Table of Contents
The first large-scale leaching and precipitation of copper was probably at Rio Tinto in Spain about 1752. The method employed comprised open-air water leaching of weathered piles of copper-bearing ore followed by precipitation of the copper by iron. A description of leaching and precipitation of copper at Rio Tinto in the 20th century is given by Taylor and Whelan. This method is essentially the same as that practiced at present by most of the copper-leaching companies in the Western United States, Nearly 12 percent of the total copper production in the Western States in 1965 was obtained from the precipitation of copper from leach liquors by metallic scrap iron. Almost all of the cement copper produced was shipped to smelters and used as feed in reverberatory furnaces.
The purpose of this report is to describe and discuss the different leaching and recovery methods currently employed in the production of copper from low-grade ores in the Western States. The data included in this report were obtained from a literature search and from visits to most of the larger leaching operations in the West. Topics discussed include the physical and mineralogical types of material being leached, the physical nature of dumps and heaps, the emplacement of dumps, methods of introduction of leach solutions, the nature of the leach solutions and the copper-bearing liquors, and methods of recovery of copper from solution.
Mineralogy of Raw Materials
Copper in the Western United States is produced from low-grade ore bodies found predominately in igneous host rocks, chiefly quartz monzonite, quartz porphyry, monzonite porphyry, granite porphyry, and quartz diorite. Less important deposits are found in dacite porphyry, rhyolitic flows, conglomerate, diabase, limestone, sandstone, and schist. Most of the open-pit deposits encompass facies of different mineral composition and alteration.
The principal copper sulfide minerals in the host rocks are chalcopyrite and chalcocite. Minor amounts of bornite, covellite, and enargite are present. The principal copper oxide minerals are azurite, chrysocolla, and malachite. Varying amounts of cuprite, native copper, and tenorite also are present.
Chemical Reactions
Oxidized copper ores, mixed oxide-sulfide ores, and those sulfide ores too low in grade to concentrate by froth flotation are treated by various copper leaching methods. The principal copper oxide minerals dissolved by leaching are azurite, chrysocolla, and malachite, although varying amounts of cuprite, tenorite, and native copper also are known to be contained in some ores and are simultaneously dissolved. The copper sulfide minerals prevalent in many sulfide ores and dissolved during leaching are chalcocite, chalcopyrite, and covellite. Pyrite is an important mineral found in many sulfide ores and is oxidized during leaching to form ferrous sulfate and sulfuric acid.
Reactions by which specific copper minerals are dissolved in leaching, either with sulfuric acid or sulfuric acid plus ferric iron were given by Van Arsdale:
Azurite
Cu3(OH)2·(CO3)2 + 3H2SO4 ↔ 3CuSO4 + 2CO2 + 4H2O
Malachite
Cu2(OH)2·CO3 + 2H2SO4 ↔ 2CuSO4 + CO2 + 3H2O
Chrysocolla
CuSiO3·2H2O + H2SO4 ↔ CuSO4 + SiO2 + 3H2O
Cuprite
Cu2O + H2SO4 ↔ CuSO4 + Cu + H2O
Cu2O + H2SO4 + Fe2(SO4)3 ↔ 2CuSO4 + H2O + 2FeSO4
Native Copper
Cu + Fe2(SO4)3 ↔ CuSO4 + 2FeSO4
Tenorite
CuO + H2SO4 ↔ CuSO4 + H2O
3CuO + Fe2(SO4)3 + 3H2O ↔ 3CuSO4 + 2Fe(OH)3
4CuO + 4FeSO4 + 6H2O + O2 ↔ 4CuSO4 + 4Fe(OH)3
Chalcocite
Cu2S + Fe2(SO4)3 ↔ CuS + CuSO4 + 2FeSO4
Cu2S + 2Fe2(SO4)3 ↔ 2CuSO4 + 4FeSO4 + S
Covellite
CuS + Fe2(SO4)3 ↔ CuSO4 + 2FeSO4 + S
Chalcopyrite will slowly dissolve in acid ferric sulfate solutions and also will oxidize according to the following reactions:
CuFeS2 + 2O2 ↔ CuS + FeSO4;
CuS + 2O2 ↔ CuSO4.
Pyrite, a prevalent mineral found in many ore deposits, oxidizes according to the following reaction:
2FeS2 + 2H2O + 7O2 ↔ 2FeSO4 + 2H2SO4.
The preceding are only a few of the many and complex chemical reactions that take place within leach dumps.
Studies relative to leaching various copper minerals have been made. Patents concerned with the recovery of copper and other metals from ores by chemical means have been issued. Results of these investigations show that several copper sulfide minerals are soluble to some extent in sulfuric acid solutions containing ferric sulfate and that many copper oxide minerals are soluble in ammoniacal solutions. This information has served as the basis for the current leaching technology.
Other metallic minerals and many products of alteration of the host rocks are found associated with the copper minerals. Noncarbonate gangue is usually unaffected by leach solutions but metals other than copper; for example, aluminum, uranium, and zinc are found in the pregnant liquors from leaching. Their presence is the result of the partial or complete dissolution of the other metallic minerals and the alteration products. The present study is concerned principally with leaching and recovery of copper; however, the occurrence of these additional metallic elements in the pregnant liquors indicates that appreciable quantities of these metals also might be recovered.
Methods of Leaching
The principal methods of leaching used at present are dump, heap, in-place, and vat. These methods are interrelated, and many items considered necessary for effective leaching by one method are also applicable to the other methods.
Choice of leaching method depends upon the chemical and physical characteristics of the specific material to be treated. The grade of the ore, the solubility of the copper minerals, the amount of acid-consuming associated gangue material, the size of the operation, and the mode of occurrence of the copper-bearing minerals are some of the important factors to be considered.
Dump leaching is used to extract copper from waste material produced during the large-scale open-pit mining of copper ore deposits. Almost all of the copper oxide and sulfide minerals encountered in such deposits are leachable by this method, since the leach cycle is measured in years.
Heap leaching is used primarily to extract copper from uncrushed porous oxidized copper ore which has been piled on prepared drainage pads. The cop¬per oxide minerals in the ore material are readily soluble in sulfuric acid solutions, and the leach cycle for this method is measured in months.
Inplace leaching involves the leaching of broken ore in the ground as it occurs. Current inplace leaching employs the dissolution of copper minerals contained in underground mines that previously utilized block-caving mining methods. Copper minerals leachable by inplace leaching are the same as those leachable by dump leaching, again since the leaching cycle is measured in years.
Vat leaching principally employs the dissolution of copper oxide minerals by sulfuric acid from crushed, nonporous ore material that has been placed in confined tanks. This method is used when rapid extraction of copper is desired, since the leach cycle is measured in days.
Brief descriptions and illustrations of dump, heap, inplace, and vat leaching operations are given in the following sections.
Dump Leaching
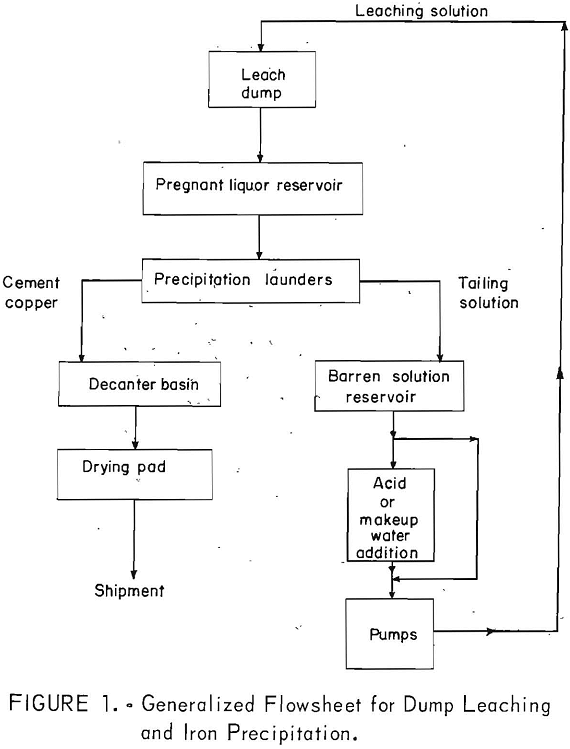
Dump leaching is used for low-grade and waste material stripped from open- pit operations. Run-of-mine ore materials containing copper in amounts less than the cutoff grade (usually 0.4 percent copper) deemed necessary for the profitable recovery by the usual mining and concentrating methods are leached by this method. A description of the technology of leaching waste dumps has been given by Malouf and Prater. Figure 1 presents a generalized flowsheet for dump leaching and iron precipitation.
Emplacement of Dumps
Ground Preparation
Most leach dumps are deposited upon the existing topography. The locations of the dumps are selected to assure impermeable surfaces and to utilize the natural slope of ridges and valleys for the recovery and collection of the pregnant liquors (fig. 2). Examples of this method of dump emplacement are found at numerous leaching operations in Arizona, Nevada, New Mexico, and Utah (figs. 3, 4, 7).
In several instances leach dumps have been deposited on specially prepared areas. Ground preparation for dump emplacement is represented by the following specific examples.
The original surface in the dump area of The Anaconda Company, Butte, Mont, is composed of 5 to 80 feet of alluvium which has been deposited on quartz monzonite. Because solution loss through the alluvial material would have been substantial, an impervious pad (fig. 6) to prevent solution loss was constructed in steps as follows: (1) The vegetation was removed by bulldozers; (2) the scraped surface was graded and then compacted using sheepsfoot and vibratory rollers; (3) a 4-inch layer of minus 1-½-inch slag was, placed on, the surface and compacted with a vibratory roller; (4) a coating of asphalt primer was placed above the slag; (5) a compacted 3-¼-inch layer of asphalt (blacktop) was placed on top of the primed surface of the slag; (6) a 1/8-inch layer of asphaltic sealant then was blown onto





