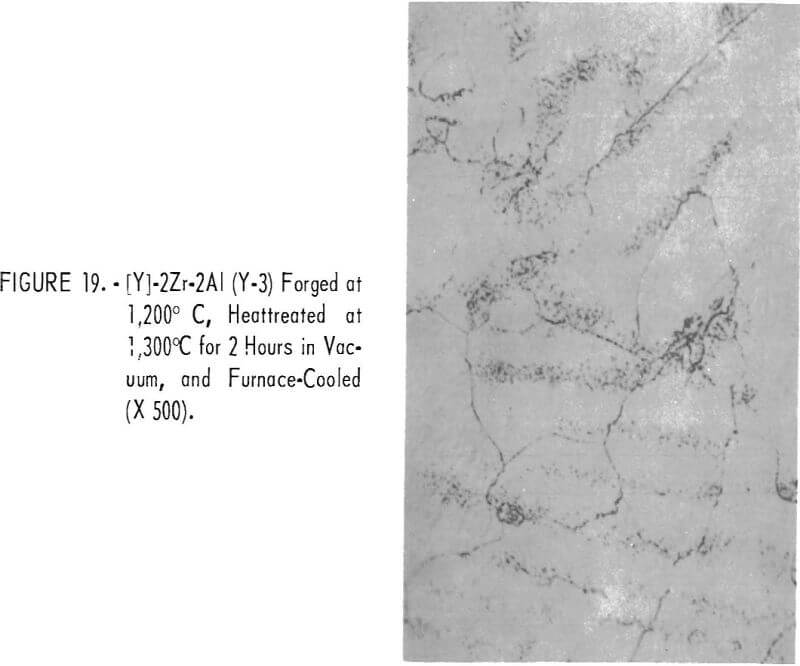


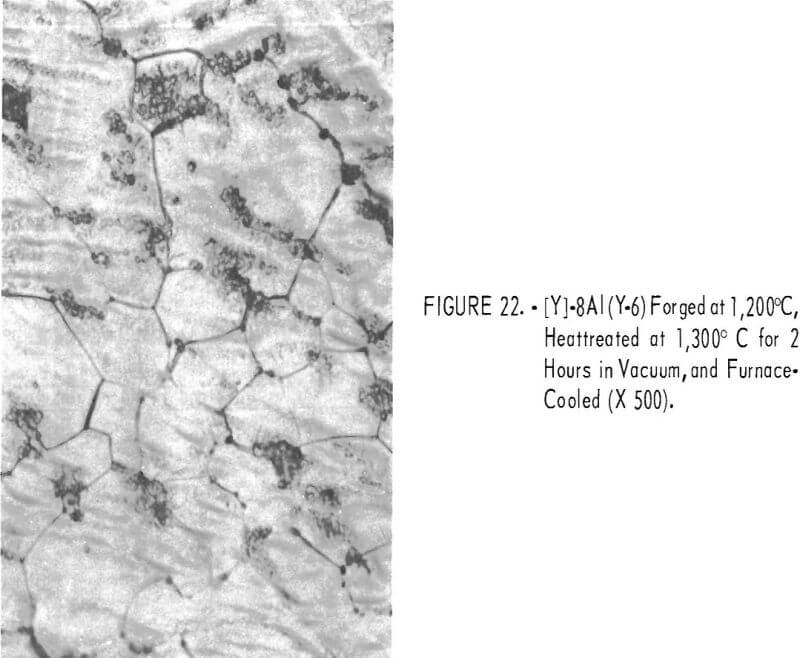
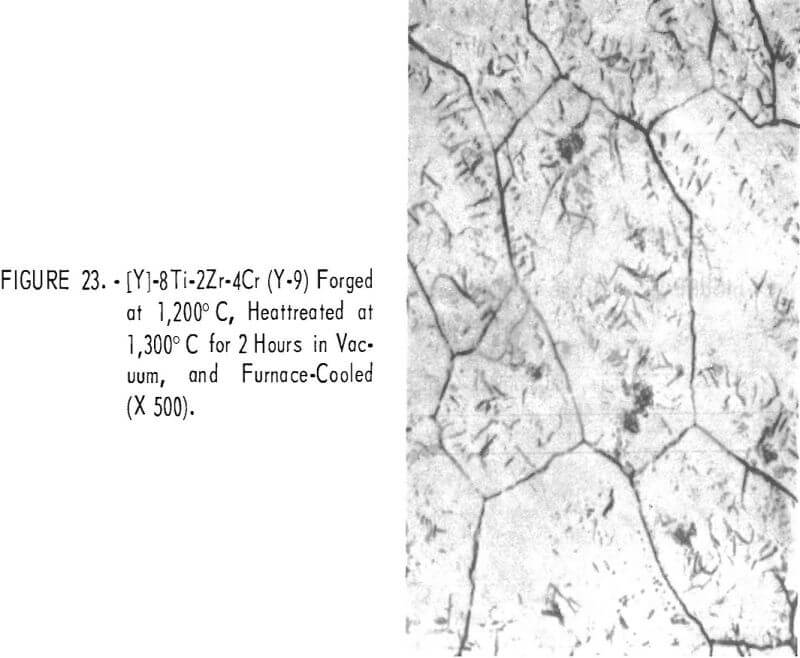

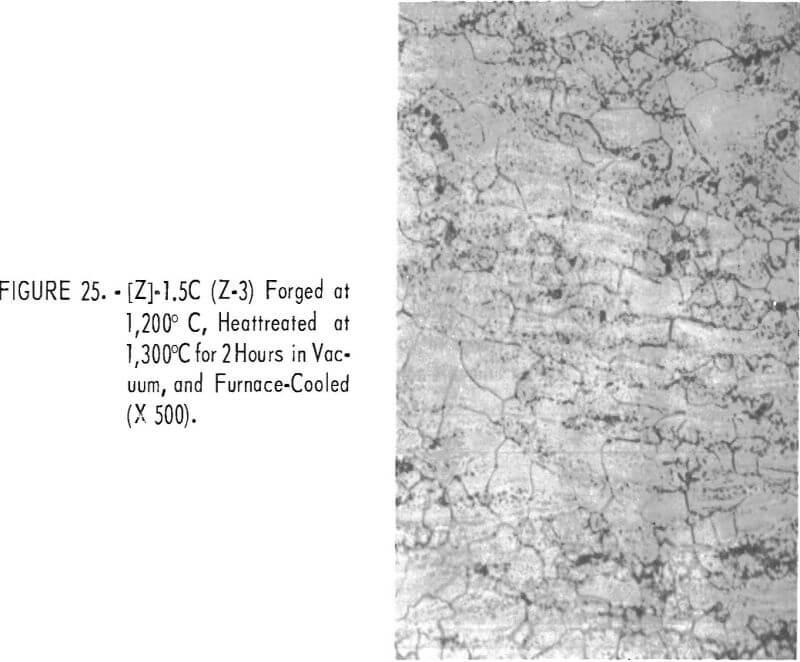


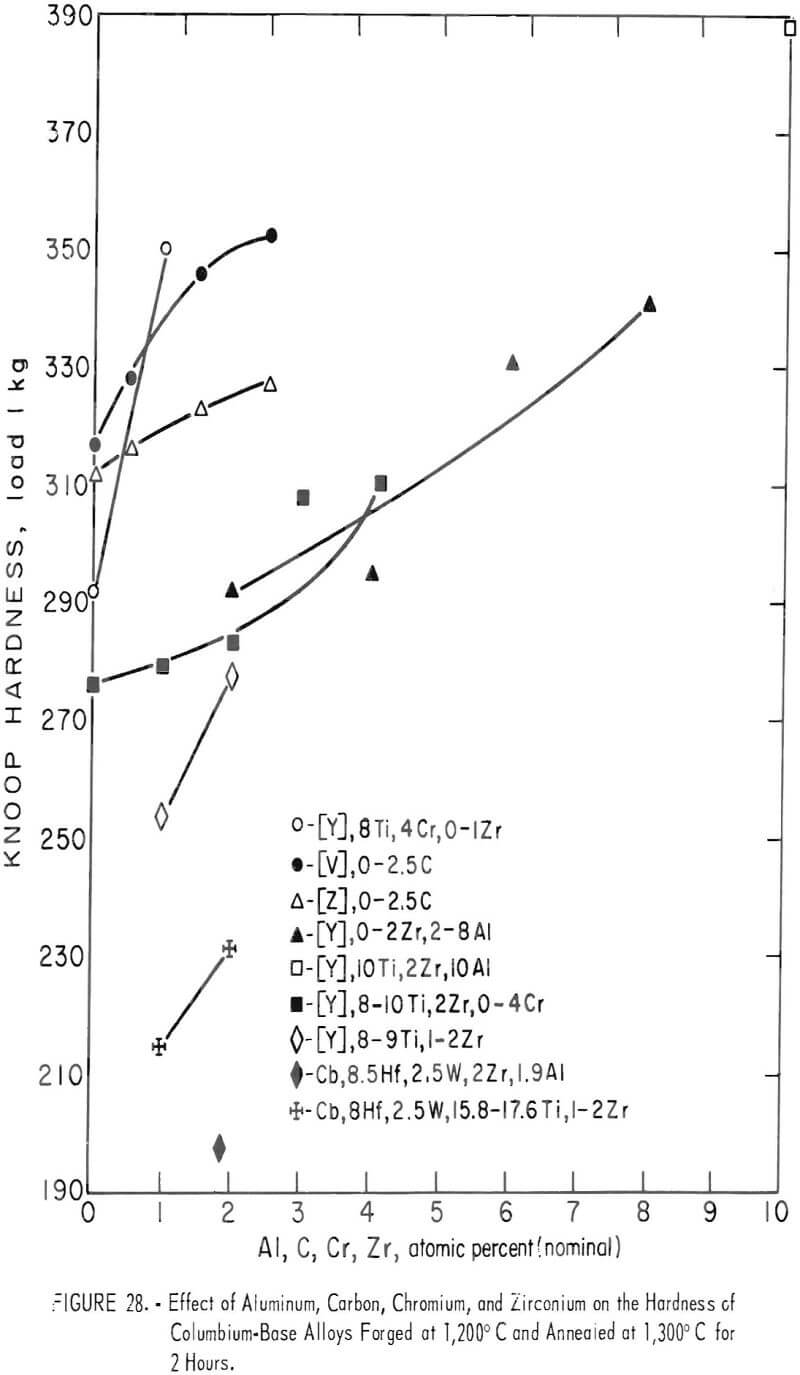
The hardness values of the alloys forged at 1,200° C and then annealed at 1,300° C for 2 hours are seen in table 7 and in figure 28. By comparing figure 17 with 28, it is evident that the hardness values of annealed alloys becomes generally less than the hardness of the as-cast alloys, and the effect of the alloying elements for hardening is the same in both alloys.
Recrystallization
A test on the recrystallization of cold-rolled alloys was made to appraise the effect of alloying elements at high temperatures.
