Table of Contents
The design, operation, and performance of an integrated pilot plant for recovering zinc and copper from a complex sulfide ore are described. Metallurgical processing comprised selective sulfate roasting in a fluid bed, leaching in a weak sulfuring acid solution, recovering copper by cementation with scrap iron, air oxidation to remove soluble iron, more-or-less conventional purification for removal of impurities deleterious to zinc electrolysis, recovering zinc by electrolyzing solutions, and recycling spent electrolyte to the sulfating roaster.
Pre-Pilot Plant Investigations
From a thermodynamic viewpoint, it is feasible to sulfate selectively a host of metals, including zinc, copper, lead, cadmium, nickel, and cobalt, in an iron sulfide ore without sulfating iron. Of course, a number of conditions such as roasting temperatures and gas composition must be controlled. In addition, as first disclosed by Hybinette, an alkali metal salt is frequently beneficial in promoting the sulfaction reactions.
The bench-scale investigations, following procedures similar to those outlined by Hanway, were successful in detailing the principal processing steps for a method of treating the ore. The process consisted of the following steps:
- Converting the zinc and copper sulfides to soluble sulfates by autogenuous roasting in a fluid bed.
- Leaching the roasted calcines with weak (10 to 50 grams per liter) sulfuric acid solution to dissolve the copper and zinc.
- Recovering the copper from solution by cementation with iron.
- Purifying the solutions by air oxidation of iron and more-or-less conventional purification procedures for the removal of other elements deleterious to zinc electrolysis.
- Recovering zinc by electrolysis
- Recirculating spent zinc electrolyte to the fluidized bed roaster and to the calcine leaching circuit.
- Recovering lead by brine leaching of calcine residues and precipitating the lead as a basic hydroxide.
Description of Pilot Plant
The Pilot Plant was subdivided into five operating sections, each of which served a particular function. These were as follows:
This circuit was designed to treat minus ¼” ore as received from the mine in a wet grinding circuit to produce a classified product of approximately 65% minus 325 mesh particle size.
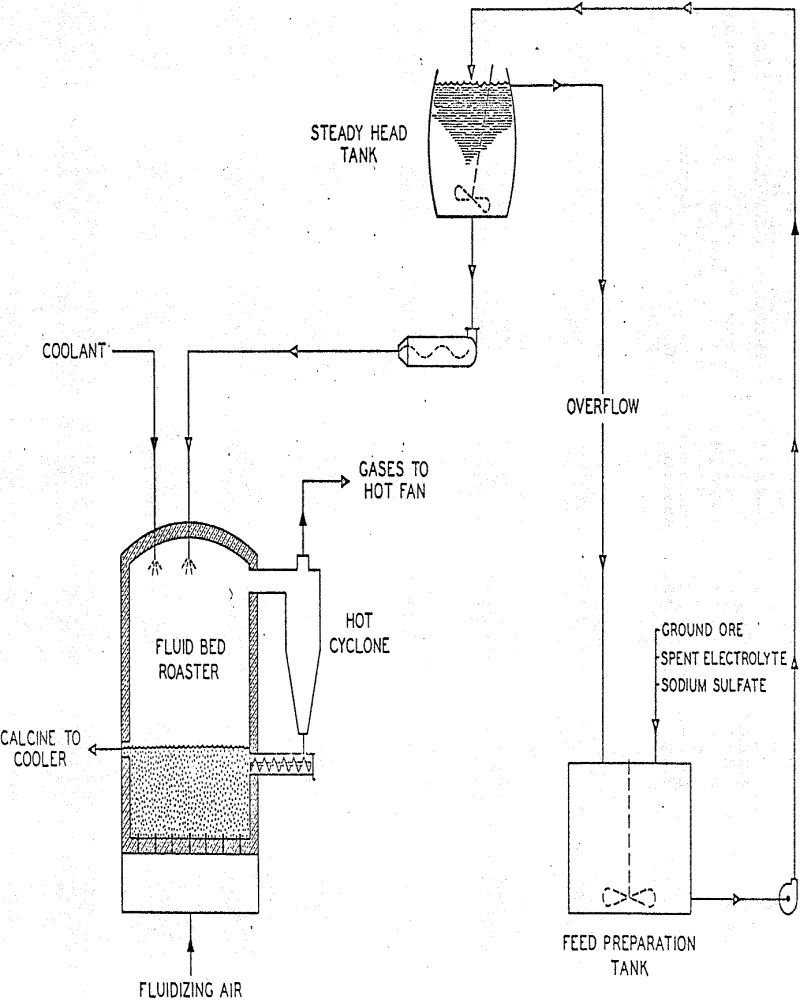
The fluid bed roaster had a free board diameter of seven feet, a bed section diameter of five feet, and a height of 21 feet-6 inches from dome to grid. Bed depth was held at 60″ throughout the pilot plant campaign.