the surface of the blacktop; (7) a 12-inch minimum thickness of fine pit-run material was placed on the asphalt mat to protect the surface from rupture by heavy truck travel; and (8) a 5- to 6-foot thickness of coarse pit-run material was placed on the fine material to provide protection against damage of the pad by boulders cascading down the slope of the high dump. Recovery of leaching solutions from the dumps constructed on this impervious pad has been very high.
The leach dumps at the Weed Heights, Nev., operation of The Anaconda Company were placed on a dry lakebed after the lakebed had been leveled by bulldozer and compacted by sheepsfoot rollers.
Method of Dump Emplacement
Leaeh material, in most operations, is hauled from the open-pit mines to the dumps by trucks or trains. Bulldozers are used to level the surfaces and edges of the dumps. Large dumps usually are raised in lifts of 50 to 100 feet (fig. 5), in a manner to keep haulage minimal with respect to both grade of ascent and distance of haul and to form a porous dump favorable for percolation of leach solutions.

Physical Nature of Dumps
Size of Leach Material
The majority of the material currently placed on leach dumps is run-of-mine material that has been loaded by power shovels as part of the normal mining operation. The leach material therefore includes boulders as large as several feet in diameter and weighing many tons; however, most of the material is less than 2 feet in diameter and includes many fine particle sizes. Leach material being placed on dumps at present generally is smaller in size than that placed on dumps in the past. The reduction in size is chiefly the result of improved blasting techniques which provide better rock fragmentation.
Geometry and Volume of Dumps
The shape of most leach dumps is that of a truncated cone. This configuration is developed by the method of dump emplacement used in most operations. The leach material forms a slope having an angle of repose peculiar to the type and size of material.
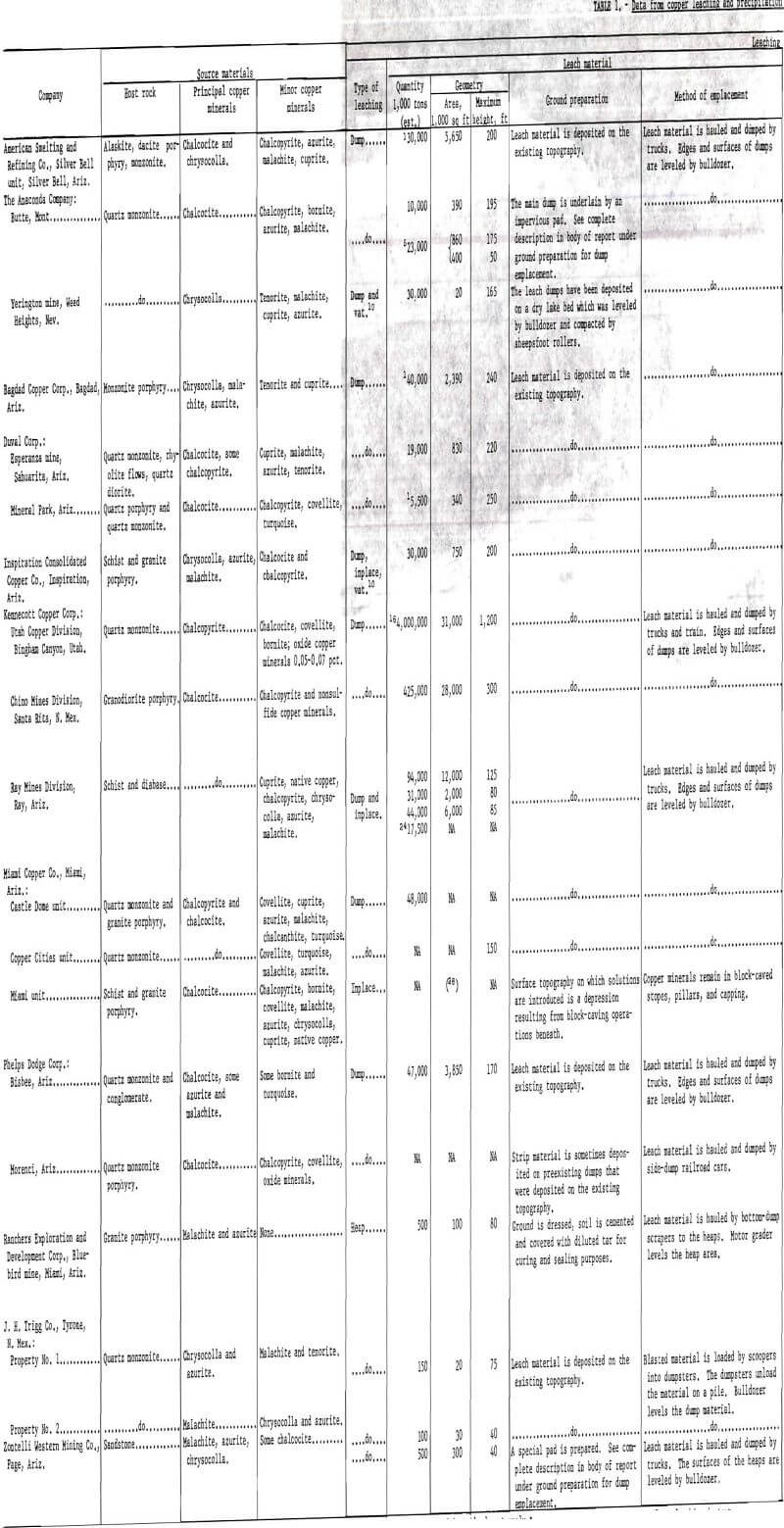
The tonnage of the major leach dumps in the Western States varies approximately from 5.5 million tons at the Mineral Park, Ariz., operation of the Duval Corp., to 4 billion tons at the Bingham Canyon, Utah, operation of Kennecott Copper Corp. More complete figures on the tonnage and geometry of leach dumps together with other pertinent data concerning leaching and precipitation are given in table 1.
Settling of Dumps
Most leach dumps settle after the leach solutions have been introduced because (1) the percolating solutions tend to transport the finer particles to the voids between larger particles and thereby compact the dump as a whole, (2) the total weight of the dump is increased by the weight of the solutions which causes a downward compression of the dump, and (3) the dissolution and disintegration of minerals in the dump causes the dump to settle.
Leaching Operation
The leaching operation comprises the introduction of a sulfuric acid solution containing varying amounts of ferrous and ferric sulfate onto the surface or into the interior of the dumps for the purpose of effecting the dissolution of copper minerals. The end product of the leaching operation is a pregnant liquor containing copper in the form of copper sulfate.
Leach Solution
Most companies recirculate the tailing solutions from their precipitation operations (fig. 8) along with makeup water and concentrated sulfuric acid to be used as the leach solution. Analyses of tailing solutions from various operations are given in table 1. Makeup water is added to replenish the water lost by evaporation or seepage during leaching. Sulfuric acid is added to lower the pH of the leach solution generally to the range of 1.5 to 3.0. The reasons for maintaining this pH are (1) to have adequate acid for dissolution of the copper minerals, (2) to prevent the destruction of bacteria in the dumps, and (3) to minimize the hydrolyzation and precipitation of iron and other metals in pipelines and on the surfaces or in the interior of the dumps.
Water added to the leach solution comes from several sources, such as wells, springs, underground mines, or streams. Polluted stream waters from nearby towns are sometimes used; however, the organic material contained in sewage waters is thought to be a deterrent to bacterial activity and therefore is not used in many leach circuits.
The sulfuric acid used in most of the larger leaching operations is produced and supplied by the operating company. In smaller operations the acid is obtained from commercial suppliers. The development of contact sulfuric acid plants coupled with a constant supply of high-grade sulfur has simplified the manufacture of sulfuric acid. A typical example of a contact sulfuric acid plant is the Bagdad Copper Corp. facility, Bagdad, Ariz. (fig. 9), located adjacent to the company’s precipitation plant. A thorough description of this semi automatically controlled acid plant has been given by Bogart.
Methods of Solution Introduction
Leach solutions are introduced onto or into dumps by means of spraying, flooding, or vertical pipes. The method chosen for a given operation is based on careful consideration and knowledge of climatic conditions, dump height,

surface area, mineralogy, scale of operation, and size of leach material. Brief descriptions of each method, with examples, are presented in paragraphs that follow.
Spraying.-Leach solutions at the different operations are introduced onto the dumps by various methods of spraying. The advantage of spraying is that it provides a uniform distribution of the leach solutions over the surface area of the dumps. The principal disadvantage of this method is the evaporation loss which in arid regions may range up to 60 percent.
The commonest spraying method involves the use of perforated plastic pipes. The Inspiration Consolidated Copper Co., Inspiration, Ariz. (fig. 10), and The Anaconda Company at Weed Heights, Nev. (fig. 11), use this method. The solutions are pumped to the surface of the dumps through 8- to 12-inch cement-asbestos, plastic-lined pipelines and are carried from the main distributing lines in 2- to 4-inch perforated plastic pipes. The pressure created in the pipelines causes the leach solutions to be discharged through the perforations in the form of a spray. Height and distance of coverage of the sprays are functions of the pipeline pressures.