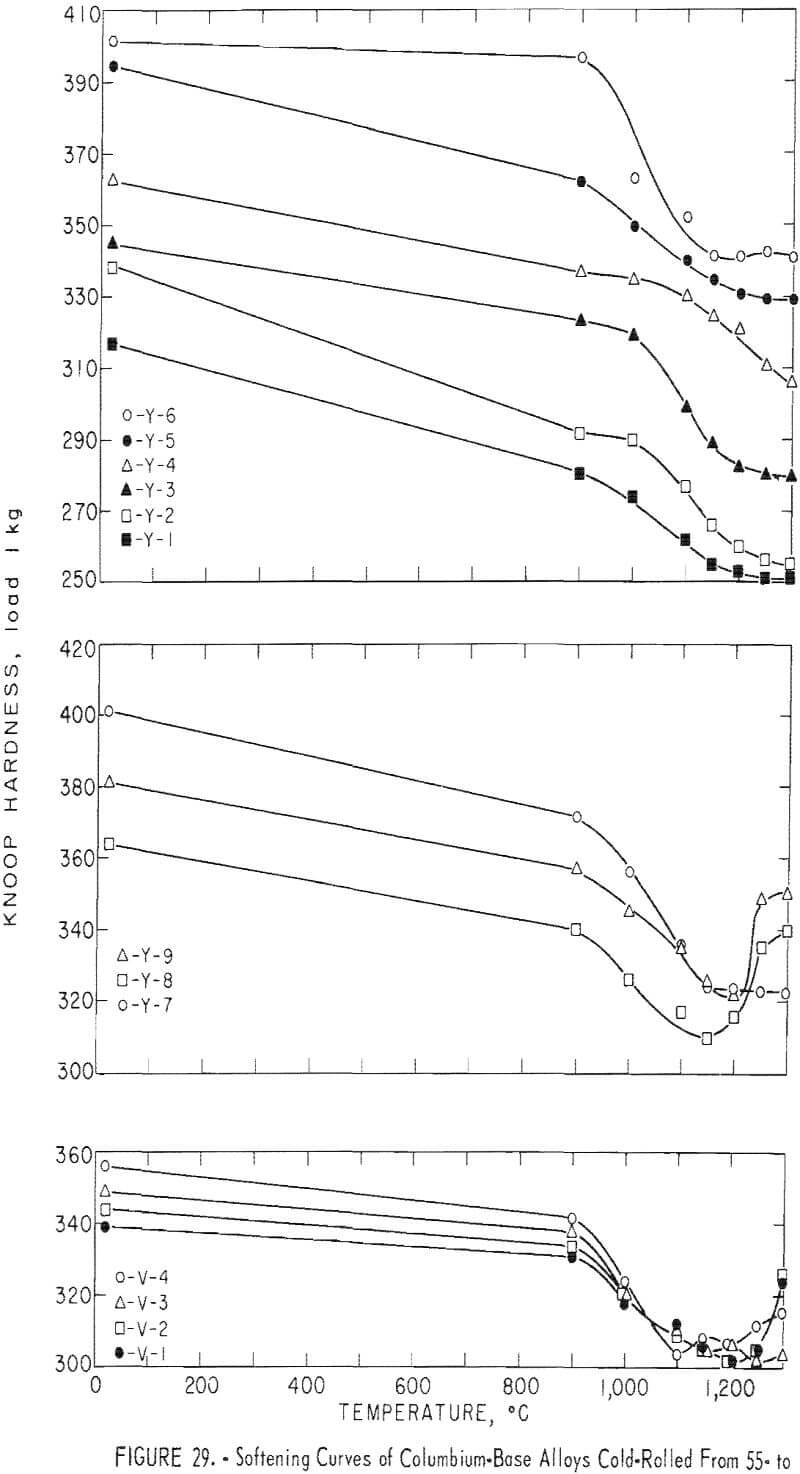
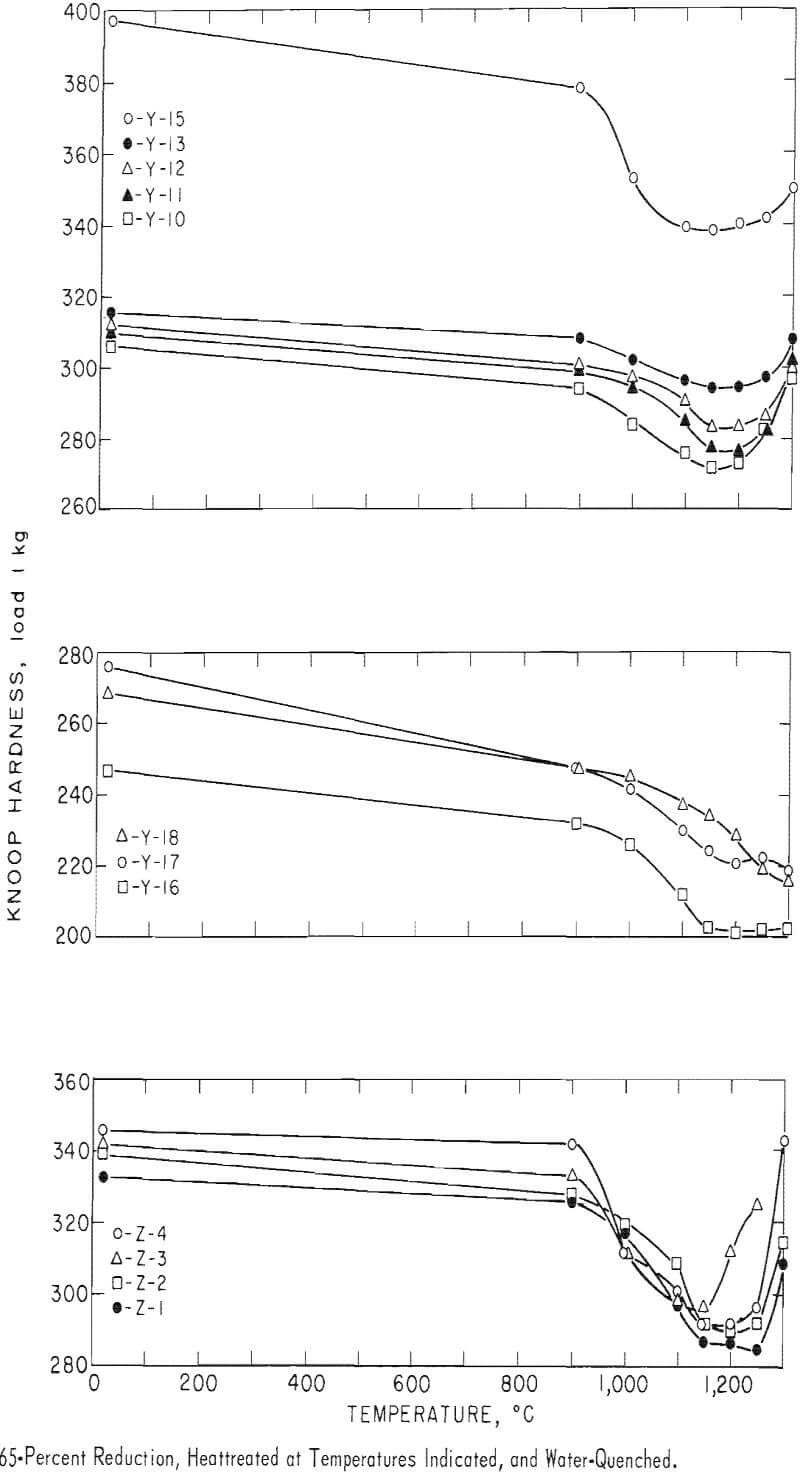
The alloys that were cold-reduced from 55 to 65 percent in thickness were heated for 1 hour at 900°, 1,000°, 1,100°, 1,150°, 1,200°, 1,250° and 1,300° C, respectively, and water-quenched. The specimens were glass-coated by a method mentioned previously. The heat treatment was carried out in the atmosphere. By examining the microstructure, hardness, and softening curves of the alloys, the recrystallization temperature and dissolving temperature of the precipitate were determined.
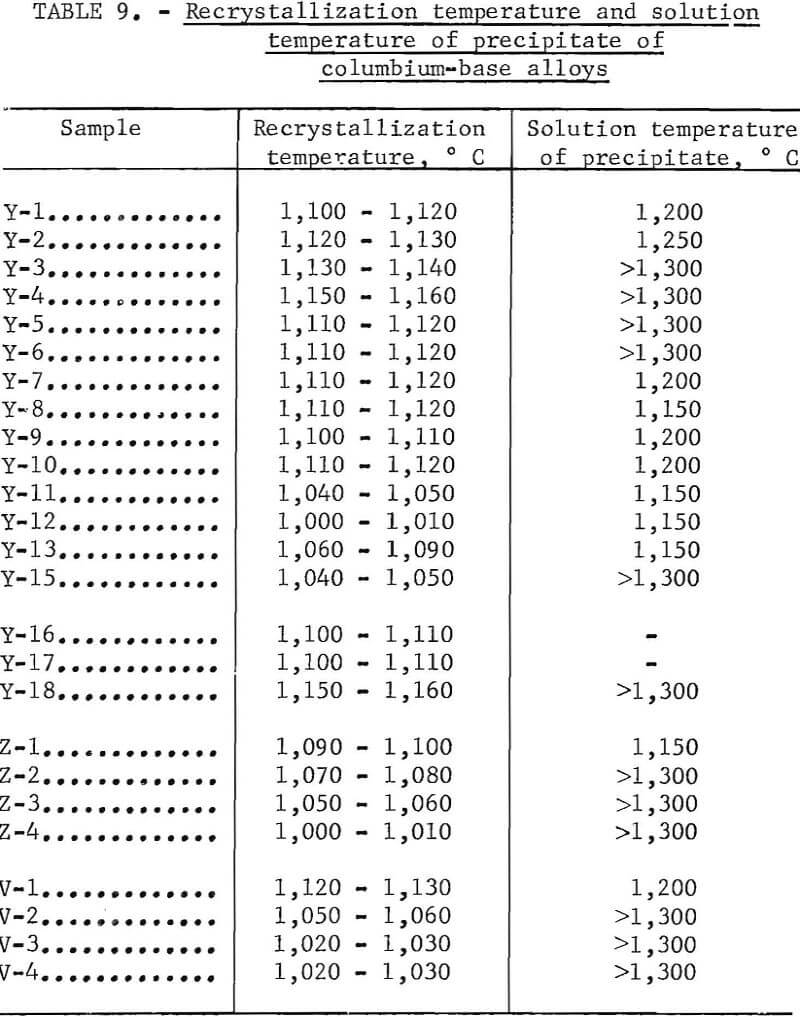
Recrystallization data are shown in table 8 and figure 29. The recrystallization of sample Y-1 begins about 1,000° C and finishes about 1,150° C. The precipitate that existed in the alloy in the as-rolled condition dissolved almost completely at 1,200° C. Also, Y-1 does not show any evidence of a second phase in the as-cast structure.
For a 1-hour test period, the recrystallization of the alloys containing a small amount of aluminum begins at 1,100° C and finishes at about 1,200° C. The precipitate that exists in the alloy at the first state does not dissolve even at 1,300° C. Therefore, the recrystallization temperature of the alloy increases with the addition of a small amount of aluminum, and the precipitate is stable to higher temperatures, such as 1,300° C, under these experimental conditions. When the alloy contains a large amount of aluminum, the recrystallization temperature decreases. Recrystallization of the alloy containing 6 percent aluminum begins about 1,000° C and finishes about 1,150° C, and the precipitation is stable to 1,300° C. The recrystallization temperature of the alloy containing 4 percent chromium begins about 1,000° C, is about 30 to 40 percent complete at 1,100° C, and is complete at about 1,150° C, The precipitation that existed in the alloy before heat treatment dissolved at 1,150° C, At a higher temperature, this alloy was contaminated because of the heating in air. As far as the alloy in the category containing 4 percent chromium is concerned, the recrystallization results were investigated for alloys containing small amounts of carbon. The recrystallization of low-carbon alloys begins about 1,000° C and finishes about 1,150° C. The precipitate does not dissolve even at 1,250° C. This alloy was contaminated at 1,300° C with the amount of precipitate decreasing because of possible decarburization.