Calcine overflowing the roaster discharged into a three-foot diameter fluid bed cooler. Heat removal in the cooler was effected by circulating cooling water through stainless steel tubes. Cooled calcine discharged into the quench tank of the Leaching Circuit.
Calcine was leached with water and sufficient sulfuric acid to yield an acid concentration in pregnant solutions of approximately 10 grams per liter. Retention time in the leaching tanks was between 2 and 3 hours and temperature was maintained at 60 – 70° C. by means of steam coils.
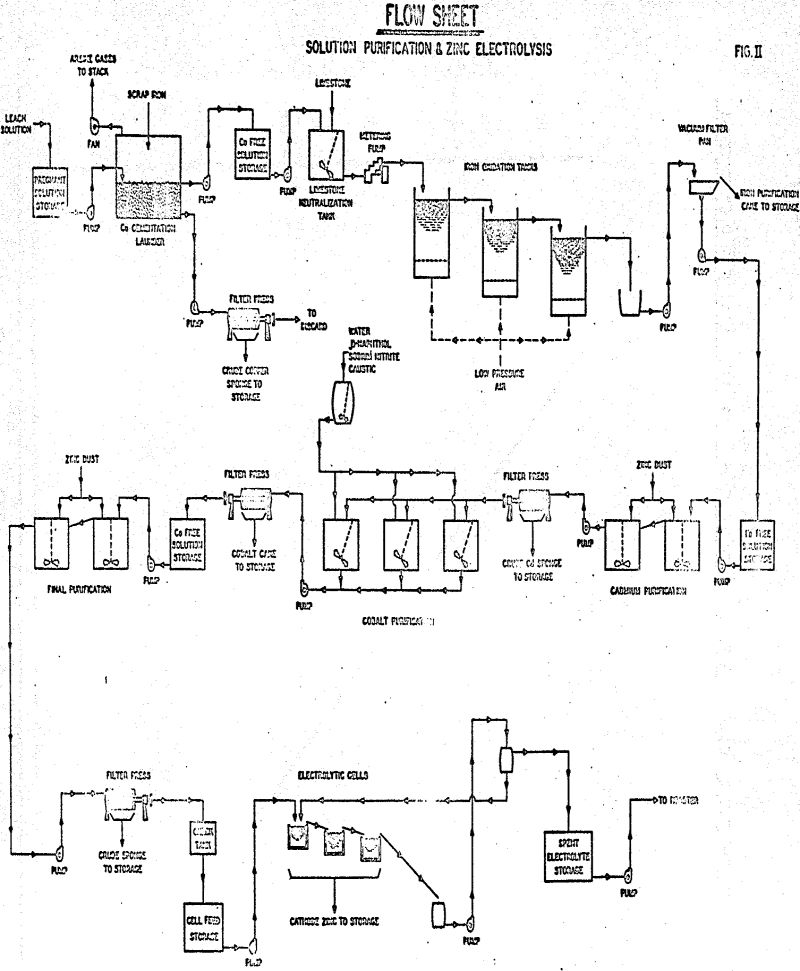
Zinc-rich liquor from the leaching circuit was treated in five individual stages of purification to remove impurities which would be harmful in the production of electrolytic zinc. Solution from the leaching section was pumped to the copper cementation launder, a 17 x 11-½ x 3 ft. rectangular wooden tank with a capacity for about 7,000 lbs. of sheet iron trimming.