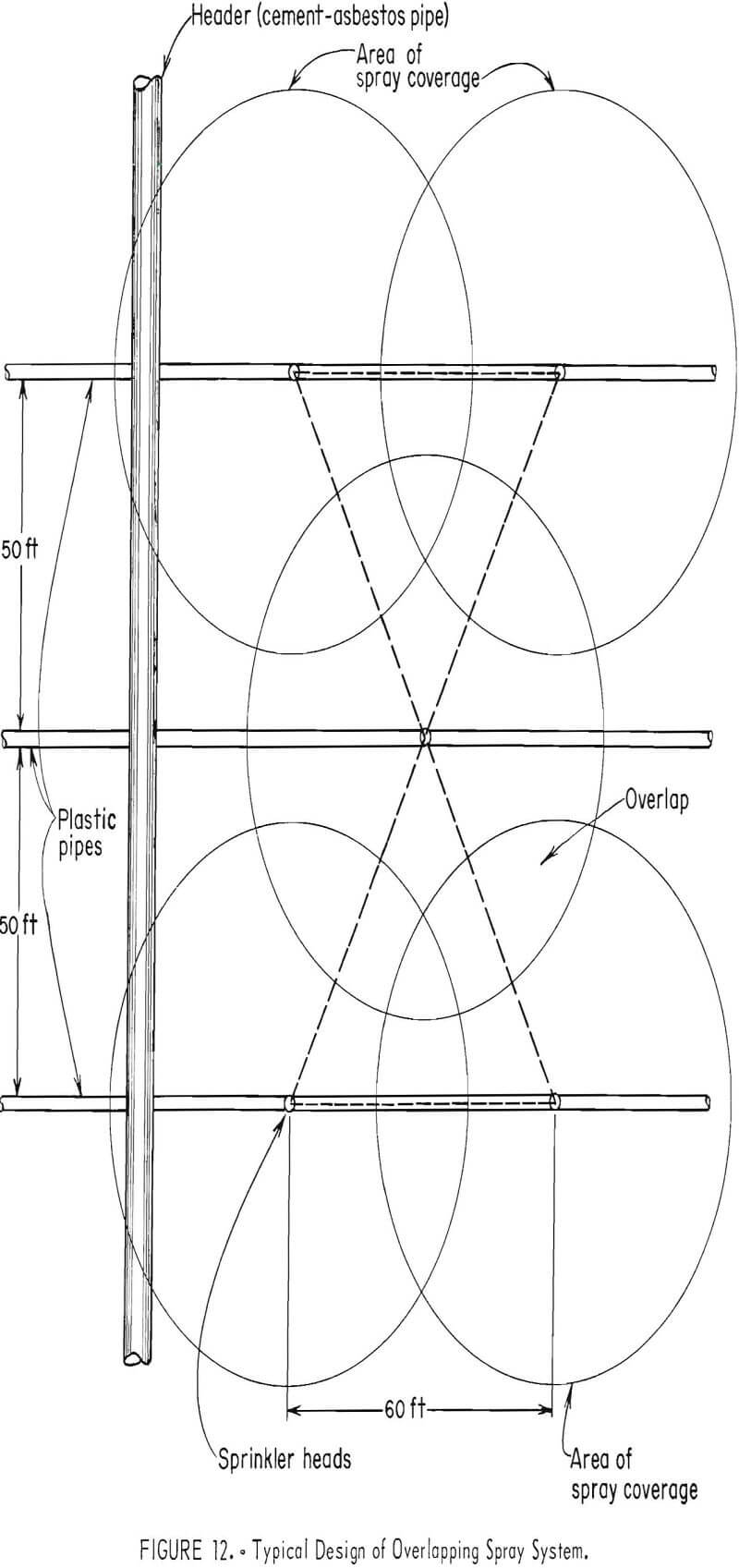
Metal or plastic sprinkler heads available commercially are used at the Esperanza operation of Duval Corp., Sahuarita, Ariz., to distribute the leach solutions in an overlapping circular pattern (fig. 12). The size of the water droplets determines the amount of evaporation to be expected. The larger the droplets in the spray, the less the amount of evaporation.
Bagdad Copper Corp. has experimented with and is using a form of sprinkler head made of surgical tubing. The tubings are attached to reducers at the end of the plastic pipes that carry the leach solution from the main distributing lines to the surface of the dump. The tubing is very pliable and reacts vigorously to the solution flow. A nominal length (about 18 inches) is used to impart a random oscillating motion to the tubing whereby a circular pattern of spray coverage is created (fig. 13).
Spraying methods also are used to distribute leach solutions over the slope portions of dumps that are being leached by flooding methods. Lengths of small-diameter plastic pipe are placed on the slope area from the top to the base of the dumps. Proper coverage of the slope area is extremely difficult because the solutions tend to form channels down the slope and rapidly flow from the slopes without penetrating the surface. Large tonnages of material in the slope area therefore are not contacted by the leach solutions.

Flooding.-Leach solutions are introduced onto the surfaces of dumps by flooding or ponding by forming furrows, ditches, ponds, or channels on the surfaces of the dumps by bulldozer or front-end loaders. The leach solutions are channeled into these conduits and allowed to build up to a certain depth in order to create a substantial flow of solution onto the dumps. Several companies use this method of solution introduction; for example, the Silver Bell, Ariz., operation of American Smelting and Refining Co. (fig. 14). Selected areas of the dump surface are saturated for a specified period, followed by a period with no solution addition. In actual practice, a section of a dump is flooded with leach solution until such time as the copper content of the pregnant liquor obtained from that section falls below a predetermined percentage. The flooding then is discontinued on that section and begun on another. Hudson and Van Arsdale and Van Arsdale state that alternating periods of wetting and drying of leach dumps produces the most efficient dissolution and removal of copper from the leach material.
Benches or furrows made by bulldozers on the slopes of the leach dumps are used in conjunction with sprays at the Castle Dome operation of Miami Copper Co., Miami, Ariz. The leach solution is ponded in the furrows and allowed to percolate through the slope area in an attempt to increase distribution throughout the slope area.

Vertical Pipes.-The newest method of solution introduction is that practiced at the Butte, Mont., operation of The Anaconda Company. The leach solution is introduced through perforated plastic pipes into the interior of the dumps (fig. 15). Six-inch churn-drill holes are drilled at the intersections of a 100-foot grid pattern and cased to a depth of about two-thirds the dump height. A 4-inch perforated plastic pipe is then placed inside the casing over the entire depth of each hole. The casing is then removed. The spacing of the churn-drill holes has varied from a 100-foot grid pattern to a 50- by 25-foot pattern. Leach solution flows to the dump area from solution reservoirs at a higher elevation. Ditches are provided along the grid lines to permit the solution to flow into the top of the plastic pipes and down the drillholes. The method promotes a thorough distribution of the leach solution and air within the dumps.
The slope areas of the high dump at the Butte operation are also wetted with leach solutions through cased churn-drill holes that have been drilled on side-slope roads constructed by bulldozers.
Distribution of Solutions in Dumps
One of the most important requirements in all dump leaching operations is the thorough distribution of the leach solutions throughout the dumps. To attain satisfactory recovery of the copper, the leach solutions must contact

as much of the copper-bearing material as possible. An ideal distribution will fill all existing voids within the dump. Numerous problems inherent in most leaching operations, however, prevent attaining ideal distribution.
A natural sorting of coarse and fine material is created in most dumps, because many of the larger particles roll down the dump slopes as the leach material is unloaded from trucks or trains. Bulldozer leveling of many dumps creates layering while the leach dumps are constructed. The overall effect is to produce alternating layers of coarse and fine material within the dumps. The leach solutions follow courses of least resistance in penetrating the dumps. Hence, when the dumps contain layers of fine and coarse material, the solutions may travel horizontally within the dumps and discharge from the sides instead of from the base of the dumps. The problem of complete distribution of leach solutions within the dumps remains unsolved.
Temperature of Leach Solutions
The temperature of the leach solutions is an important factor that affects the rate of dissolution of copper from leach dumps. Temperatures of leach solutions introduced onto dumps varies from a low of 38° F during January and February at the Page operation of Zontelli Western Mining Co. to a high of 90° to 95° F during the summer at the Bingham Canyon operation of Kennecott Copper Corp. and the Bisbee, Ariz., operation of Phelps Dodge Corp.

