Table of Contents
In extractive metallurgy, separation of insoluble solids from solutions is an important economic factor in almost all flowsheets. This separation step can employ sedimentation, filtration (vacuum and pressure), or centrifugation. Since practically all systems require a relatively complete separation, multiple unit, countercurrent thickeners or vacuum filters are generally used.
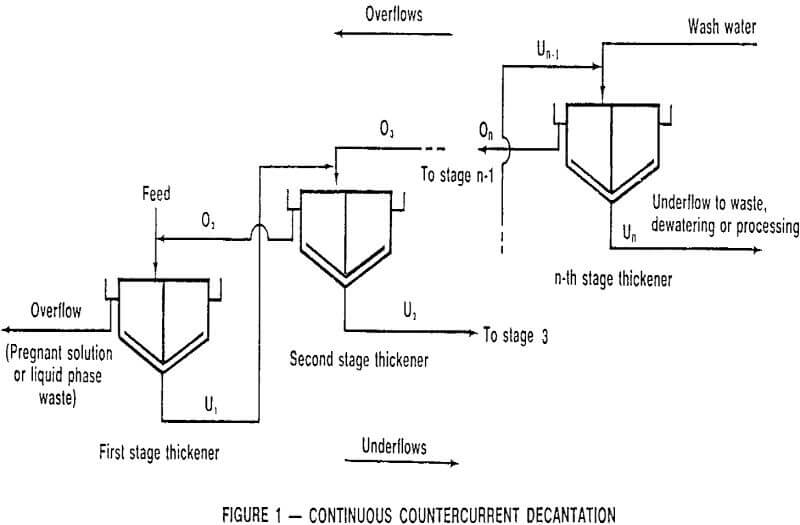
Continuous Countercurrent Decantation
In its simplest form, washing in a thickener can consist of mixing solids and associated solution with water, settling the solids, decanting the clarified solution, and then repeating the process as required until the dissolved material is removed. The logical extension of this procedure is to use the more dilute, decanted solutions as wash liquid in earlier stages, a procedure which lends itself to continuous operation, and, hence, continuous countercurrent decantation.
The controlling factors in such a procedure are:
- The number of mixing-settling stages;
- The relative amounts of liquid in the feed and underflow; this can be expressed as the ratio of overflow volume to volume of liquid in the underflow, which is often termed “wash ratio”;
- The “efficiency” of each stage, referring to the completeness of mixing of the underflow and overflow solutions which enter a thickener (defined quantitatively in later text).
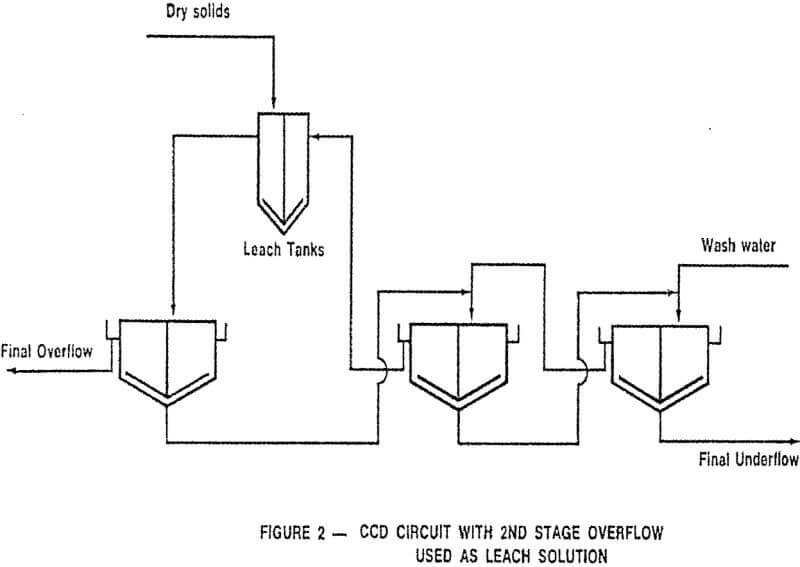
CCD flowsheets can take many forms; the most common is the straight forward procedure illustrated in Figure 1 in which a leach pulp enters the primary thickener, after being diluted with second stage thickener overflow, with the solids proceeding through the series of thickeners in a direction countercurrent to the wash solution.
