Table of Contents
Most mercury ores are directly roasted or retorted for recovery of metallic mercury without preliminary concentration. Recoveries are high, often approaching 100 percent; the cost is nominal. The tenor of domestic mercury ores, however, has been dropping steadily, so that the estimated average heads are now about 0.35 percent Hg or 7 pounds per ton of ore, compared with 14-pound mercury ores being treated in Italy and 120-pound ores in Spain.
Many laboratory experiments and mill runs have been made on concentration of mercury ores, yet aside from a few small operations in California, little concentration is practiced. Walter W. Bradley conducted a large number of concentration tests on mercury-bearing materials at the University of California. He concluded that the ultimate decision between a straight furnace reduction and concentration and roasting of concentrates will be a matter of comparative costs and comparative extractions.
The advance in flotation technique made since Bradley’s work was reported has made possible excellent concentration results from some ores as reported in comparatively recent papers by Shaffer, Newton and Fahrenwald and by Rey and Brevers. When considering concentration of mercury ores, however, it should be remembered that ore dressing must be as simple as possible and highly efficient in both recovery and ratio of concentration, so that only a small proportion of the feed will, require retorting and so that the over-all recovery will still approach that of direct roasting.
Gravity methods are rarely applicable for concentration of mercury ores in spite of the high specific gravity of cinnabar. Cinnabar tends to slime when crushed, and considerable loss is involved if the slime is discarded; low-grade concentrate containing considerable gangue-slime results if the slime fraction, is combined with the gravity concentrate. Such concentrates, carrying large amounts of gangue slime, are not only difficult to filter and dry but also present difficulties in subsequent distillation.
Cinnabar is readily amenable to flotation, as are finely floured metallic mercury and synthetic mercury sulfides found in treating old dumps from amalgamation or quicksilver-condenser plants. Laboratory tests show tailings containing 0.2 to 1.0 pound Hg per ton not uncommon, and usually high-grade concentrates are obtained that can easily be retorted for the recovery of metal. Grinding to a fineness suitable for flotation, however, is a major disadvantage, and in most cases the grinding cost prohibits the use of flotation for beneficiation of mercury ores.
Flotation methods employed during the studies of the mercury ores from Idaho, California, Nevada, and Oregon conformed to standard laboratory practice. Xanthates commonly were used as collecting agents, with lime and cyanide to depress pyrite when needed. One ore did not respond readily to xanthate flotation without the addition of a mercuric salt as activator.
Physical
Engineers of the Bureau of Mines examined the War Eagle mercury mine, situated 26 miles north of Medford, Jackson County, Greg. A sample of the ore was submitted to the Salt Lake City laboratories for flotation and distillation.
The War Eagle ore is unusual, in that it contains appreciable amounts of arsenic. Microscopic examination revealed that the ore is a replacement of rhyolitic rock with deposition of’quartz containing pyrite and sporadic inclusions of cinnabar, orpiment, and molybdenite. The yellow arsenic sulfide, orpiment, together with incipient grains of pyrite are associated with cinnabar on the fracture planes. The molybdenite occurs in the small quartz veins and stringers ‘that traverse’ the rhyolitic gangue. Traces of chalcopyrite, arsenopyrite, and magnetite were identified.
The petrographic study indicated that minus 200-mesh grinding would be necessary to achieve optimum liberation.
Chemical
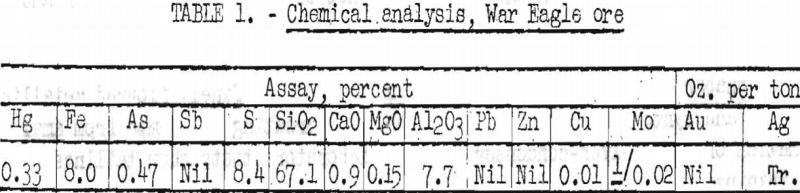
The chemical analysis shown in table 1 is indicative of the composition of the ore sample, tested.
Concentration
Flotation
Although microscopic study indicated that extremely fine grinding would be necessary to achieve mineral liberation, preliminary flotation tests showed that grinding finer than minus 100-mesh created large amounts of gangue slime, which interfered with cinnabar recovery.
Lime and cyanide were effectively employed to depress pyrite, but it was found necessary to use an activator such as mercuric chloride to produce concentrates of reasonable grade.
Best results by flotation were achieved by stage-grinding in a laboratory rod mill to minus 100-mesh and selectively floating the cinnabar at a low pulp density, using American Cyanamid reagent 242 as collector. Results are shown in table 2.
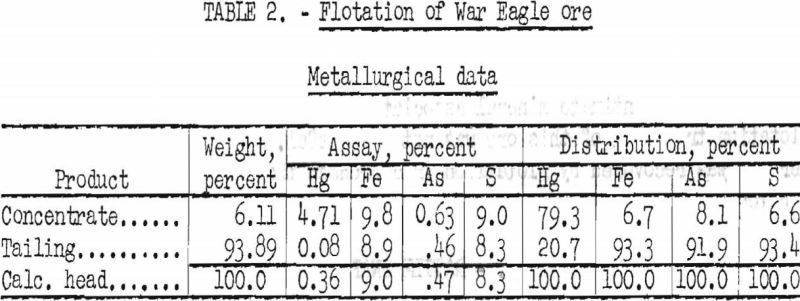
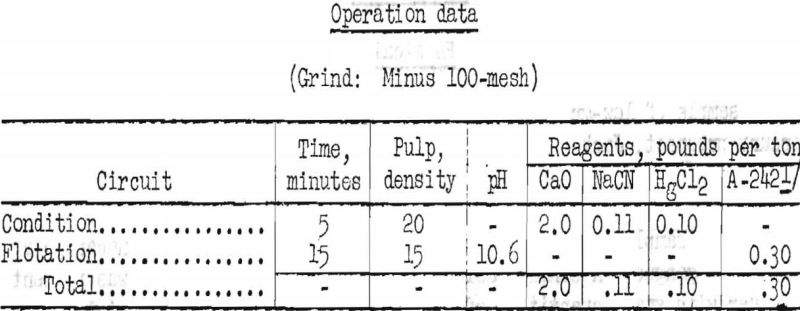
By flotation, over 90 percent of the objectionable pyrite and orpiment were rejected, but only 79.3 percent of the mercury was recovered in a concentrate that assayed 94.2 pounds Hg per ton (4.71 percent).