The structures of the alloy containing large amounts of carbon were also investigated. The recrystallization of high-carbon alloys is about 40 to 50 percent complete at 1,000° C and is complete at about 1,100° C. This alloy was contaminated by oxidation at 1,300° C, but a large amount of carbide remained because of the large carbon content. Recrystallization structures were examined on alloys containing vanadium and carbon. Grain refining was accomplished by adding vanadium to the alloy that contained carbon; also, the larger the carbon content, the lower the recrystallization temperature. The carbide is stable to 1,300° C.
The results of these metallographic observations are summarized in table 8, The annealing curves are shown in figure 29. In these curves, the increase of aluminum content is found to have a pronounced effect.
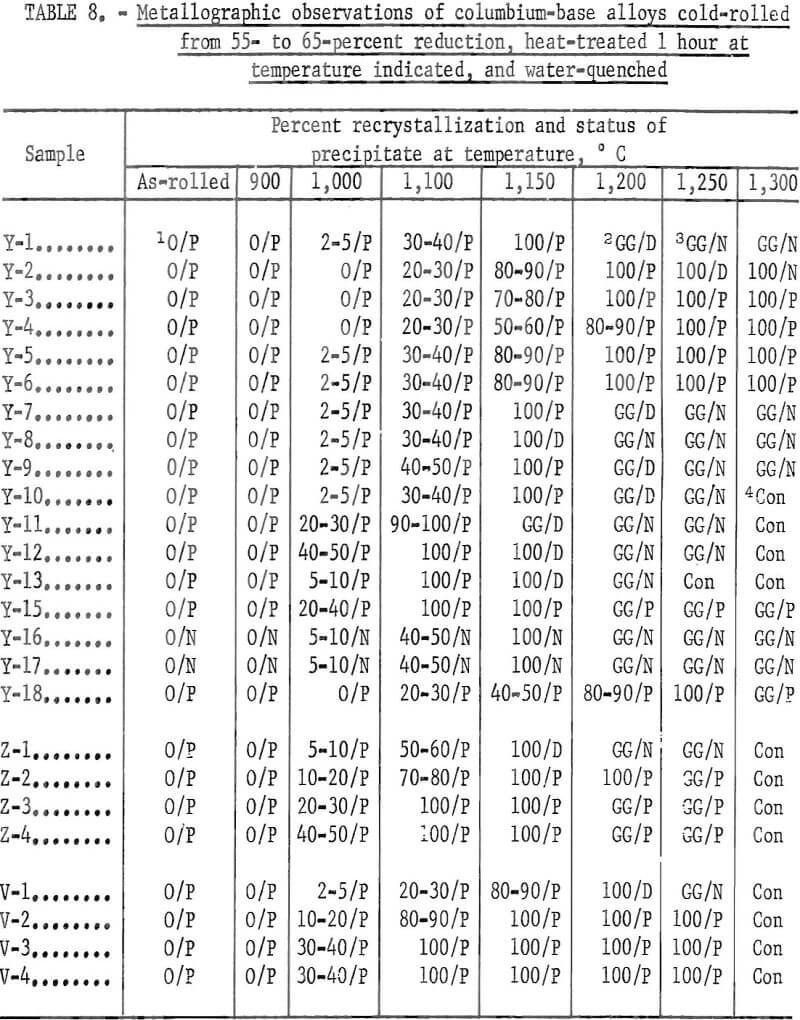
Note: Value to left of slash = percent recrystallization.
Value to right of slash = status of precipitate.
Table 9 shows the temperature at which the recrystallization is about 50 percent complete. This is also the solution temperature of the precipitate. The narrow range shown for the recrystallization temperature is only arbitrary; the actual range may be somewhat wider than shown in table 9. The recrystallization temperature increases with the addition of a small amount of aluminum but decreases with the addition of large amounts of aluminum and small amounts of chromium or carbon. The solution temperature of the precipitate is 1,150°
to 1,200° C in the alloy containing chromium but is above 1,300° C in the alloy containing aluminum or carbon.
Strength
The alloys that were fabricated into sheet were cut into standard tensile specimens all of which were from 0.03 to 0.04 inch thick, from 0.24 to 0.25 inch wide, and 5 inches long with a 1-inch gage length. After the specimens were prepared, they were annealed at 1,300° C for 2 hours and furnace-cooled. The specimens were tested on a high-temperature tensile-testing machine with a strain rate of 0.05 inch per minute. The testing was done in a vacuum, and the method of heating was by self-resistance. A platinum-platinum-10 percent rhodium thermocouple was spot-welded to the sample to sense the temperature of the test section. The time required to heat the specimen was from 15 to 30
seconds, after which the sample was held at temperature for an additional 15 to 30 seconds before testing.