Results
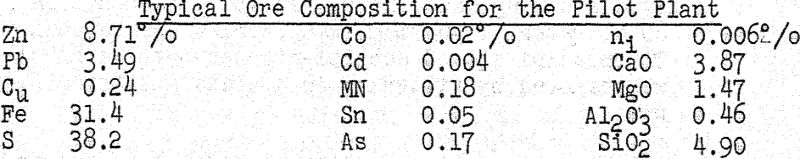
Data obtained from the processing of 1508 tons of ore was subjected to a detailed statistical study. Typical composition of ore used in the pilot plant is shown in the following table :
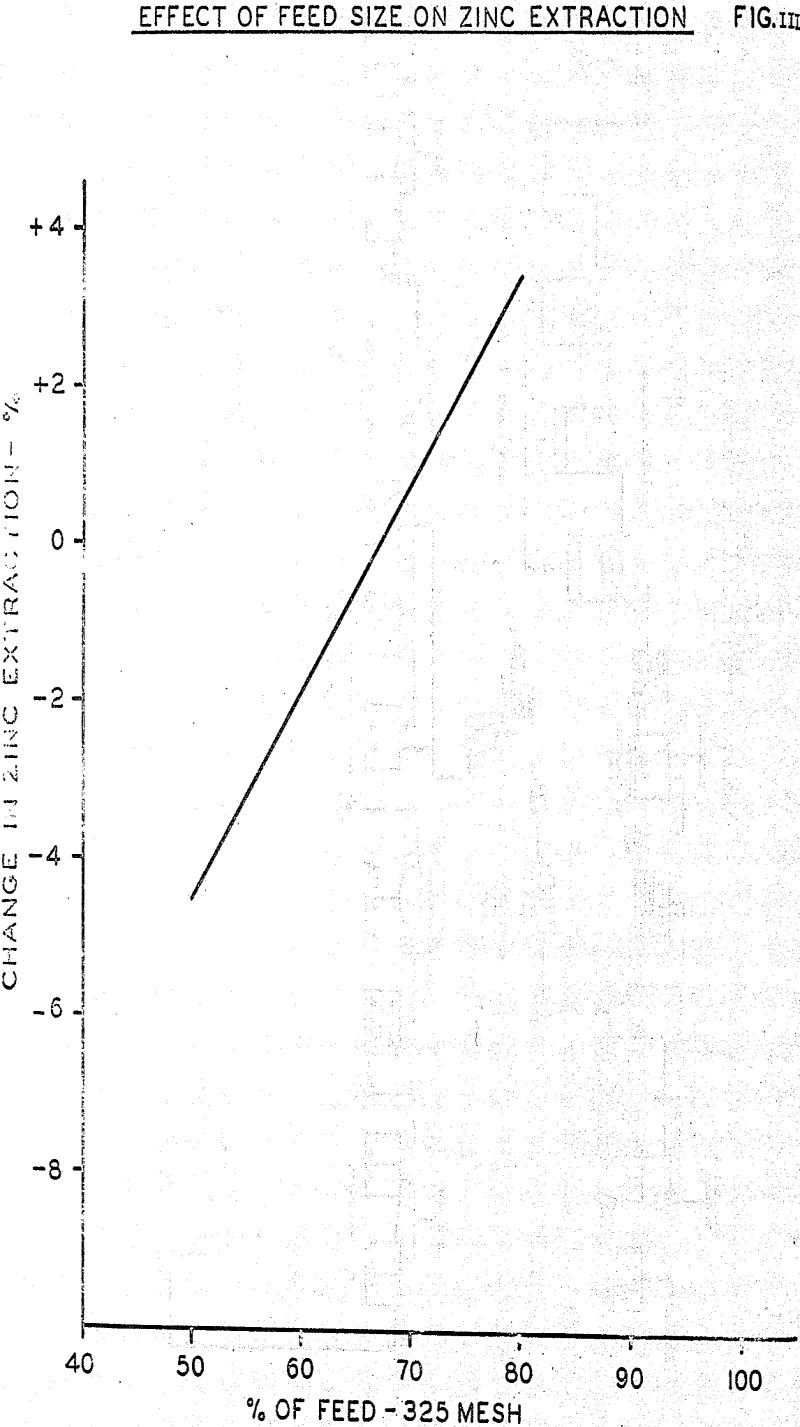
Pilot plant experience demonstrated that ore particle size had considerable influence on the extraction of zinc. Increasing the fineness of ore grind improved zinc extraction; a 10% increase in in percent minus 325 mesh increased extraction approximately 2.7%.
Use of a sulfation promoter to lessen ferrite formation was shown to be beneficial to zinc extraction. Optimum extraction was achieved with sodium sulfate additions equal to about 2% of the weight of ore. Increasing sodium, sulfate addition to 2% from a level of 1% improved zinc extraction 8%. Higher addition rates did not improve extraction.