A similar test, in which no mercuric chloride was used, gave a recovery of 84.4 percent of the mercury, but the concentrate contained only 31.2 pounds Hg per ton.
Distillation
The owners of the property reported that the ore appeared to be amenable to distillation for the recovery of metallic mercury but that corrosive action was excessive and frequent replacement of iron contact parts was necessary.
For laboratory distillation tests, ore crushed to minus ¼-inch was used. In each test the ore was treated in an iron retort at a dull red heat for 3 hours. A moderate current of air was passed through the retort to remove vapors.
In the first test, 94 percent of the mercury was extracted, but corrosive action destroyed the bottom of the retort. A second test, made in a similar manner but with 10 percent quicklime mixed with the ore, gave an indicated recovery of 99 percent with no noticeable corrosion of the retort. The metallic mercury recovered assayed only 0.043 percent As.
Summary
The war Eagle ore is amenable to direct distillation, provided lime is added to minimize the corrosive effect of sulfur dioxide formed during the process. Although the sample tested contains a relatively high amount of arsenic, the mercury collected from a lime-retort test assayed only 0.043 percent As.
Because of intimate mineral association and. interference of gangue slime, flotation treatment of this ore was not successful. Only 79 percent of the mercury was recovered by flotation in a product that assayed 94 pounds Hg per ton.
Physical
A sample of low-grade mercury ore was obtained from the Rattle Snake Mercury prospect, Jackson County, Oreg., by engineers of the Bureau of Mines, and was submitted to the Salt Lake City laboratories for beneficiation.
In the sample received, mercury was present as smears of cinnabar on a calcareous gangue. A considerable amount of readily slimed clay was present, together with small quantities of pyrite and arsenic-bearing material.
Chemical
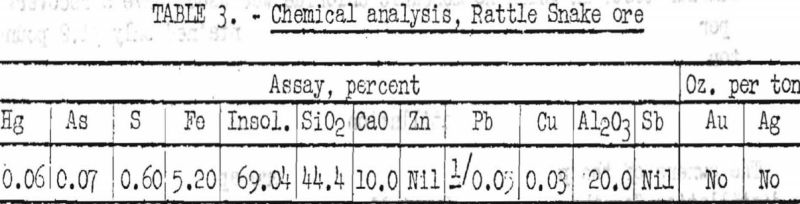
The chemical analysis of the sample treated in the laboratory is given in table 3.
Concentration
A sample of ore was crushed to minus 10-mesh and sieve-sized wet. The minus 200-mesh fraction was deslimed by decantation. Results are given in table 4.
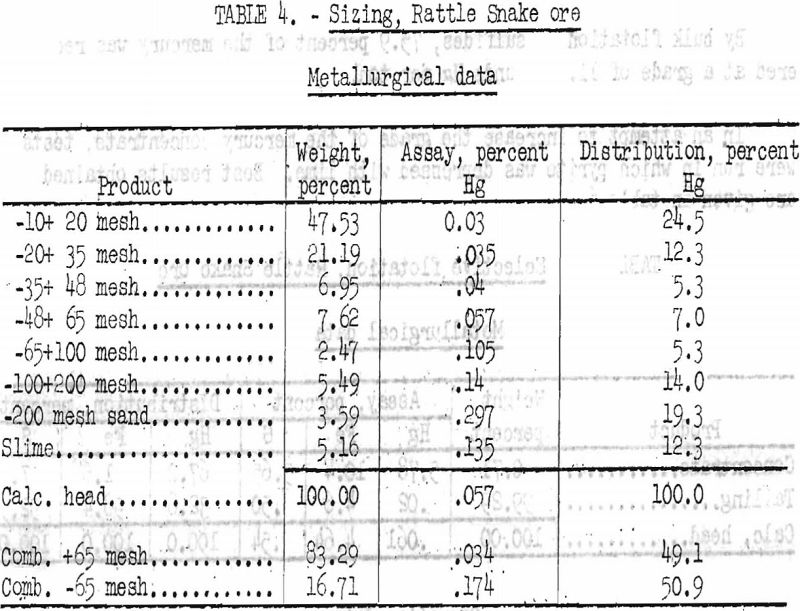
As shown in table crushing to minus 10-mesh and sizing showed a definite concentration of mercury in the finer sizes. Retention of only minus 65-mesh material would recover 50.9 percent of the mercury in 16.7 percent of the total weight. The product would assay 3.48 pounds Hg per ton.
Attrition grinding tests were tried in the attempt to recover a better percentage of mercury by sizing; however, the gangue material was soft and slimed readily, so that no successful concentration was made by this method.
Several bulk flotation tests were run with xanthate or thio-phosphate reagents as collector for sulfides. Best results are shown in table 5.