Temperature is one of the most important environmental factors in a microbial reaction. Corrick and Sutton have shown that the optimum temperature for maximum microbial oxidation of pyrite is 35° C (95° F), and Bryner, Walker, and Palmer also have shown that 35° C is the optimum temperature for the biological oxidation of copper sulfide.
Influence of Bacteria
The role played by micro-organisms in the formation and alteration of mineral deposits is presented by Kuznetsov and coworkers. The importance of certain iron oxidizing bacteria during cyclic leaching processes has been described by Zimmerley and coworkers. The use of bacteria in leaching copper sulfide and other metallic sulfides has been investigated and described. The presence of certain bacteria in the leach solutions or within the dumps promotes and accelerates oxidation of pyrite to ferric sulfate and sulfuric acid and the oxidation of copper sulfide minerals to copper sulfate. Experiments by many of the aforementioned investigators have shown that oxidation rates are accelerated by as much as 2,000 to 1. Factors mentioned by the investigators as important controls in bacterial leaching are shielding from light, thorough aeration of bacterial solutions, and high rates of solution circulation.

Control of Iron Salts in Solution
Probably the most troublesome problem encountered in the leaching operation is precipitation of iron salts in pipelines, on the surface of dumps (fig. 16), or within the dumps. These salts precipitate from solution when the pH of the solution is higher than 3.0. A pH of 2.4 is required to prevent the formation of iron salts and a pH of 2.1 is required to redissolve the salts after they have been precipitated. Strict control of iron content and pH is necessary to minimize the precipitation of the iron salts.
Pipelines constricted by precipitated iron salts are cleaned with mechanical devices such as balls and chains (“pigs”) or cutting tools. Precipitated iron salts which have plugged the surfaces of dumps periodically are furrowed or scraped and pushed over the edge of the dumps. At the Silver Bell unit of American Smelting and Refining Co., however, the iron precipitates (fig. 17) are not removed from the surfaces of the ponds. During the “drying-out”


period, the iron precipitates dry and crack and present only slight resistance to solution flow at the time the next flooding period is initiated.
Iron salt precipitation within the dumps is extremely difficult to overcome. The normal result of iron salt precipitation here is the formation of impervious layers which prevent movement of leach solutions within the dumps. Copper-bearing materials below the layers have no contact with leach solutions. Operating personnel of several companies (Miami Copper Co. and others) have stated that large tonnages of iron are being deposited daily within the leach dumps. The chemical reactions involved during the precipitation operation provide the major quantity of iron in the circulating solutions. Some of the iron in the pregnant liquors is derived from the dissolution of the iron-bearing minerals within the dumps.
Most operators recirculate the tailing solutions from their precipitation plants to the dumps without purification. Some companies, however, purify the solutions prior to introduction to the dump surfaces to control the iron content. At the Castle Dome operation of the Miami Copper Co., the tailing solution flows through shallow ponds and is cascaded down the banks to precipitate basic iron salts and thereby lower the iron content. The resultant solution is collected and pumped to the dumps and used as the leach solution.
Tailing solutions from the precipitation plant at the Mineral Park property of Duval Corp. and the Silver Bell operation of American Smelting and Refining Co. (fig. 18) are collected in settling ponds where some iron salts are allowed to precipitate. The brown color of the water is typical of such tailing solutions which have low copper and high iron contents. At the Silver Bell operation, the water is pumped from the pond to the leach dumps by float- mounted pumps. A new reservoir has been constructed as part of the new leaching and precipitation plant at the Bingham Canyon operation of Kennecott Copper Corp. The reservoir will hold 500 million gallons of solution. Half of the tailing solution from the precipitation operation will be fed to this reservoir. The primary purpose of the new reservoir is to impound and provide makeup water to the leach solution distribution system. A second purpose is to allow time for oxidation of some of the iron from the ferrous state to the ferric state and thereby precipitate some of the contained iron.
Pregnant Liquor
Composition.-Chemical analyses of typical pregnant liquors from several operations (see table 1) show a wide variation in copper content, total iron, ratio of ferrous to ferric iron present, total acid, and pH. Copper contents of pregnant liquors vary from 0.75 to 2.16 grams per liter. Total iron varies from 0.20 to 6.60 grams per liter. An interesting point to note when considering the iron content of the pregnant liquors is the extreme variation in the ratio of ferrous to ferric iron from one operation to another.
Temperature.-The temperature of the pregnant liquors in nearly all leaching operations is generally from 5° to 20° F higher than that of the leach solutions. A rise in temperature is to be expected because of the exothermic reactions taking place during the oxidation of sulfide minerals within the