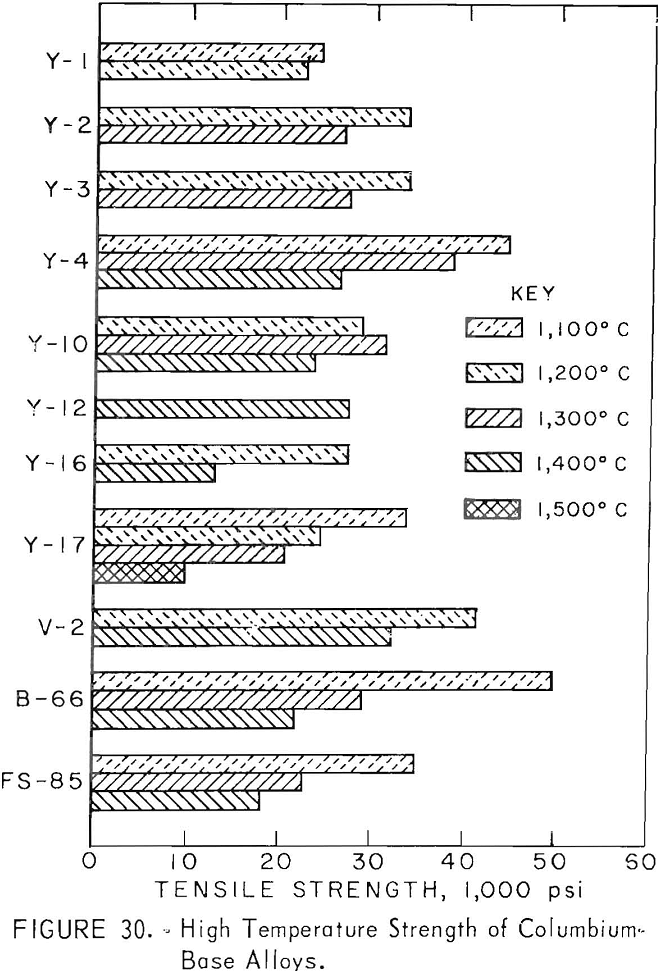
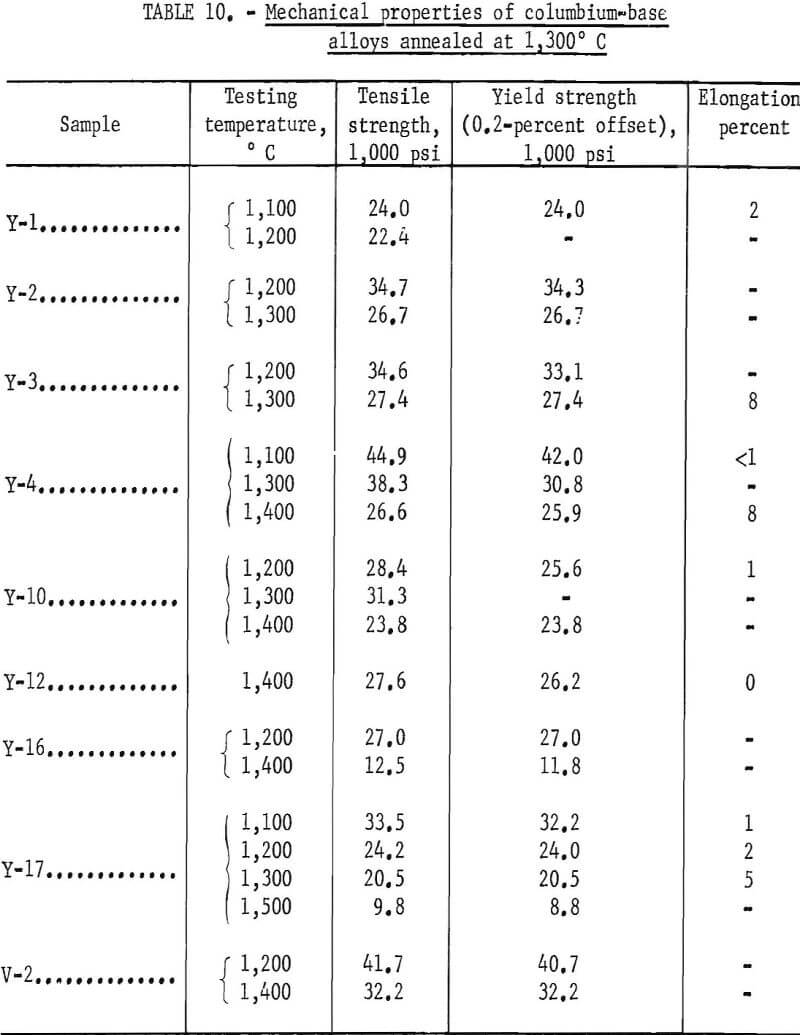
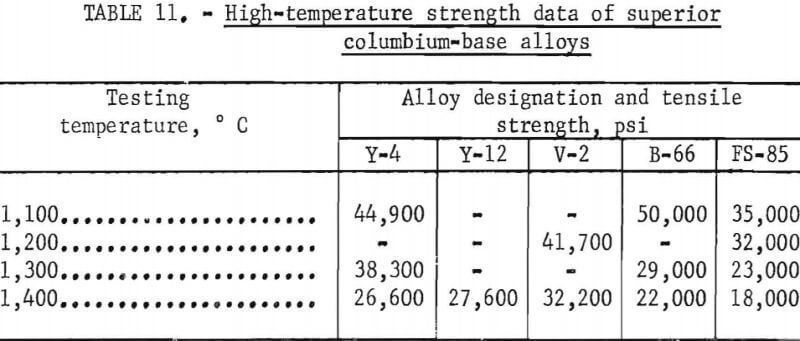
Strength data could not be obtained for all alloys because the total length of some specimens was insufficient. Table 10 shows data on high-temperature strength. The relationship between the tensile strength and temperature is presented in figure 30. The strength of the base alloy (Y-2), containing 2 per-cent zirconium is higher than the strength of the sample (Y-1) containing 1 percent zirconium. The strength of alloy Y-3, in which 8 percent titanium is replaced with 2 percent aluminum, is the same as for sample Y-2. But for Y-4 where 4 percent aluminum is added, the strength is the highest. Y-12 has excellent strength at 1,400° C and is equally as strong as Y-4. Alloy V-2, containing nine components, displayed a strength superior to all other alloys tested with a value of 32,200 psi at 1,400° C. The strength of samples Y-16 or Y-17 containing large amounts of titanium is low at high temperatures. The ductility as shown in table 10 appears to be low for all the alloys tested. Y-4, Y-12, and V-2 are comparable in strength with the well-known columbium alloys B-66 and FS-85. The comparison may be easily seen in table 11.