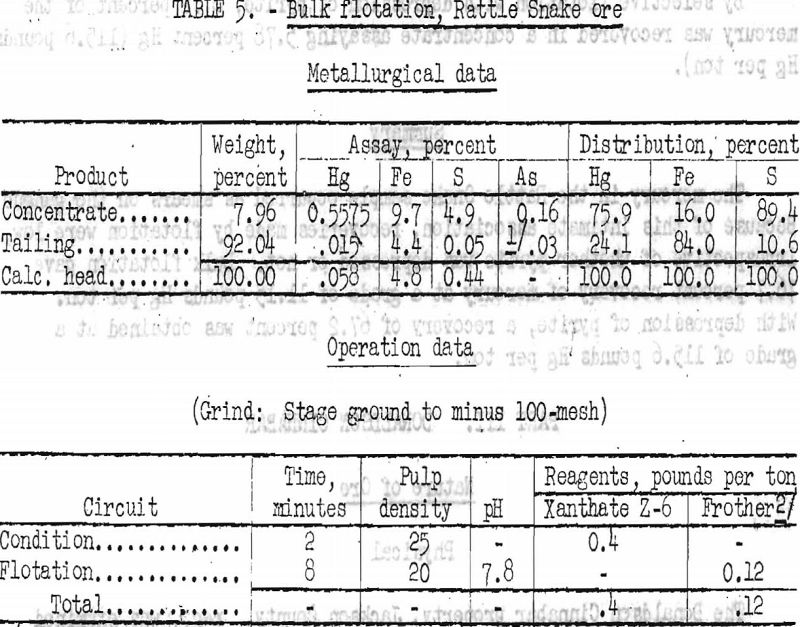
By bulk flotation of sulfides 75.9 percent of the mercury was recovered at a grade of 11.15 pounds Hg per ton.
In an attempt to increase the grade of the mercury concentrate, tests were run in which pyrite was depressed with lime. Best results obtained are given in table 6.
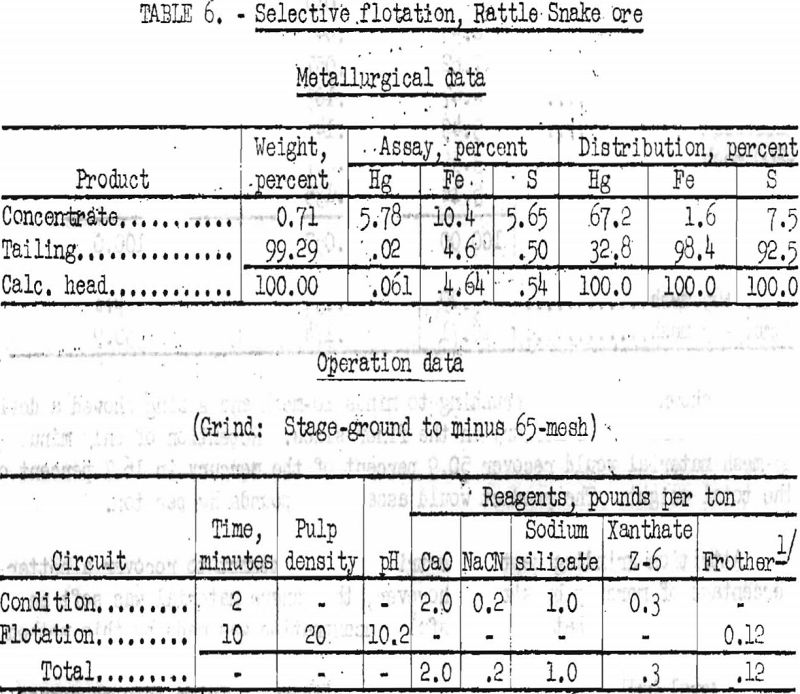
By selective flotation with depression of pyrite, 67.2 percent of the mercury was recovered in a concentrate assaying 5.78 percent Hg (115.6 pounds Hg per ton).
Summary
The mercury in the Rattle Snake sample occurred as smears on the gangue. Because of this intimate association, recoveries made by flotation were low, irrespective of whether pyrite was depressed or not. Bulk flotation gave 76.7 percent recovery of mercury at a grade of 11.15 pounds Hg per ton. With depression of pyrite, a recovery of 67.2 percent was obtained at a grade of 115.6 pounds Hg per ton.
Physical
The Donaldson Cinnabar property, Jackson County Oreg., was examined by engineers of the Bureau of Mines, and a sample was submitted to the Salt Lake City laboratories for flotation beneficiaticn tests.
The sample was very low-grade. Cinnabar was present as fine-grained deposits in interstices and in the fracture planes of the quarzitic gangue. Microscopic examination indicated that a small portion of the cinnabar could be liberated by grinding to pass a 65-mesh sieve, but that complete liberation could be achieved only by extremely fine grinding.
Chemical
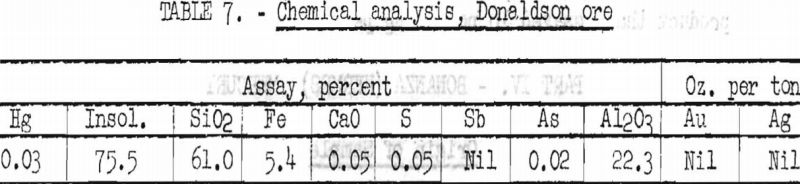
The chemical analysis of a representative head sample, shown in table 7, is indicative of the composition of the sample submitted for flotation studies.
Concentration
Preliminary studies included a wet screen analysis of ore crushed to minus 10-mesh. Results of the screen analysis showed a slight concentration of mercury in the minus 200-mesh sand fractions and indicated that minus 200-mesh grinding would be required for adequate liberation of the cinnabar from the gangue.
Subsequent flotation tests, however, showed that fine grinding increased the loss of mercury in the tailing product owing to the formation of excessive cinnabar slime. Best flotation results were obtained from ore stage-ground in a laboratory ball mill to minus 65-mesh. Operation data and metallurgical results are shown in table 8.