dumps. The air temperature is known to be as high as 135° F within one leach dump at the Bingham Canyon operation of Kennecott Copper Corp. High temperatures reflect accelerated oxidation rates in the dumps and presumably account for the dissolution of some of the difficultly soluble minerals, more especially chalcopyrite.
Operational Control During Leaching
Control of the pH of the leach solutions is mandatory for effective leaching. Sulfuric acid is added in many leaching operations prior to the introduction of the leach solutions onto the dumps. Numerous monitoring devices are used and acid addition is controlled automatically. Pregnant solutions are sampled periodically and choice of sections of the dumps to be leached is governed by analyses of these solutions. When the copper content of the pregnant liquor falls below a predetermined amount, leaching is discontinued in the sections of the dumps being leached and begun on new sections. Additional amounts of acid usually are added to the leach solutions when the tailing solutions from the precipitation plants have a pH of more than 3.0.
Heap Leaching
Heap leaching is employed to dissolve copper from porous oxide ore that has been placed on a prepared surface. Heap leaching contrasts with dump leaching principally in that ore material is leached instead of waste material. This method is not applicable to the dissolution of copper sulfide minerals or to ores containing large amounts of carbonate or other acid soluble gangue minerals. Companies currently employing heap leaching are Ranchers Exploration and Development Corp. at the Bluebird mine near Miami, Ariz., the J. H. Trigg Co. operation at Tyrone, N. Mex., and Zontelli Western Mining Co. operation at Page, Ariz.
Emplacement of Heaps
Ground Preparation
Leach heaps are deposited primarily on areas after the ground surface has been prepared. One exception to this procedure is at the heap leaching operation of the J. H. Trigg Co., where the leach heaps are deposited on the existing topography. Examples of prepared surfaces are given by the following.
At the Bluebird mine of the Ranchers Exploration and Development Corp., the ground was first cleared of vegetation; then the soil was cemented and covered with diluted tar for curing and sealing purposes.
The area used for emplacement of the leach heaps at the Zontelli Western Mining Co. operation was leveled by bulldozer, with a slope maintained toward the pregnant liquor sump adjacent to the precipitation vats. After the leveling operation was completed, a 10-inch layer of fine sand was spread on the surface, followed by a layer of 10-mil polyethylene plastic. A 12-inch layer of fine sand then was placed on the polyethylene plastic. Asbestos pipe perforated with ½-inch holes and protected by wooden cribbing then was placed on the 12-inch sand layer at 15-foot intervals across the width of the heaps.
These drainpipes extend from the ends of the heaps and are alined to taper toward the pregnant liquor sump.
Method of Heap Emplacement
The material for heap leaching operations is blasted or ripped and then pushed by bulldozer or hauled by bottom dump scrapers and dumpsters to the heaps. The method employed at the Bluebird mine of Ranchers Exploration and Development Corp. is typical of this type of operation. This method was designed to produce minimum compaction in the heaps which are built in 15- to 18-foot lifts. When starting a new heap, scrapers place the leach material in a 30-foot-wide strip along the centerline of the new heap. Subsequent loads are dumped along the outer edge of the strip and pushed over the side with a motor grader to build the heap to its full width without compaction. In this method only the top few feet of the heap becomes compacted, and this layer is subsequently loosened by a bulldozer equipped with a ripper.
Physical Nature of Heaps
The leach material at the J. H. Trigg Co. property No. 1 is less than 6 inches in size and that at property No. 2 is less than 2 feet in size. The maximum size of heap leach material at Ranchers Exploration and Development Corp. is approximately 12 inches. The bulk of the material, however, is less than 4 inches. The leach material at Zontelli Western Mining Co. is crushed to minus 4 inches.
The volume of leach material on heaps is considerably less than that of leach dumps and varies from 100,000 tons at J. H. Trigg Co. to 500,000 tons at Zontelli Western Mining Co. and Ranchers Exploration and Development Corp.
Leaching Operation
The overall heap leaching operation is similar in many respects to that of dump leaching, with a few exceptions.
More concentrated sulfuric acid solutions are used in heap leaching because of the acid strength necessary for dissolution of the copper oxide minerals. Acid is not regenerated within the heaps because of the absence of pyrite in the oxidized ore. As much as 50 grams per liter of sulfuric acid is added to the leach solution at the Ranchers Exploration and Development Corp.’s Bluebird mine.
A different approach for removal of iron produced in the precipitation plant is conducted by Ranchers Exploration and Development Corp. In addition to high-grade material placed on heaps for leaching, material containing less than 0.3 percent copper is placed on mine waste dumps. The tailing solution from the precipitation plant is circulated through the waste dumps to allow some of the iron to precipitate. Some acid is consumed in this process, but copper also is dissolved. The solution from the waste dumps then is returned to the leach heaps to be used as the leach solution.
Because of the higher grade of the heaps, the stronger acid solution, and the ready solubility of oxidized copper minerals, the pregnant solutions produced generally are much higher in copper content than pregnant solutions obtained from dump leaching. Copper content of the pregnant liquor at Ranchers Exploration and Development Corp. varies from 3 to 7 grams per liter.
In-place Leaching
Inplace leaching techniques are applicable to leaching oxide and sulfide ores of copper and other metals. The basic principles involved are similar to those for dump or heap leaching. The leach solutions must pass downward through the ore and contact and dissolve the copper-bearing minerals. Adequate arrangements are necessary to collect the resultant copper-bearing liquors without appreciable loss. Provision usually must be made to pump the liquors to the surface where the copper can be recovered.
Leaching inplace and precipitation of copper at the Ohio Copper Co. mine, Bingham Canyon, Utah, has been described by Anderson. Leach solutions were distributed upon the surface of a large rockfill overlying a portion of the caved zone of the underground mine. Slow percolation was used in the leaching because it was believed this method would promote the oxidizing conditions deemed favorable for continued and rapid dissolution of the copper sulfide minerals.
Thomas has described the inplace leaching of copper from worked-out areas of the Ray mines in Arizona. Sprays that were moved from one area to another were used to uniformly distribute fresh water to the caved area. This practice promoted the oxidation of pyrite and the copper minerals and therefore their dissolution. The copper-bearing liquors were pumped to the surface and the copper was recovered by precipitation with iron.
The Miami mine of the Miami Copper Co. is the best example of a current inplace leaching operation. The company is leaching copper from the under-ground ore bodies which previously were mined principally by block-caving methods. Various amounts of copper remain in the capping, in the stopes below the capping, and in the crushed pillars in the mine. A description of the inplace leaching operation at the Miami mine is given by Fletcher. Acid is added to tailing water from the precipitation plant, and this solution is distributed by sprays onto the surface area which overlies the former block-caving operation (fig. 19). The leach solution percolates downward through 600 feet of material, the bottom 150 feet of which contains the mixture of ore and waste with the largest percentage of copper. The solution moves from the surface to the 1,000-foot level of the mine within 3 to 4 weeks. Pregnant liquor that is collected in a reservoir on the 1,000-foot level is emptied periodically into a sump from which the liquor is pumped to the precipitation plant on the surface.
Vat Leaching
Vat leaching is employed to extract copper from oxide or mixed oxide sulfide ores containing more than 0.5 percent acid-soluble copper. This method is used in preference to heap leaching if the ore material is not porous and crushing is necessary to permit adequate contact between the leach solution and the copper minerals. Despite increased costs necessary for crushing and screening, numerous advantages are achieved by vat leaching. Higher copper recovery in shorter periods is realized; pregnant liquor losses are negligible; and copper content of the pregnant liquor is much higher, permitting recovery of the copper by means other than precipitation by iron.
Seidel has discussed numerous factors which influence vat leaching processes. Percolation flow rates are affected chiefly by the texture or permeability of the ore bed in the vats. Air or other gas bubbles present in the ore beds reduce the percolation rates of the leaching solutions. Higher temperatures of aqueous leach solutions will produce higher percolation rates. Numerous variables, such as particle size, particle porosity, temperature, reagent strength, and percolation rates, greatly affect the mass transfer operations during the dissolution of the mineral particles and the washing and recovery of the dissolved copper from the leach residue.
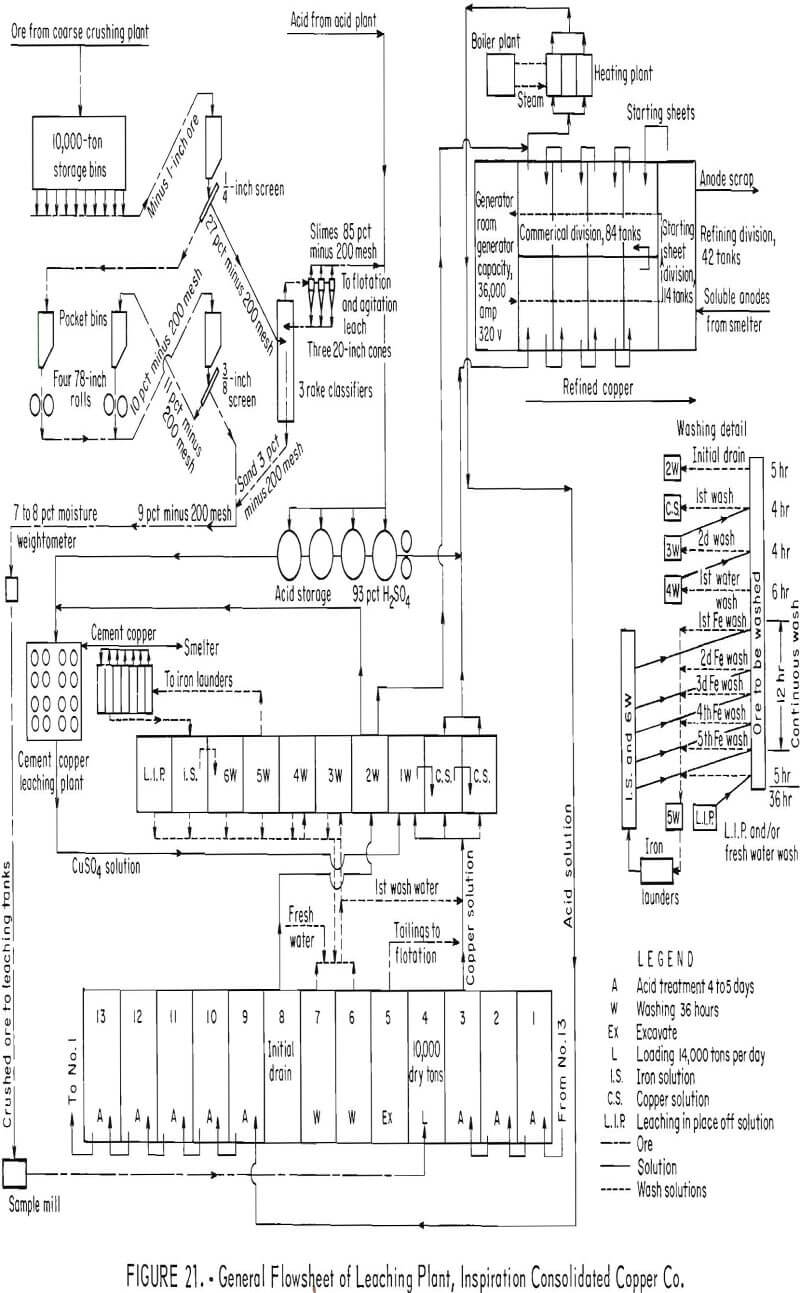
Ores processed by vat leaching are made suitable for placement in the vats by crushing and screening. Descriptions and illustrations of the vat leaching operations of The Anaconda Company, Weed Heights, Nev. , and the Inspiration Consolidated Copper Corp., Inspiration, Ariz., are given in the following sections.
The Anaconda Company
The ore treated by vat leaching at the Weed Heights operation of The Anaconda Company (fig. 20) is predominately oxide ore containing 0.59 percent copper. Approximately 86,000 tons of ore is treated per week in eight vats, each measuring 120 feet wide, 139 feet long, and 19 feet deep. Descriptions of this operation have been given by Huttl and in “Mining World”. The oxide ore is crushed and screened to minus 7/16 inch. Water sprays are used to increase the moisture content of the minus 7/16-inch material to 5 or 6 percent as it is layered on the conveyor belt that moves the material to the leaching vats. Because of the increased moisture content the fine particles tend to adhere to the coarser particles and also to cling together to form larger particles. Agglomerating the fines in this manner obviates the necessity for removing the fines which normally would reduce the porosity of the ore beds in the vats and therefore prevent effective leaching.
The principal copper-bearing mineral in the ore is chrysocolla. Dilute sulfuric acid solutions containing from 30 to 35 grams per liter of acid are used to leach the ore. The pregnant liquors and wash waters obtained from the vat leaching operation are pumped to a precipitation plant where the copper is recovered by use of iron scrap.
Inspiration Consolidated Copper Co.
The ore treated in the vat leaching operation of the Inspiration Consolidated Copper Co. (fig. 22) is mixed oxide-sulfide ore. Presently chrysocolla is the predominant oxide mineral and chalcopyrite is the predominant sulfide mineral. Early methods of leaching Inspiration ore have been described by Aldrich and Scott and Van Arsdale. The design of the original leaching plant to treat underground ores, which consisted of approximate pro-portions of 60 percent oxide minerals and 40 percent chalcocite, was based on the ready solubility of chalcocite in a solvent containing sulfuric acid and ferric sulfate. The ore mined and treated contained an increasing amount of chalcopyrite as the method of mining was gradually changed from underground to open pit. Chalcopyrite is only slowly soluble in a ferric sulfate leach, and it became necessary to employ a system to recover copper separately from both the oxide and sulfide minerals.
In the present operation the ore is crushed, screened, passed through two sets of rolls, and then rescreened. The final sand product is suitable for leaching with a maximum size of three-eighths of an inch and with a minimum amount of fine material detrimental to the flow of leach solutions. The slime fraction obtained from the rake classifiers and cyclones in the circuit contains 85 percent minus 200-mesh material and is used as a feed to a separate slime leaching operation. A generalized flowsheet of the Inspiration leaching plant is given in figure 21.
Approximately 9,600 dry tons of ore is loaded into each of 13 leaching tanks by a gantry-mounted clamshell bucket. Each tank is approximately 68 feet wide, 175 feet long, and 18-½ feet deep. The oxide minerals are batch leached by using the return solution from the electrolytic tankhouse as the leach solution. The leach solution is heated to about 53° C and is introduced into the ore at a rate of 2,400 gallons per minute. The leach solution and the pregnant liquor derived from the leaching operation is represented by the following typical chemical analyses with the data expressed in grams per liter:


Chalcopyrite is not readily soluble in the leach solution, but is recovered later from the vat tailing. The tailing from the vat leaching operation is excavated by clamshell and transported by train to the company’s concentrating plant where the chalcopyrite is recovered with other sulfides by conventional flotation methods.
During 1965, Inspiration Consolidated Copper Co. installed the latest innovation in bulk-handling systems which consists of a bucket line reclaimer used to continuously empty the leach tanks at the rate of 1,100 tons per hour and feed a 7,400-foot overland belt conveyor system connecting the leach tanks with the concentrating plant. A description of this innovation is given in “Engineering and Mining Journal”.
Copper is recovered from the vat leach liquors by electrolytic deposition. Copper is recovered from the slime fraction mentioned earlier by flotation of the sulfides and by subsequent leaching of the oxides.
Other Methods of Leaching
Several minor methods of leaching presently are used to recover copper from mixed oxide-sulfide ores or from the slime fraction obtained from crushing oxide ores used in vat leaching. These methods are referred to as leach-precipitation- flotation (LPF) and slime leaching, respectively, and are described in the following sections. Ammonia leaching has been used in the past to recover copper from copper oxide minerals associated with carbonate gangue material. Descriptions of a former ammonia leaching operation have been given by Duggan and Eddy. Leaching of copper minerals by cyanide solutions has been proposed by Lower and Booth, but as yet has not been used in practice.
Leach-Precipitation-Flotation
The original LPF process, according to Bean, was conceived and developed by F. W. Maclennon and Harmon Keyes of the Miami Copper Co. during the years 1929-34. The process was designed to supplement a conventional flotation operation by adding a leach circuit to the mill.
Many of the porphyry-type copper deposits in the Western United States are highly altered and contain readily acid-soluble oxidized copper minerals associated with sulfide copper minerals. The nonsulfide minerals often occur as coatings and stains on the gangue and ore minerals and not as discrete grains in the rock as a whole. To recover both the oxide and sulfide copper minerals, leaching and froth flotation methods have to be employed. In some plants the sulfide minerals are recovered by froth flotation and the resultant tailing then is leached to recover the remaining copper. More common practice is to acid leach the oxide minerals, precipitate the dissolved copper with iron, and recover the resultant cement copper and the sulfide minerals in the same flotation circuit. Descriptions of LPF circuits have been given by Franz, Huttle, and Milliken and Goodwin.
The first unit operation in the LPF process comprises crushing and grinding to liberate the sulfide minerals. The finely ground ore is classified in bowl classifiers, drag classifiers, and cyclones to produce a uniform feed for leaching and flotation.
The ground pulp is agitation leached in tanks for about 30 minutes with a sufficient amount of sulfuric acid to maintain a solution pH between 1.5 and 2.0. From the leach tanks the pulp is introduced into a series of precipitation tanks or cells where finely divided iron is added to precipitate the dissolved copper. The pulp is then moved to acid-proof froth flotation cells to recover a concentrate containing both cement copper and copper sulfides. The pH of the flotation pulp in most plants is regulated to about pH 4 by the addition of lime, although metallic iron also has been used for this purpose. Because the flotation feed is acidic, dixanthogen is the commonly used collector. Copper recovery by the LPF process is generally about 90 percent.
The main improvement in recent years in the LPF process has been the availability of more durable equipment. Flotation cells, pumps, and tanks are coated with new types of organic and rubber materials which prevent excessive corrosion and erosion of the equipment.
Slime Leaching
The slime fraction in the ore obtained from the preparation of the vat leach feed at the plant of Inspiration Consolidated Copper Co. is treated first by conventional flotation methods to recover the sulfide copper minerals. The flotation tailing then is thickened to about 40 percent solids and sent to the slime leach plant for extraction of copper from the acid-soluble oxidized copper minerals. The process has been described by Power. The slime plant consists of three 20-foot-diameter redwood leach tanks. Each tank is equipped with a mechanical agitator having a 4-inch airlift in the shaft of the agitator for removal of the leached pulp. Separation of the resultant copper sulfate solution from the acid insoluble residue is accomplished by countercurrent washing in four 150-foot concrete traction thickeners, each equipped with three 4-inch airlifts on the spigot product and one 4-inch lead-lined centrifugal pump on the overflow. The pregnant copper sulfate solution is pumped to an iron launder where the copper is precipitated by scrap iron. The sulfuric acid requirements for leaching the slimes ranges from 3.9 to 4.2 pounds of acid per pound of copper recovered. Iron consumption for precipitating the cement copper amounts to 1.6 pounds of iron per pound of copper precipitated. Copper recovery from the slime leach plant feed is about 95 percent.
Copper Recovery
Copper presently is recovered from leach liquors almost entirely by means of precipitation by metallic iron. Copper in the vat leach liquors at Inspiration Consolidated Copper Co. is recovered by electrolytic deposition. Other methods of precipitation have been suggested by Baarson and Ray and Croasdale. Numerous investigators, including Forward, Peters and Hahn, and Schaufelberger, have shown that at elevated temperatures and pressures, copper can be precipitated from solution by hydrogen reduction.
Bagdad Copper Corp. is constructing a plant to produce copper powders by application of hydrogen reduction principles. Other methods of recovering copper from solution, suggested by Agers and coworkers. Fletcher and Flett, Quarm, and Swanson and Agers, are by solvent extraction and ion exchange. At present, however, copper recovery is accomplished in practice only by the methods of precipitation by metallic iron and electrolytic deposition, and the following descriptions focus attention on these methods.
Precipitation by Iron
Metallic iron precipitates copper from solution according to the well-known reaction: CuSO4 + Fe ↔ Cu + FeSO4. In practice copper is recovered from copper-bearing pregnant liquors almost entirely by application of the principle of this equation.
Sources of Iron
Shredded scrap cans (fig. 23) are the chief source of iron used as a precipitant in the recovery of copper from copper-bearing solutions. They are