Filtration
Batch pressure filters are used in minerals processing, usually where pulps are dilute in solids, or hot and possibly saturated in dissolved components. Thorough cake washing is rarely practical, and usually some degree of further processing for solution recovery is required. Hence, batch filtration achieves its recovery mainly by producing a well dewatered filter cake. Relatively high pressures, around 200 psi, can be employed in plate and frame type filters to achieve a high solids concentration, usually 5-10% greater than can be obtained on a vacuum filter. On leaf filters, blowing the cake with steam can often produce the same result, although at a considerable cost in terms of heat requirements.
Continuous vacuum filtration, generally employing cake washing, is far more commonly used because of the relative ease of control, greater effectiveness and stability, and reduced cost of operation and labor.
Washing filters can consist of single stage, two stage, or even three stages of drum filters. The latter system usually employs countercurrent washing, and Figure 3 illustrates a typical circuit. Horizontal units, either belt, tilting pan, or table filters, are used for relatively fast filtering materials where countercurrent washing of the filter cake is desired. The higher cost of installing and operating horizontal filters encourages their use principally under conditions where drum filters are not applicable.
Choice of a System
In designing a hydrometallurgical process, selecting a washing system is probably one of the more difficult tasks facing the engineer. Ultimately, the choice is largely an economical one; an approach for obtaining all the data to determine the cost can proceed along these guidelines:
Some materials simply either will not filter or not settle, and the choice may be obvious. Potash slimes are typical of the former (while filtration is possible, the rates are very low, and if the slime content of the ore is relatively high, which is often true in the deposits now available for mining, the filter area required is excessive). On the other hand, the solids will usually flocculate and settle, although underflows are often very dilute, and a multi-stage CCD system is generally employed.
Filtration may be indicated in some systems where the retention time in a thickener affects the process chemistry. For example, metastable salt crystallization processes, such as in the Na-K-Mg-Cl-SO -H2O system, can take advantage of the slow rates of crystallization of some salts from super¬saturated solutions to effect a separation. Filters (or centrifuges) are more suitable than thickeners because of the limited hold-up times possible.
The flowsheet employed may also dictate the circuit to be utilized. For example, in a leach circuit where more than one component is recovered, with the principal one of these removed by solvent extraction, it could be necessary to recycle the raffinate to build up the concentration of minor components, removing a bleed stream from the circuit for their precipitation. To minimize processing costs, it is desirable to remove as small a fraction of this circulating solution as possible, as the cost of the subsequent processing steps will increase with the volume.
Good washing is required at the solid-liquid separation step as the loss of secondary components will be magnified to the extent of the ratio of leach liquor to bleed stream, which can be 10 fold. Under these conditions a 1% soluble loss in reality would be a 10% loss of the minor components.
Complex flowsheets with numerous processes and operations are often more amenable to filtration. Simple flowsheets without complicated liquid balances lend themselves better to CCD operation. Similarly, large quantities (i.e. 5000 TPD solids and up) are often more economically handled with CCD systems, because of the lesser number of units required and the reduced operating attention necessary. Filters have the advantage of obtaining maximum recovery after start-up in the shortest period of time because of the short detention time. This is particularly important with high dollar value solubles.
Recently designed plants have tended to proceed in the direction of coarser grinds and higher throughputs per unit area, often without classification circuits which would provide separate processing of the sands. This puts a much greater load on the thickening mechanism and demands closer control of the operation to prevent failure of a unit.
Operational Problems and their Cures
The well designed solid-liquid separation section of a modern metallurgical plant should start up the first time that ore comes into the processing plant, recover 99.9% of the dissolved values throughout the life of the ore-body, and be shut down only for planned maintenance work. This would be, of course, a pipe dream, and reality seems to be far removed. Some of the difficulties that beset thickeners and filters are impossible 10 anticipate; however, many of the problems that occur are worth reviewing in the hope that future plants can be designed to avoid some of these troubles.
Flocculant use in all CCD circuits is now almost universal practice. Some of the high molecular weight reagents produce a strong flocculent structure which, while resulting in rapid thickening, is highly resistant to repulping, with corresponding loss in washing efficiency. In one six stage CCD circuit, the following mixing efficiencies were recorded in each successive stage, starting with the first one: 100%, 88%, 83%, 65%, 41%, and 30%. These low efficiencies occurred despite the inclusion in the plant of interstage mixers, whose use was discontinued because of “too much trouble”.
Dissolved value loss on washing filters can generally be attributed to these causes:
- An inadequate wash volume being applied;
- Filtrate blowback;
- On even wash pattern due to plugged nozzles, channelling, etc.;
- Blinded filter media, or excessively thick filter cakes, restricting the volume of wash which will pass through the cake.