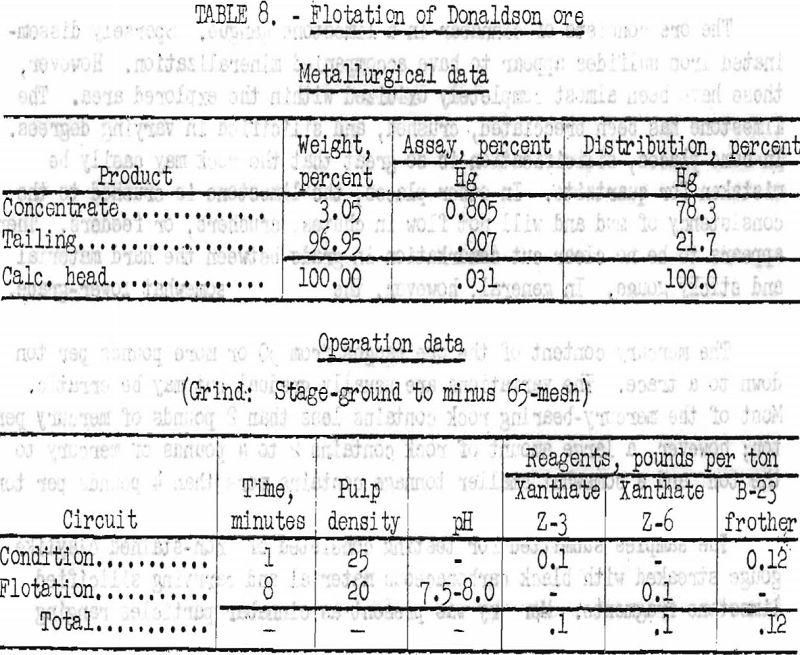
By flotation, 78.3 percent of the mercury in the Donaldson cinnabar sample was recovered in a rougher concentrate that assayed 0.805 percent Hg or 16.1 pounds Hg per ton. No attempt was made to clean the rougher concentrate to produce a higher-grade product.
Further testing was considered unwarranted, as only preliminary tests had been requested and because the ore was definitely submarginal in grade.
Summary
Although the ore from the Donaldson property assayed only 0.6 pound Hg per ton, flotation of 65-mesh ore recovered 78 percent of the mercury in a product that assayed 16 pounds Hg per ton.
Origin of Sample
Early in 1941, the Bureau of Mines started an extensive diamond-drilling campaign to determine the size and value of mercury deposits located near the head of Cinnabar Creek in Valley County, Idaho. Almost simultaneously, the Bonanza Mines, Inc., began development on these deposits, which resulted in production at a rate of 200 flasks of mercury per month by September 1942.
During the course of examination and development, it was disclosed that the mine contains considerable tonnage of soft, gougelike ore that is not amenable to direct furnace treatment. Samples of the gouge material were submitted to the Salt Lake City laboratory for metallurgical study.
Nature of Ore
Physical
The ore consists of cinnabar in a limestone gangue. Sparsely disseminated iron sulfides appear to have accompanied mineralization. However, these have been almost completely oxidized within the explored area. The limestone has been brecciated, crushed, and silicified in varying degrees. In some places, siiicification is so great that the rock may easily be mistaken for quartzite. In other places, the limestone is crushed to the consistency of mud and will not flow in chutes, crushers, or feeders. There appears to be no clear-cut demarkation in grade between the hard material and sticky gouge. In general, however, the gouge is somewhat lower-grade.
The mercury content of the ore ranges from 50 or more pounds per ton down to a trace. The variations are usually gradual but may be erratic. Most of the mercury-bearing rock contains less than 2 pounds of mercury per ton; however, a large amount of rock contains 2 to 4 pounds of mercury to the ton, and a somewhat smaller tonnage contains more than 4 pounds per ton.
The samples submitted for testing consisted of iron-stained claylike gouge streaked with black-carbonaceous material and carrying silicified limestone-fragments. Mercury was present as cinnabar particles ranging from 65-mesh to minus 280-mesh, mainly in the gouge fraction but some in the silicified limestone.
Chemical
Disintegration and sizing tests were made on a composite of several “mine-run” samples ranging in mercury content from 1 to 8 pounds per ton. The calculated assay of the composite was 0.163 percent Hg (3.26 pounds per ton).
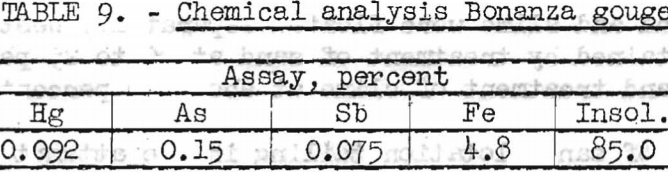
Preliminary concentration tests were conducted on a sample of gouge, the chemical and analysis of which is shown in table 9.
Concentration
Preliminary investigation showed that 35 to 40 percent by weight of the gougelike ore may be discarded as a low-grade slime. Use of sodium silicate or calgon as a dispersing agent was found to be beneficial.
Disintegrating and hydraulic sizing tests were run on several samples of ore ranging in grade from 1 to 8 pounds of mercury per ton. Data given in table 10 summarizes the results to be expected on ore assaying 0.18 percent Hg (3.6 pounds per ton).
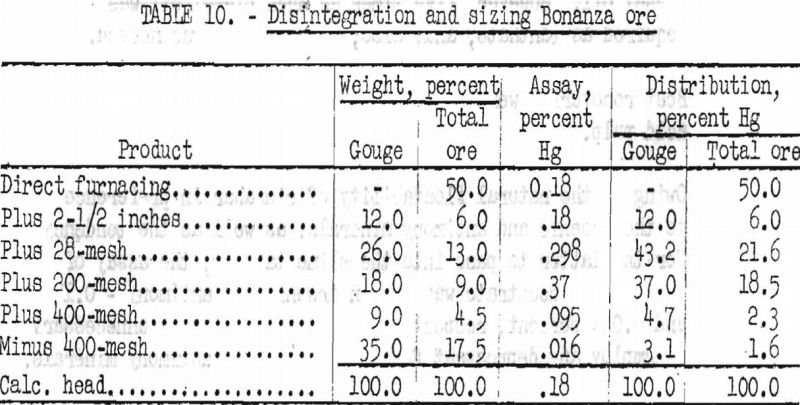
Table 10 gives an average of numerous tests conducted on mine-run ore. Apparently, about 50 percent of the ore is gouge material containing broken rock fragments. By disintegration and sizing of the gouge fraction, 35 percent of the material treated (17.5 percent of the total ore) was rejected as minus 400-mesh slime that assayed only 0.016 percent Hg.