Oxidation Resistance
The alloys that were rolled were cut into samples approximately 0.1 by 2 by 2 cm, cleaned, and annealed at 1,300° C. The apparatus used for oxidation tests consisted of an automatic balance, a recorder, a tube furnace and a controlling potentiometer. This apparatus gave a continuous measurement of the specimen weight, which increased as the oxide film formed and adhered to the specimen. A quantitative indication was also given if there was a weight loss through the spalling of oxide.
The furnace was brought to the test temperature at 1,000° C, the weight of the sample was equalized with weights on the right-hand pan, and the recorder was adjusted to read zero. After all the adjustments were made, the specimen was placed in the middle of the furnace, using a platinum wire, and heated in air for 1 hour at 1,000° C. Only 1 sample of each heat was tested on this survey oxidation analysis.
As shown in table 12, these alloys have reasonably good oxidation resistance. If 8 to 10 percent titanium is replaced with a small amount of aluminum, the weight gain by oxidation is small; also, resistance to oxidation is improved by adding 4 percent chromium or carbon.

Discussion of Results
As shown in figures 17 and 28, the elements aluminum, carbon, chromium, vanadium, and zirconium have a hardening effect on the as-cast or annealed columbium-base alloys. The effects of vanadium and zirconium are solid-solution hardening. Aluminum and carbon result in precipitation hardening because the solubility of these elements is low in columbium. The solubility of chromium in columbium is approximately 12 weight-percent at 1,500° C and decreases to 4 weight-percent at 800° C. Because of the comparatively low solubility of chromium, the hardness curves as shown in figures 17 and 28 rise rapidly in columbium-base alloys containing more than 3 or 4 percent chromium. The effects of precipitation hardening of aluminum, chromium, and carbon are supposed from metallographic observation as shown in figures 2 to 6, 10 to 16, and 19 to 27. It is probable to consider that these precipitates may mainly be Cb3Al and Cb2Al for aluminum, CbCr2 for chromium, and Cb2C and CbC for carbon. However, when the precipitated phase in each sample was examined by microprobe, no carbide or intermetallics were identified.
The fabrication data in table 6 show that the maximum alloying concentration where cold-working is possible is 4 percent aluminum and 2 percent chromium. Cold-working is difficult however for the alloys containing carbon. The alloys containing 8 percent aluminum, 2.5 percent carbon, and 4 percent chromium can be fabricated by hot-working. These results show that, out of all the alloying elements which induce precipitation hardening, the addition of aluminum has a relatively small effect on fabricability.
The most interesting results are seen in the recrystallization data shown in table 9. The recrystallization temperature of columbium-base alloys decreases with the addition of carbon, large amounts of aluminum, and small amounts of chromium. The addition of small amounts of aluminum raises the recrystallization temperature of the alloy. Examination of the microstructure revealed that recrystallization always nucleated in the neighborhood of the precipitate. The larger the amount of precipitate or the larger the size of the precipitate, the lower the temperature requirement for recrystallization. The kind, amount, size, and rate of dispersion of the precipitate are important factors that control the recrystallization temperature. With small amounts of aluminum, it is probable that the precipitate has an effect that raises the recrystallization temperature. The recrystallization temperature will be raised by the solid-solution effect with a small amount of aluminum. The aluminum and carbon precipitates are stable to high temperatures above 1,300° C, but the precipitate containing chromium dissolves in the matrix at a temperature of 1,150° to 1,200° C. The precipitate containing aluminum and that containing carbon has the same relative stability. It has been thought that the recrystallization temperature decreases with the addition of carbon; but, on the contrary, it increases with the addition of small amounts of aluminum. This fact shows that this kind of precipitate has a large effect on the recrystallization temperature. It is probable that the decrease of the recrystallization temperature with a small amount of chromium may be traced to the existence of a precipitate that is introduced with the addition of chromium and is due to the instability of the precipitate of chromium. Moreover, comparing Y-1 with Y-2 and Z-1 with V-1 as shown in table 9 reveals that zirconium and vanadium raise the recrystallization temperature.
The strength results shown in figure 30 account for the relationship between the recrystallization temperature, the solution temperature of precipitate, and high-temperature strength. The high-temperature strength is low for alloys Y-1, Y-16, and Y-17 that have a low recrystallization temperature or a low solution temperature of precipitate; the strength increases for the alloys Y-2 and Y-3 that have a higher recrystallization temperature and a higher solution temperature of precipitate; and, finally, the alloy Y-4 that has the highest recrystallization temperature and a higher solution temperature of precipitate has the highest strength. Alloy V-2 with strengths even higher than Y-4 has a lower recrystallization temperature than that of Y-4, but the solution temperature of precipitate (carbide) is higher than 1,300° C. A high-temperature strength for alloys containing a larger amount of carbon cannot be expected because of the decrease in the recrystallization temperature.
The study of columbium-base alloys containing aluminum was performed by R. T. Begley and A. I. Lewis. The alloys investigated were Cb-1.5Al (weight-percent) as a binary system and Cb-10V-1.5Al (weight-percent) as a ternary system. Cb-1.5Al has low strength at high temperatures, and Cb-10V-1.5Al cannot be fabricated satisfactorily. Samples Y-4, Y-12, and V-2 obtained by our study are new alloys. These new alloys attain high strength and oxidation resistance at high temperature by substituting aluminum for titanium and by having a complex alloy using the strengthening mechanisms in one alloy. These new alloys have three properties that are necessary for refractory alloys : excellent high-temperature strength, good oxidation resistance, and good fabricability. The designations Y-12, Y-4, and V-2 were given to these alloys.