Particle size of Calcine, as judged by the plus 48 mesh size distribution, was the most important single variable influencing zinc and copper extraction.
The results show optimum zinc and copper extraction correspond with calcine particle size in the range 45 to 55% plus 48 mesh. It is fortunate (and perhaps not a coincidence) this range in particle size also corresponds to that for optimum fluidizing characteristics in the roaster.
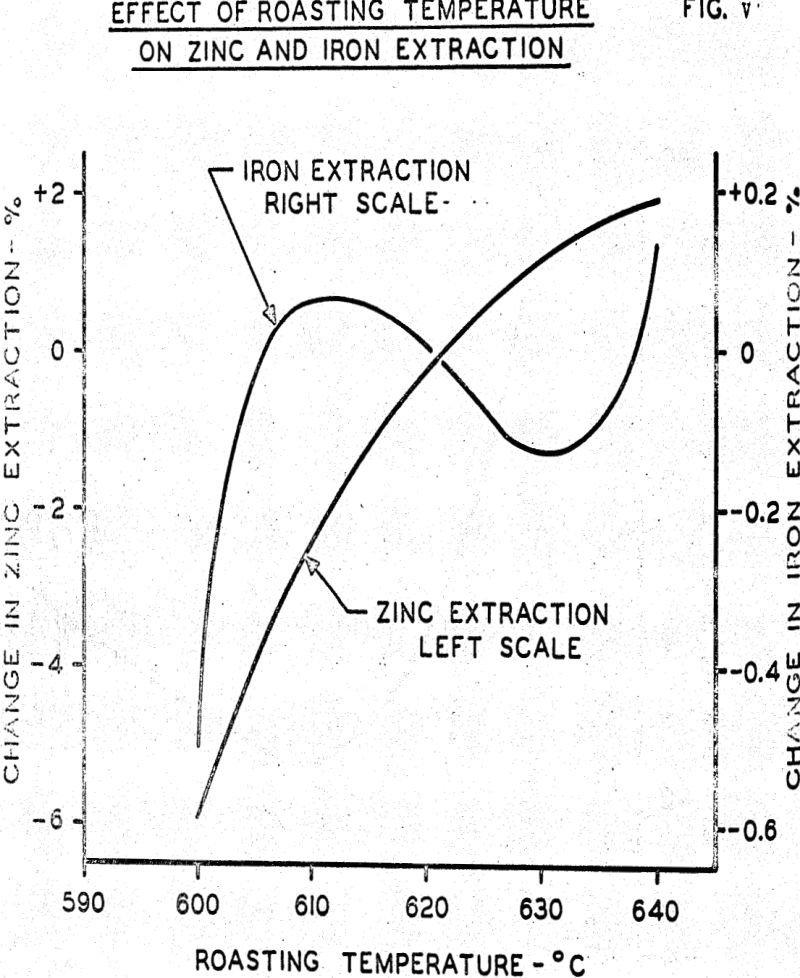
Results of the regression analysis permit predicting extraction of zinc, copper, and iron for various conditions of roaster operation. For example, using 75% excess air (corresponding to an SO2 content of approximately 6.4%), recycling spent electrolyte to the feed preparation tank with sufficient additional sodium sulfate added to yield 2% sodium sulfate as percent of ore feed, grinding the ore to 75% minus 325 mesh, operating the roaster feed gun to achieve 50% of plus 48 mesh particle size in calcine, operating the roaster at a bed temperature of 630°C., and feeding the roaster with ore containing 20% gangue should achieve approximately 95% solubilization of zinc, 97-½% of copper, and 2.1% of the iron.
Purification of Leach Solutions for Zinc Electrolysis
Zinc solutions from the leaching circuit contained about 10 grams per liter sulfuric acid and had the following range in metal compositions:

Treatment of leach solutions with scrap iron in the copper launder removed an average of 96.6% of the copper with an associated solubilization of 1.81 grams of iron per gram of copper removed. A major portion of the arsenic was also removed in this cementation step.
The zinc produced in the electrolytic cells was of acceptable chemical composition running almost entirely above; 99.97% zinc with production the last two months of pilot plant operation being about 99.99% zinc. Lead was the major contaminant in the metal which probably reflects the effects of new anodes and the fact that no lead depressant reagents were used.