Table concentration tests were run on gouge ore that was disintegrated, and the plus 20-mesh fraction was crushed to pass a 20-mesh sieve. Results were not encouraging; only 30 to 67 percent of tire mercury was recovered in concentrates averaging 4.0 percent Hg.
The following conclusions were derived from study of the results of preliminary flotation tests:
- Flotation of undeslimed minus 100-mesh feed resulted in recoveries ranging from 82 to 93 percent, but concentrate grade and resulting ratio of concentration were low. Best products obtained by this method of treatment assayed only 0.756 percent Hg and gave a ratio of concentration of 12 to 1.
- When sand and slime were floated separately, best results were obtained by treatment of sand at 20 to 25 percent solids and treatment of slime, at about 10 percent solids.
- Tabling of sand, flotation tailing in the attempt to obtain additional recovery of slow-floating, carbon-coated cinnabar is unwarranted. In one test, 48.8 percent of the total feed was contained in the sand flotation tailing, which, when tabled, yielded only 1.6 percent of the total mercury at 0.2. percent Hg grade (4.0 pounds per ton).
- Optimum amount of sodium silicate to use as a dispersing agent was established as 3.5 pounds, per ton of ore.
- Arayl and pentasol xanthate were successfully employed as cinnabar collectors. Equal results were obtained by using Diesel oil. However, five times as much Diesel oil was required as xanthate and also more frother was needed.
- Best recoveries were obtained from neutral or slightly acid pulp.
- Owing to the natural floatability of cinnabar in preference to the arsenic and antimony -minerals, as well as the tendency for the latter to pass into the slime tailing, the assay of the final concentrate was low in arsenic and antimony – 0.175 and 0.053 percent, respectively. It was therefore unnecessary to employ any depressant for the arsenic and antimony minerals.
- Mercury recovery was not improved by making low-grade concentrate with lower ratios of concentration. In all cases, contamination was due largely to barren clay, and the mercury losses were not affected by the inclusion or rejection of the clay.
Best results were obtained by using the following treatment procedure:
A sample of ore was- log-washed at 30 percent solids, agitated with 3.5 pounds sodium silicate per ton, and screened on 100-mesh. The oversize was ground to minus 100-mesh. The ore was deslimed by decantation. The sand and slime fractions were floated separately, and the slime flotation concentrate was cleaned once. Results are shown in table 11.
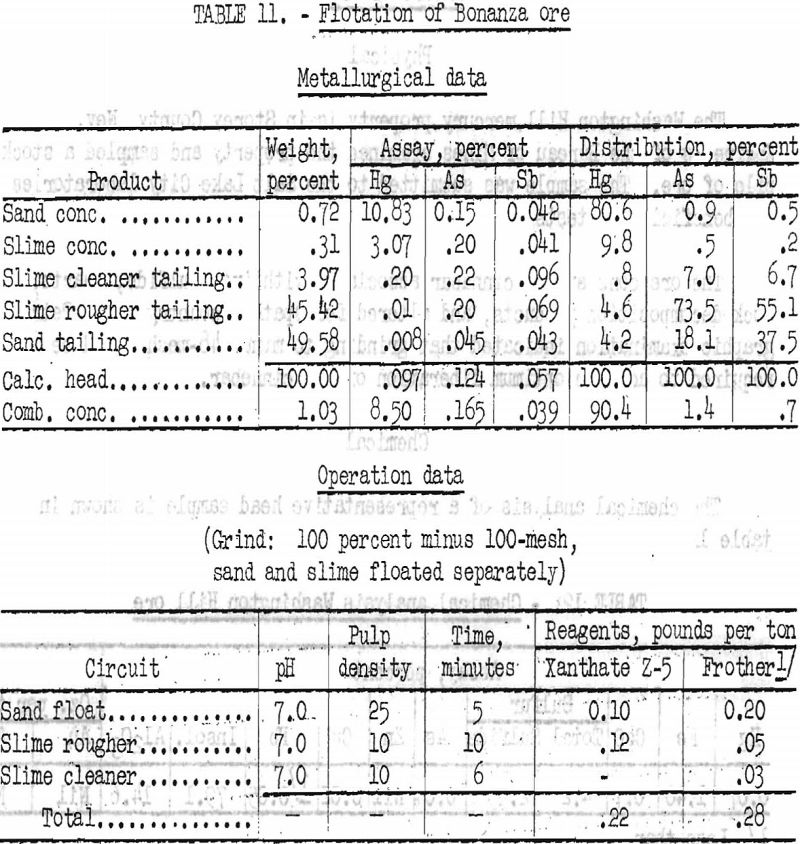
By separate sand and slime flotation, 90.4 percent of the mercury was recovered in a combined concentrate that contained 170 pounds Hg per ton (8.50 percent).
Summary
Laboratory studies indicated that the sticky gouge ore from the Bonanza property could be treated by disintegration, desliming, and direct furnacing of the sand fraction. This treatment would-reject 35 percent of the ore as slime with a loss of only 1.6 percent of the total mercury.
Flotation of mercury from the ore was successful but of doubtful economic value in view of the excellent recovery obtained-by disintegration and direct furnacing of sand. Owing, however, to changing grade of ore or lower economic limit for direct furnacing, flotation of a portion of the ore such as the fine sand fractions and/or slime might be desirable to reduce tonnage furnaced and to increase the grade of furnace feed.