first burned to remove paper labels and organic material. The tin content of scrap cans is often removed by a caustic leach process. The principal Western suppliers of shredded, detinned cans are Proler Steel Corp., with plants at Houston and El Paso, Tex., and San Francisco, Calif., and the Los Angeles By-Products Co., with plants at Bakersfield and Los Angeles, Calif.
Scrap punchings and clippings produced during the manufacture of cans has attained significant importance during recent years as a source of iron for copper precipitation. These materials are good precipitators for copper because of their relative uniformity in size and absence of deleterious impurities, such as organic materials and other solids. Proler Steel Corp. has recently erected a shredding plant near Copperton, Utah, to supply the Bingham Canyon precipitation operation of Kennecott Copper Corp. The product will be used in the newly installed cone precipitators.
Another source of iron that has been used as a precipitant is turnings from lathes and other cutting machines. This type of precipitant has proved unsatisfactory, however, because of inclusions of oil and other lubricants. An additional deterrent to the use of turnings is the slow precipitation of copper attained because of the low surface-to-weight ratio of the iron.
Heavy scrap iron has been used as a precipitant for copper in several operations. Low cost of the scrap iron obtained from the mine and milling operations has made this type of precipitant attractive. Deterrents to using heavy scrap as a precipitant, however, are the difficulty in handling the scrap during loading and cleaning of the precipitation launders and the small surface area per unit of weight available for reaction. A few operators have used heavy scrap in the head section of the launders to reduce the ferric iron content of the pregnant solution and thereby reduce the shredded can consumption in the remaining cells, since ferric sulfate and sulfuric acid are responsible for the iron consumed in the launders according to the reactions :
Fe2(SO4)3 + Fe ↔ 3FeSO4;
H2SO4 + Fe ↔ H2 + FeSO4.
Finely divided sponge iron is an effective precipitant of copper, although at present it is not competitive in cost with available shredded scrap cans. The principal advantage in using sponge iron seems to be the relatively faster copper precipitation rate obtained with particulate iron as compared to that obtained with more massive scrap iron products. According to Back, sponge iron is most effective as a precipitant for copper when used in conjunction with cone-type precipitators. High-grade magnetite, iron ore, and pyrite cinders are the chief sources for producing sponge iron suitable as a reactive precipitant for copper. Kennecott Copper Corp. is investigating the feasibility of producing sponge iron for copper precipitation by the reduction of the iron in copper reverberatory slag from the firm’s smelter.
Types of Precipitation Plants
Copper is precipitated from solutions in a series of gravity launders or in cone precipitators. Descriptions and examples of each type currently employed are given in addition to illustrations of certain aspects of operating practices.
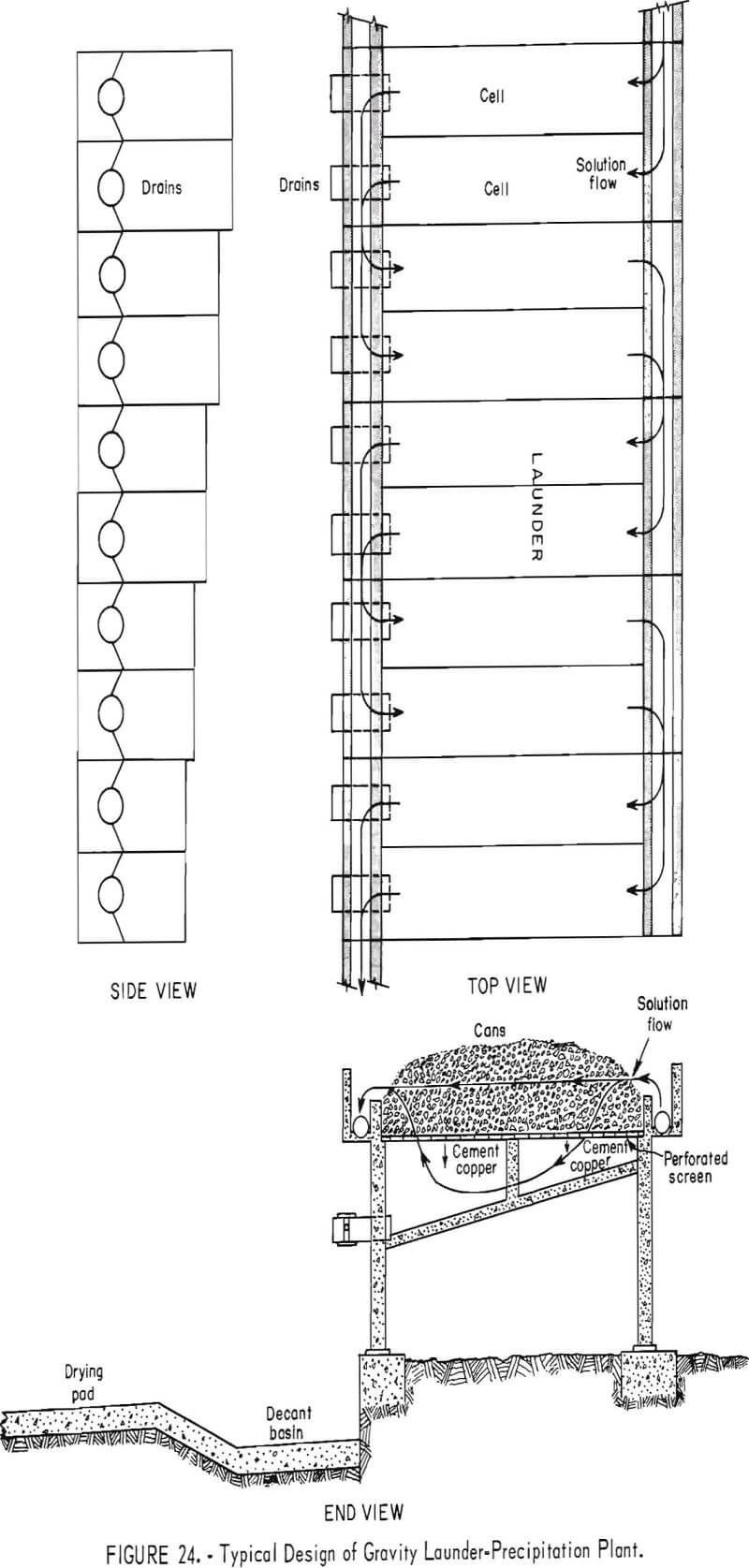
Precipitation Launders
Current precipitation practice is typified by the use of gravity launders. Typical top, end, and side views of a series of gravity launders are shown in figure 24. Launder dimensions at numerous operations are given in table 1.
Ballard, Jacky, and Power have described the precipitation launders of The Anaconda Company and the American Smelting and Refining Co. Many launders are of concrete construction, with wooden planks to protect the top of the launder walls. Plywood and/or stainless steel gratings are used to support the iron precipitant within the launders.
The launders of the precipitation plants are charged with cans by means of different mechanical devices. The launders of the Copper Cities precipitation plant of the Miami Copper Co. are loaded with cans that are discharged from a belt conveyor by a moving tripper. A crane-mounted magnet is used to charge and clean launders at the Silver Bell precipitation plant of the American Smelting and Refining Co. A gantry-mounted clamshell bucket is used to load cans into the launders at the Butte precipitation plant of The Anaconda Company. Front-end loaders are used at the Morenci precipitation plant of Phelps Dodge Corp., and at the Mineral Park plant of the Duval Corp. Part of the cells at the latter plant are loaded by a belt conveyor (fig. 25). Kennecott Copper Corp. at its Bingham Canyon original launder precipitation plant uses a forklift loader to load the launders with cans.
Copper-bearing liquors are introduced into the upper end of gravity launder plants through a solution feed launder and allowed to trickle downward through the shredded cans. Launder arrangement is such that solution flow is in series. Baffles and pumps are utilized in order that any launder may be bypassed and solutions returned to any particular launder. The copper, ferric iron, and free sulfuric acid content of the pregnant liquors and the surface area of iron available for reaction are the more important factors in determining the rate and degree of recovery of copper in each particular launder.
Copper usually is washed three times a week from the first few launders, which in most plants precipitate more than 60 percent of the total copper. The copper precipitated in the remaining launders is removed usually from once a week to once a month. Tailing (barren) solutions discharge by gravity from the lower ends of the plants.
The “Yerington-type” plant differs from those described in that the pregnant solution flow is upward through the iron precipitant. The pregnant liquor is introduced into the launders under pressure through three parallel, 4-inch plastic tubes which lie in gutters in the floors of the launders. The tubes have been perforated with 5/8-inch holes spaced from 1 to 3 feet apart

(fig. 26). High-solution velocities are maintained in the launders and a coarser cement copper product is formed. Higher purity and lower moisture content are synonymous with this coarser product.
Pregnant liquors introduced into, and tailing (barren) solutions dis-charged from, precipitation plants are sampled continuously. The solutions are analyzed for pH and copper, iron, and acid content by wet chemical methods, although other methods are being adopted such as X-ray, electrolytic, and atomic absorption. Typical analyses of such solutions from numerous precipitation plants in the Western States are given in table 1. The pH of the pregnant liquors varies from 1.4 to 3.5, copper content from 0.75 to 7.0 grams per liter, ferrous iron content from 0.00 to 3.60 grams per liter, ferric iron content from 0.05 to 3.00 grams per liter, and free acid content from 0.04 to 7.50 grams per liter. The recovery of copper is high in all operations, averaging well above 90 percent. The copper content of the tailing solutions therefore is low and ranges from less than 0.01 to 0.36 gram per liter. The pH of the tailing solutions is higher, in the range of 2.4 to 4.4, as a result of the decrease in acid content. The decrease in acid and an increase in total iron content (almost entirely ferrous iron) is the result of the several iron-consuming reactions that take place during precipitation.


