Table of Contents
Advances in hydrometallurgy during recent years resulted in greater attention being focused on the various unit operations involved. Some steps, similar to normal beneficiation practice—crushing, grinding, classification, etc. are well known.Others —roasting, leaching—have recently been intensively investigated from both theoretical and practical viewpoints. Less well-studied are such operations as precipitation and precipitate filtration.
Continuous Filters Suitable for Precipitates
The drum filter, or its variations, is the most widely used continuous filter for precipitates. The advantages of drum filters are the following:
- They are suitable for washing the filter cake.
- Changes in the physical characteristics of the precipitate cause less difficulty on drum filters than on other types of machines.
- Drum filters are adaptable to different methods of cake discharge, each with its own advantages, as described below:
String filters are not suitable where the cake cracks excessively, preventing the strings from lifting the cake off as a solid mass. Blinding of the filter medium is more common, as the air blow used on a standard drum filter helps maintain the cloth in a more permeable state by dislodging accumulated fines. Hence, thin, relatively permeable media are required, resulting in poorer clarity.
Tacky cakes, particularly gelatinous precipitates, are adaptable to removal by roller discharge. This device consists of a roller driven in an opposite direction of rotation and synchronized with the speed of the filter. The roller contacts the filter cake, and because of the greater-tendency of the cake to adhere to the roll it is removed by it, and then discharged from the roll by means of a scraper. With very tacky materials, the roll is usually precoated with as much as an inch of filter cake solids. The scraper is retracted accordingly, so as to trim off only the newly picked up filter cake. Less adhesive, more granular mate¬rials are better removed by employing no filter cake layer on the roll, and in these cases the use of an air blow sufficient to inflate the cloth against the roll is often desirable.

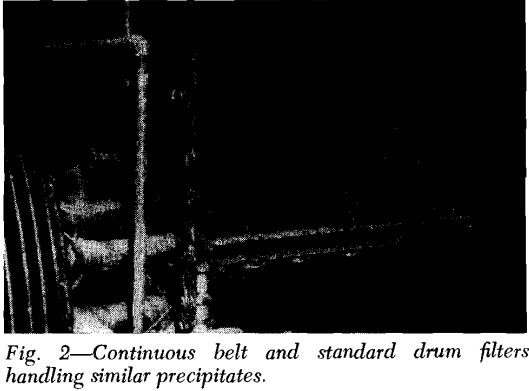
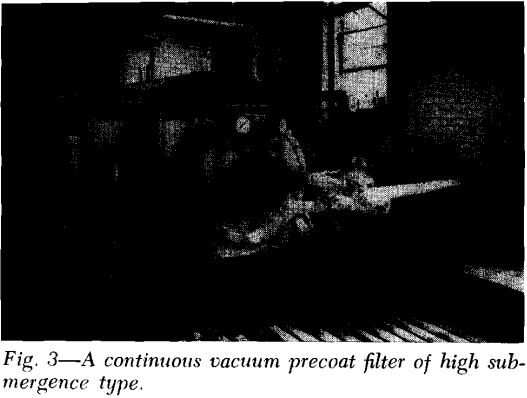
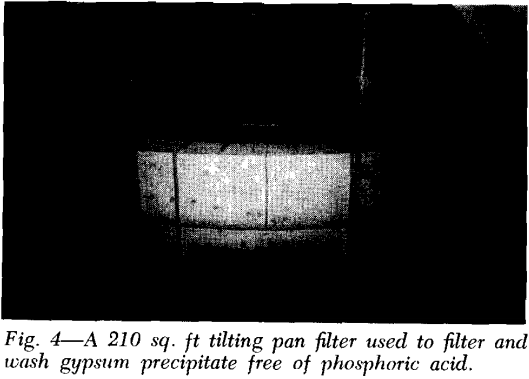
Precoat Drum Filters: This unit employs a bed of precoat—usually diatomaceous earth—initially at a thickness of up to 3 inches, to serve as the filter medium and retain the solids on its surface. A slowly advancing knife simultaneously trims off the filtered solids plus a thin (0.0005 to 0.01 in.) layer of precoat as the drum revolves.
A precoat filter should be avoided where crystallization can cause blinding of the bed, or where the solution would attack the precoat material. If the filter cake cracks badly, then it is apt to cause excess consumption of precoat because of a tendency of the cracked portions of cake to curl up, sometimes lifting about 1/32 in. of precoat material at the same time.
Filter Media Selection
Selection of the best cloth for a particular process from the hundreds of different cloths now available is confusing, to say the least. In general the synthetic fabrics will give better service on precipitates than the natural fibers, primarily because of their better discharge characteristics and lesser tendency towards blinding. Synthetic materials are usually multi or monofilament, as opposed to the spun staple natural fabrics, and hence are more resistant to penetration of the yarn strands by solids.
- Fine, slimy precipitates are best handled on thin, tight fabrics of twill or satin weave. Cloths weighing less than 5 oz per sq yd, with an average thread count of about 150 x 100, and an air permeability of less than 10 cfm per sq ft at 0.5 in. water gage, are in this class.
- Slightly coarser materials, having some particles approaching 50 micron size, will be handled well by thin, plain weave fabrics, some monofilament media, and in some instances, cotton fabrics of varying weight, but preferably of twill weaves.
- Precipitates containing a wide range of particle sizes, including slimes, are best filtered on monofilament cloths. The solids settle rapidly, and if the medium blinds due to the slimy material, the flow rate through the medium will be too low to pick-up the coarser material.
- Coarse, granular precipitates are best handled on screens, monofilament cloths, or, where clarity is important, on light weight, medium porosity, multifilament fabrics.
Precipitation Methods
The reasons for considering precipitation as an important step in any process are that this operation will have substantial bearing on the type, size, and cost of the equipment to be used to recover the precipitate, it will affect the purity of the product, and it will determine to some extent the degree of recovery (or loss) of the valuable product.
Analytical chemistry techniques for preparing easily filtered precipitates call for addition of precipitating reagents slowly, to boiling, agitated, dilute solutions, followed generally by a period of continued boiling or aging for completeness of precipitation and formation of large size crystals.
In plant practice it is rare indeed when all of these criteria can be met. However, it will generally be true that if the precipitating reagent is rapidly dispersed (but without high shear agitation that would result in the mechanical degradation of the crystals) in the solution, either dilute or concentrated, and a suitable aging time allowed, good results can be obtained.
With the magnesium oxide precipitation of uranium, the slow dissolution of the oxide causes gradual neutralization of the acid, promoting the precipitation at a higher pH of the uranium as a diuranate salt of any convenient ion present, usually sodium. Thus the precipitating reagent does not even enter into combination with the precipitate but simply controls the rate of precipitation, promoting good crystal growth.
The precipitation of gypsum, CaSO4·2H2O, from acidic solutions using hydrated lime or limestone is commonly found in many flow sheets. Plant practice has shown that the following steps are important: The reagent should be added as a slurry to insure complete reaction. Particle sizes above 100 mesh will usually not enter into the reaction sufficiently rapidly to be effective.