Nature of Ore
Physical
The Washington Hill mercury property is in Storey County, Nev. Engineers of the Bureau of Mines examined, the property and sampled a stock pile of ore. The sample was submitted to the Salt Lake City laboratories for beneficiation tests.
The ore consists of cinnabar associated with iron sulfide, quartz, rock decomposition products, and altered feldapathic country rock. Petrographic examination indicated that grinding to minus 48-mesh would be required to achieve optimum liberation of the cinnebar.
Chemical
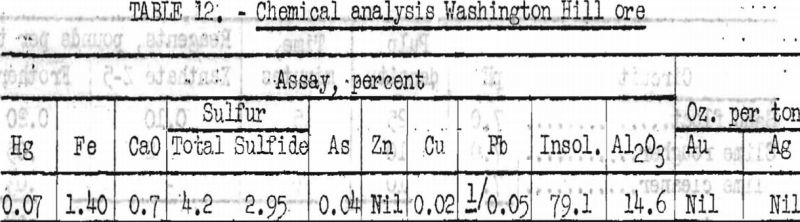
The chemical analysis of a representative head sample is shown in table 12.
Concentration
Desliming of original ore crushed to minus 10-mesh permitted the rejection of 27.6 percent of the weight with, a loss: of only 3.9 percent of the mercury. Subsequent attrition grinding tests, however, were unsuccessful owing to the ready attrition of the cinnabar.
A sample of ore was stage-ground in a laboratory ball mill to minus 35-mesh and sized on 48-, 65-, 100-, and 200-mesh sieves. Each sized fraction was tabled separately to produce a concentrate, middling, and tailing. The middling from, each sized fraction was reground to pass the next sieve in the series and redistributed into the proper fractions before the finer sizes were tabled.
The table slime product was treated by flotation with 2.5 pounds sodium silicate, 0.1 pound pentasol xanthate, and 0.06 pound cresylic-alcohol frother per ton of slime. Metallurgical results are shown in table 13.
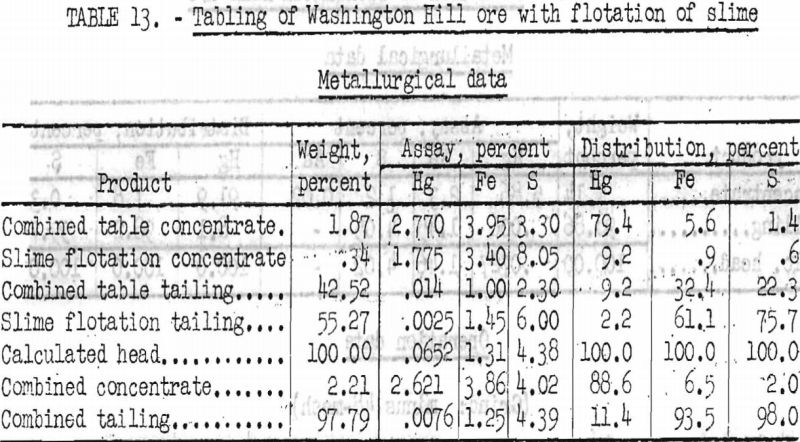
As shown in table 13, by tabling sized fractions of minus 35-mesh ore and flotation of slimes, 88.6 percent of the mercury was recovered at a grade of 52.4 pounds Hg per ton (2.62 percent). This.treatment rejected 93.5 percent of the iron and 98.0 percent of the total sulfur.
Several flotation tests were conducted. Best results were obtained from ore stage-ground to minus 48-mesh in a laboratory rod mill. Pentasol xanthate was employed as collector. The use of mercuric chloride as an activator for cinnabar appeared to give higher-grade concentrates at the expense of recovery. Data and results obtained from two typical tests are given in tables 14 and 15.
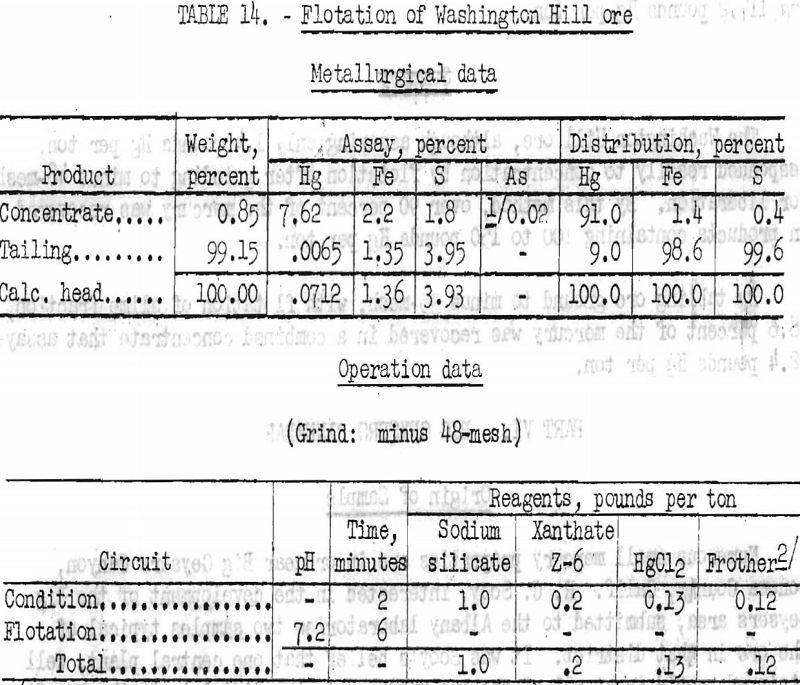
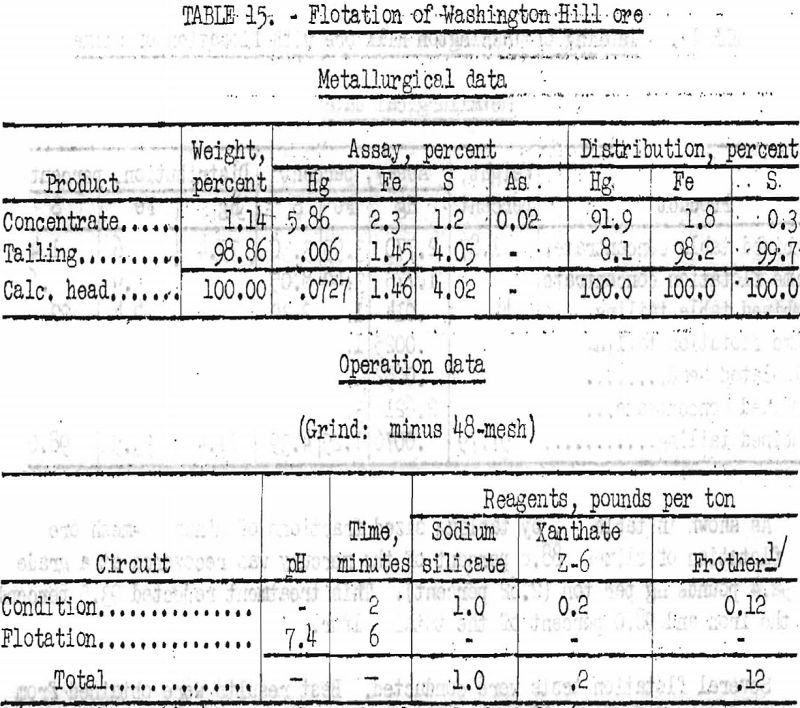
By flotation of original ore, stage-ground in a rod mill to minus 48-mesh, 91 percent of the mercury was recovered in a concentrate assaying 152.4 pounds Hg per ton; a similar test gave a recovery of 91.9 percent in a product assaying 117.2 pounds Hg per ton.
Summary