Summary
Throughout the investigation of columbium-base alloys, excellent high-temperature properties were observed on materials containing many alloying elements.
The following are some highlights of this investigation:
- An increase in hardness for columbium-base alloys may be achieved by solid-solution hardening with vanadium, zirconium, and a small amount of chromium. Precipitation hardening may be obtained by adding aluminum or carbon or a large amount of chromium.
- Cold-working is possible with an alloying concentration of 4 percent aluminum and 2 percent chromium. The alloys containing 8 percent aluminum, 2.5 percent carbon, and 4 percent chromium can be fabricated by hot-working.
- The recrystallization temperature increases with the addition of a small amount of aluminum but is lowered with the addition of carbon, large amounts of aluminum, or small amounts of chromium.
- The precipitate containing aluminum and carbide is stable above 1,300° C, but the precipitate containing chromium dissolves in the matrix at a temperature of 1,150° to 1,200° C.
- The larger the amount of precipitate and the larger the size of precipitate, the easier it is to start recrystallization. The kind, the amount, the size, and the rate of dispersion of the precipitate are important factors that control the recrystallization temperature.
- Vanadium has a more pronounced grain-refining effect on columbium-base alloys than the other alloying additions.
- As the recrystallization temperature increases, the high-temperature strength increases. Therefore, the columbium-base alloy containing small amounts of aluminum has both the highest recrystallization temperature and the highest elevated temperature strength,
- A complex alloy (V-2) using the solid-solution and precipitation-strengthening mechanisms is a successful means to obtain high-temperature properties.
- The oxidation resistance of the alloy containing small amounts of aluminum is almost equal to that of the alloy containing large amounts of titanium.
- Y-4, Y-12, and V-2 alloys have three properties that are necessary for refractory alloys: excellent high-temperature strength, good oxidation resistance, and excellent fabricability.
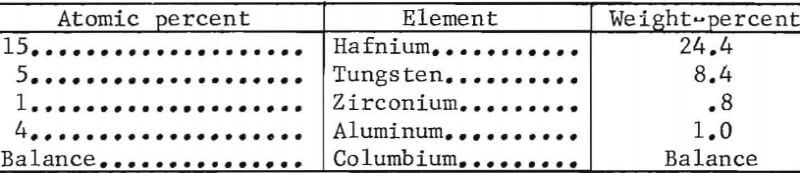
- The chemical composition for Y-4 alloy is as follows:
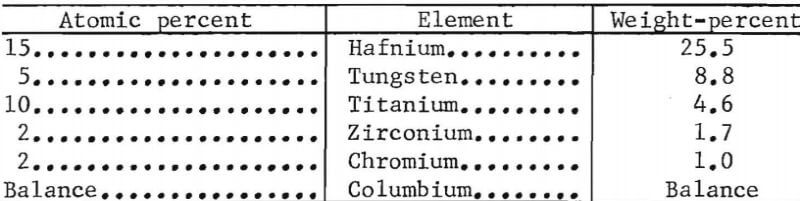
- The chemical composition for alloy Y-12 is as follows:
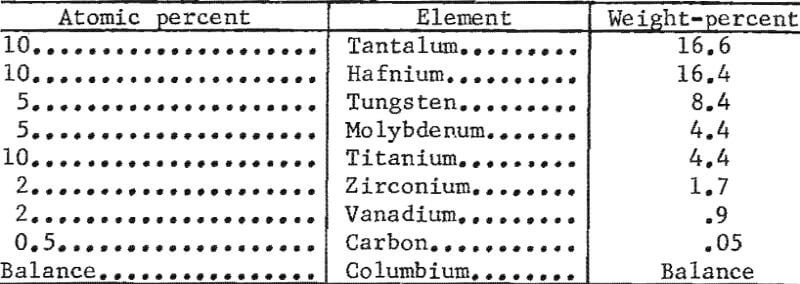
- The composition for alloy V-2 is as follows:
Proposed Future Studies
Recommendations for future columbium-base alloy studies follow:
- Development of Y-4 alloy—
Cb-15Hf-5W-1Zr-4Al
Cb-15Hf~5W-1Zr-5Al
Cb-15Hf-5W-2Zr-4Al
Cb-15Hf-5W-2Zr-5Al - Development of Y-4 alloy containing titanium—
Cb-15Hf-5W-1Zr-4Al-4Ti
Cb-15Hf-5W-1Zr-4Al-6Ti
Cb-15Hf-5W-2Zr-4Al-4Ti
Cb-15Hf-5W-2Zr-4Al-6Ti - Development of Y-4 alloy containing nitrogen (use Cb-N alloy for the addition of nitrogen)-
Cb-15Hf-5W-2Zr-4Al-1N
Cb-15Hf-5W-2Zr-4Al-2N
Cb-15Hf-5W-2Zr-4Al-4Ti-1N
Cb-15Hf-5W-2Zr-4Al-4Ti-2N - Development of Y-4 alloy containing nitrogen and carbon (use Cb-C alloy) —
Cb-15Hf-5W-2Zr-4Al-1N-1C
Cb-15Hf-5W-2Zr-4Al-2N-1C
Cb-15Hf-5W-2Zr-4Al-4Ti-1N-1C
Cb-15Hf-5W-2Zr-4Al-4Ti-2N-1C - Development of Y-12 and V-2 alloys by varying the constituents.
- Upon completion of the above study and after a more detailed investigation is conducted on alloys Y-4 and V-2, an application for a patent will be made.