The Washington Hill ore, although assaying only; 1.4 pounds-Hg per ton, responded readily to concentration by flotation after grinding to minus 48-mesh for liberation. By this method, over 90 percent of the mercury was recovered in products containing 100 to 150 pounds Hg per ton.
By tabling ore-ground to minus 35-mesh, with flotation of slime fraction, 88.6 percent of the mercury was recovered in a combined concentrate that assayed 52.4 pounds Hg per ton.
Origin of Sample
Numerous small mercury properties are in or near Big Geysers Canyon, Sonoma County, Calif. R. C. Sooy interested in the development of the Big Geysers area, submitted to the Albany laboratories two samples typical of the ore in that district. It was Sooy’s belief that one central plant, well equipped, could treat all of the ore produced in the district, to the benefit of the various owners. He was particularly interested in the amenability of Big Geysers ore to concentration by sink-float methods.
The two samples received, were composited for laboratory testing.
Nature of Ore
Physical
The Big Geysers ore consisted of small blebs of cinnabar in a friable, loosely consolidated, argillaceous sandstone. The rock was streaked with limonitic stain, but no valuable mineral other than cinnabar was identified.
A portion of the cinnabar particles was 10- to 20-mesh in size, but minus 65-mesh grinding was necessary to effect optimum liberation.
Chemical
The sample as received, was crushed to minus 1-inch, and a 50-pound sample was cut out. The 50-pound sample was further reduced in rolls to minus 10-mesh, and a pulp-size sample was cut and prepared for analysis; the reject was retained for flotation tests.
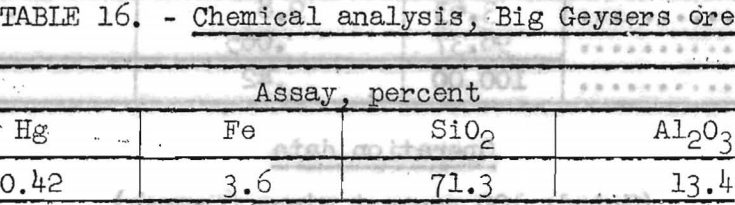
The chemical analysis of the head sample is shown in table 16.
Concentration
Dense Medium
Sink-float testing was requested specifically and was run even though examination of the ore indicated that poor results could be expected.
A sample of ore crushed to minus 1-inch was screened wet on a 9-mesh sieve to remove the fine material. The oversize was treated by sink-float methods at a medium specific gravity of 2.4. Preliminary testing showed that the entire sample would sink in a medium having a specific gravity of 2.2, whereas at a specific gravity of 2.55 the entire sample would float.
Results obtained by this method of concentration are shown in table 17.
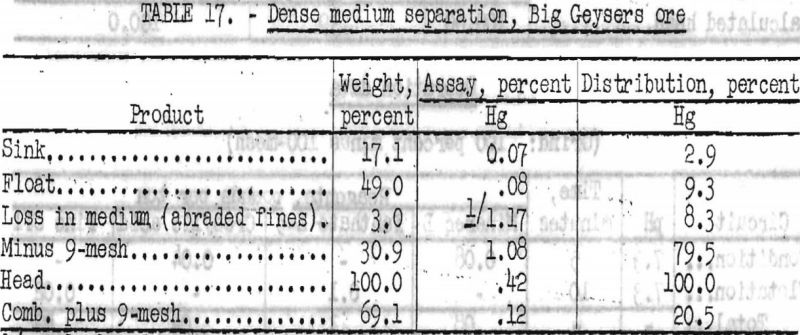
Crushing to minus 1-inch and sizing wet on a 9-mesh screen effected concentration of mercury. The undersize fraction represented only 30.9 percent of the original weight of ore, contained 79.5 percent of the total mercury, and assayed 1.08 percent Hg or 21.6 pounds Hg per ton. No concentration was made by sink-float treatment of the low-grade oversize.
Flotation
Preliminary flotation tests on the Big Geysers ore indicated that grinding to at least 65-mesh was necessary to effect liberation of the cinnabar.
Best results obtained by flotation treatment of this ore are shown in tables 18 and 19, together with the operation data for each test.
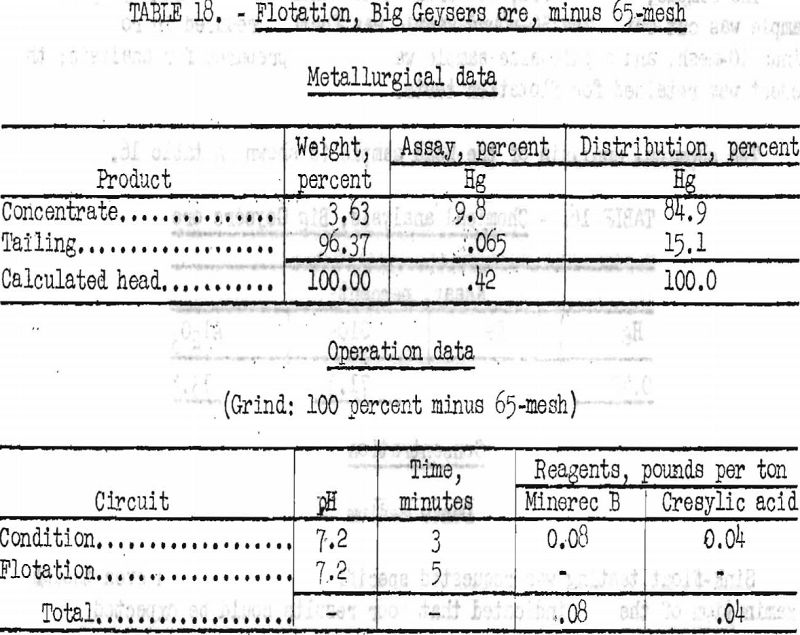
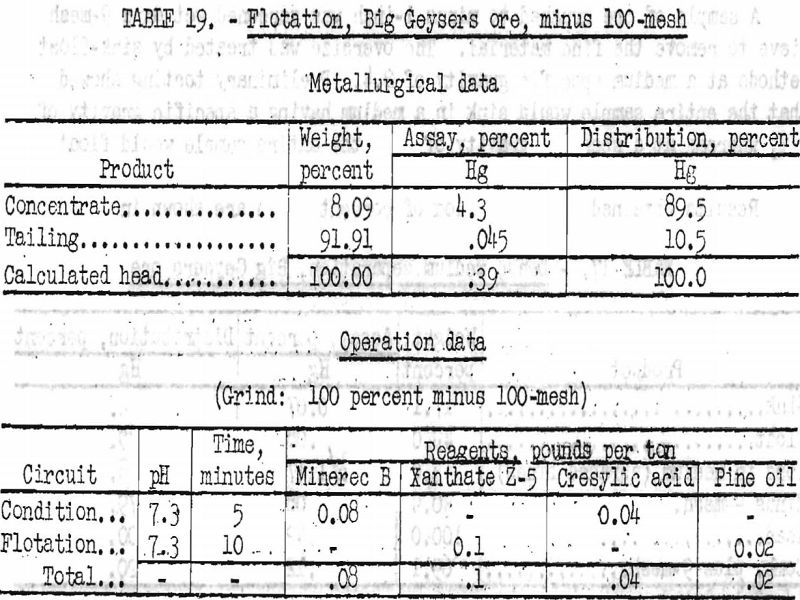
By flotation, 85 to 89 percent of the mercury was recovered in concentrates ranging from 4.3 to 9.8 percent Hg. Higher grades were obtained in some tests, but only at the expense of recovery.
Summary
The cinnabar ore from Big Geysers Canyon, Calif., was fairly amenable to concentration by flotation. Up to 89 percent of the total mercury was recovered in concentrates assaying 86 pounds Hg per ton and higher. Although at least minus 65-mesh grinding was required for liberation, the ore was very friable, and crushing was both easy and rapid.
The minus 9-mesh fraction of ore crushed to minus 1-inch and wet-screened contained 79.5 percent of the mercury at 1.08 percent Hg grade.
Dense medium separation of the ore gave no satisfactory results.
Conclusions
- The War Eagle ore was found to be amenable to direct distillation with addition of lime to the retort to minimize corrosive action. Flotation treatment of this sample was not successful.
- The occurrence of cinnabar as smears on the gangue material of the low-grade Rattle Snake ore prevented the production of mercury concentrates with high recoveries by any mechanical method.
- Flotation of ore from the Donaldson property was fairly successful in spite of the extremely low grade (0.03 percent Hg). Over 78 percent of the mercury in the ore was recovered in a concentrate containing 16.1 pounds Hg per ton.
- Mercury can be recovered from the sticky gouge portion of the Bonanza ore by disintegration, desliming, and direct furnacing of the sand fractions. Laboratory studies indicated that the ore is amenable to flotation treatment and that flotation of fine sand and slime possibly could be used as a supplement to distillation treatment of the bulk of the ore.
- The low-grade Washington Hill ore (0.07 percent Hg) was readily amenable to concentration by ore dressing. By flotation, over 90 percent of the mercury was recovered in concentrates containing 100 to 150 pounds Hg per ton. Tabling of sand and flotation of slime recovered 88 percent of the mercury at a grade of 52 pounds Hg per ton.
- The ore from Big Geysers Canyon, Calif., was fairly amenable to concentration by flotation. A concentrate containing 86 pounds Hg per ton was made with a mercury recovery of 89 percent. Crushing ore to minus 1-inch and sizing on 9-mesh produced an undersize fraction that contained 30.9 percent of the total weight and 79.5 percent of the mercury at a grade of 21.6 pounds per ton.