The amount of iron consumed per pound of copper precipitated (commonly known as the “can factor”) is an important concern in all plants. Iron consumption varies from operation to operation, but is always higher than the theoretical 0.88 pound of iron per pound of copper required for the iron replacement by copper reaction. The can factors range from a low of 1.2 to a high of 2.5 at the various operations. A list of can factors is given in table 1.
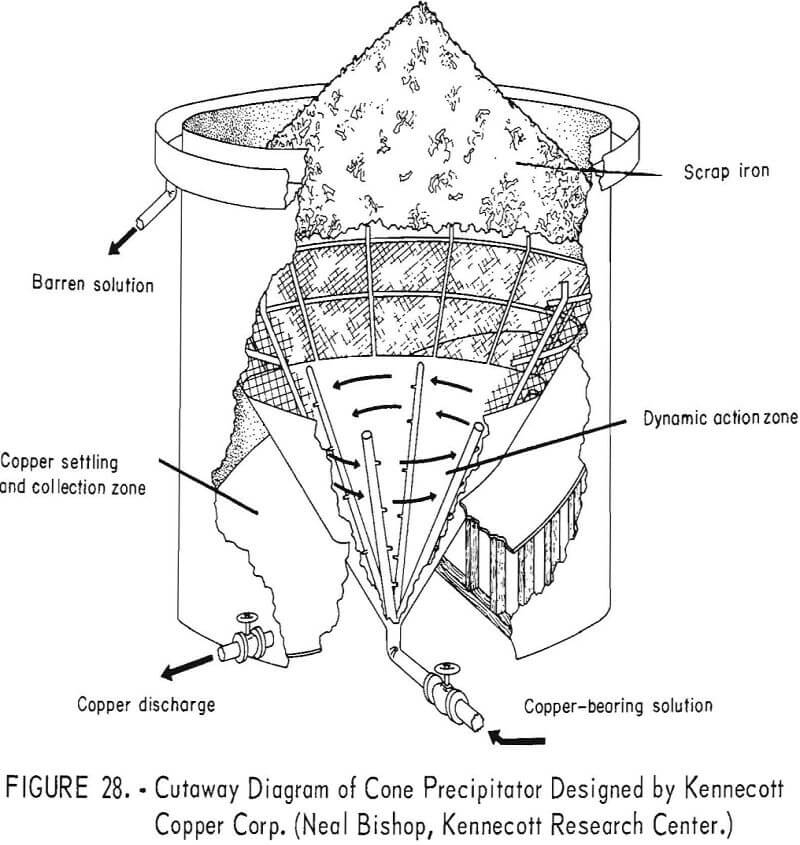
Cone Precipitators
A recent innovation in the recovery of copper from copper-bearing solutions is the cone-type precipitator developed by the Kennecott Copper Corp., and described by Spedden and coworkers. A cutaway diagram of the precipitation cone is shown in figure 28. Copper-bearing solution is pumped through a manifold system in the bottom of the cone and is injected through nozzles into shredded, detinned iron scrap, which is semicontinuously loaded into the inner cone above a heavy-gage, stainless steel screen. The injection of the solution under pressure into the iron material has a dual effect. Copper is rapidly precipitated and quickly removed from the iron surfaces by the turbulent action, creating fresh, clean surfaces of iron for continued precipitation of the copper.
Methods of Handling Cement Copper
Various methods are used to handle the cement copper following its precipitation in the launders or in the cones. The purpose in each instance is the same, to obtain as pure a product as possible with minimum moisture content.
Removal From Precipitation Unit
The most frequently used method for removing cement copper from precipitation launders is with high-pressure streams of water. The water is pumped at about 100 pounds of pressure to wash hoses equipped with quick shutoff nozzles (fig. 27). The cement copper is washed from the unreacted iron, and the resultant slurry is emptied into decant basins through drain valves in the bottoms of the cells.
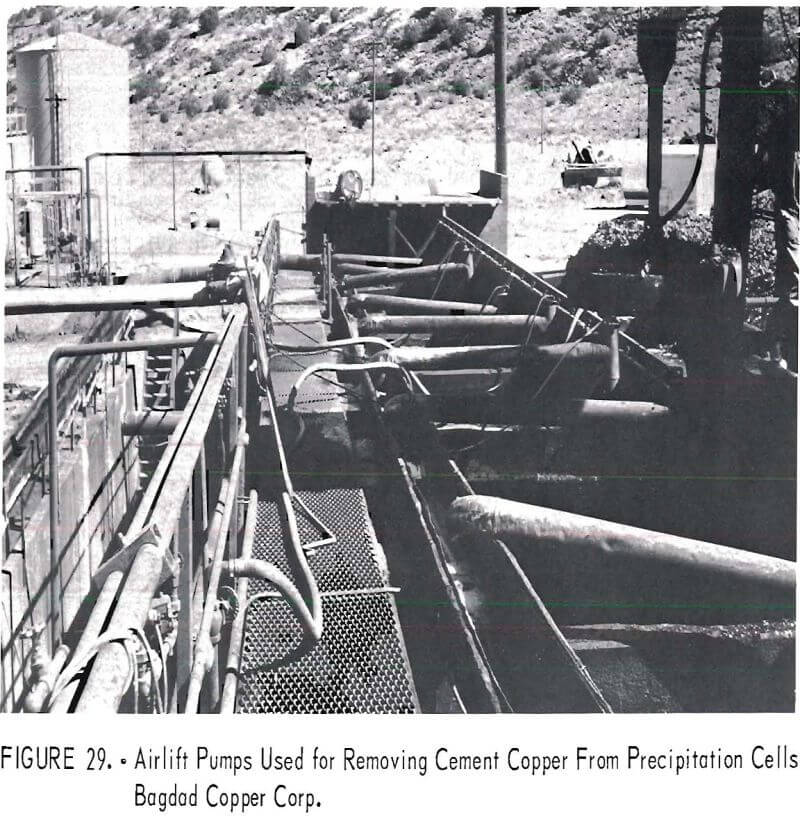
Airlift pumps (fig. 29) are used by Bagdad Copper Corp. to remove the cement copper from the bottom of the launders after the cement copper has been washed from the unreacted cans. The slurry of cement copper and water, after being discharged from the airlift pumps, flows by gravity into settling basins (fig. 30).
Hydraulic slushers (fig. 31), consisting of a trussed bridge which spans all six of the parallel launders at The Anaconda Company’s Butte precipitation plant, are used to agitate the cans in the launders, remove the cement copper from the cans, and slush (sweep) the cement copper along the launders to drop tanks. After the drop tanks are nearly full, the water is decanted by pumping and the cement copper is loaded into railroad cars by an overhead clamshell bucket.
A few operations use a gantry-operated clamshell bucket to unload the cement copper and unreacted cans from the precipitation launders. The combined material then is charged into a trommel (fig. 32) fitted with stainless steel screens having small-diameter openings to separate the cement copper and unreacted cans from one another. The unreacted cans are returned to the precipitation launders and the cement copper is discharged into settling basins.
The cement copper produced in Kennecott’s cone precipitators settles through the stainless steel screen, drops to the bottom of the tank, and is removed intermittently through a pneumatically operated valve. Higher grade products, analyzing 90 to 95 percent copper, are obtained by this method of precipitation. Other benefits from this method are a substantially lower consumption of iron and the elimination of the need for the usual labor involved in a launder-type plant.
Decanting
The cement copper is allowed to settle in basins. The clear water is decanted and returned by pumping to the precipitation launders to prevent the loss of any suspended fine particles of copper.
Drying
The cement copper is removed from the settling basins by various mechanical devices, such as front-end loaders and crane-mounted clamshell buckets. The cement copper removed from the settling basins is dried before shipment to smelters or other markets. Concrete drying pads are generally used, and in most instances are constructed adjacent to the settling basins. The cement copper at the Weed Heights, Nev., operation of The Anaconda Company is dried on large gas-fired hotplates (fig. 34).
Atmospheric drying of the cement copper reduces the moisture content from about 50 percent to between 25 and 30 percent. The cement copper dried by hot-plates at Weed Heights contains 15 percent moisture.
Plate-and-frame filter presses are used to reduce the moisture content of the cement copper produced in the cone precipitators at the Bingham Canyon




operation of Kennecott Copper Corp. The final product .contains from 8 to 10 percent moisture.
Additional Treatment
The cement copper produced in a few plants is processed further to increase the purity of the final product. The cement copper is screened through small-mesh vibrating screens by the Inspiration Consolidated Copper Co. Flotation techniques are used at the Bingham Canyon operation of Kennecott Copper Corp. to improve the quality of part of the cement copper from the launder plant for direct sale to consumers.
Electrolytic Deposition
The Inspiration Consolidated Copper Co. recovers copper from vat leach liquors by electrolytic deposition using insoluble anodes. The process is termed electrowinning. Liquors containing from 25 to 30 grams per liter of copper serve as the electrolyte for the electrowinning process. A description of the electrowinning process and operating data for the Inspiration.plant have been given by McMahon.
The principal advantages of this process are that electrolytically refined copper does not require additional treatment that is, the usual smelting operation required for copper production is eliminated and sulfuric acid and ferric sulfate needed as solvents during the leaching operation are regenerated.
Research
Most companies currently engaged in copper leaching are researching or are planning to research the following: (1) The solvent extraction of copper from leach liquors; (2) the use of other precipitants such as sponge iron and aluminum; (3) bacterial leaching; (4) the nature of dumps (by drilling and analysis of drill samples); (5) chemical relationships of dump materials and solutions; (6) effectiveness of acid and other solvent additions to the leaching solution; and (7) dump emplacement techniques.
Summary
A more rapid and more complete recovery of copper from leach dumps is the ultimate concern of all companies engaged in copper leaching. Close attention must be given to numerous problems inherent in the leach cycle to achieve a satisfactory leach rate and recovery of the copper.

Iron salt precipitation on the surface or within leach dumps is one of the principal problems encountered in leaching. Impervious layers formed within leach dumps act like blankets through which leach solutions cannot pass. Large volumes of copper-bearing material are not contacted by the leach solutions. Examples of this phenomenon can be seen in the Ohio Copper Co. mine presently exposed in the open-pit mining operation of Kennecott Copper Corp. at Bingham, Utah, and at the Inspiration Consolidated Copper Corp. at Inspiration, Ariz. (fig. 33). A partial solution to this problem seems to be to minimize the iron salt precipitation within the dumps by maintaining a low solution pH by acid additions to the leach solutions.
Channeling is created in many leach dumps as a result of the methods employed to build the dumps. Solution flow often is horizontal following alternating layers of coarse and fine material; hence, large volumes of mineralized material may not be contacted by the leach solutions. Close attention to the method of constructing the leach dump is needed to minimize size segregation.
Another important problem encountered is the loss of leach and pregnant solutions. Losses of leach waters frequently are encountered in the Southwest where evaporation is abnormally high. Pregnant solution loss is high whenever the ground upon which leach dumps are deposited is permeable or contains many fractures and faults. Use of prepared pads for dump emplacement is a partial means of solving this problem. Solution loss can result from entrapment of leach solutions within iron salt-lined cavities in the dumps. Improved pH control can partially correct this situation.
The formation of iron precipitates in pipelines and pumps has caused many maintenance problems. The solution to this problem is to prevent the iron salts from precipitating by controlling the acidity of the circulating solutions.
Numerous unsuccessful attempts have been made to build up the copper con-tent of the pregnant solution by recycling. Recent investigations by Grunig have shown that clay products within the dumps act as ion exchangers that absorb copper.
Conditions of equilibrium likewise influence the solubility of the many minerals, both ore and gangue, that are contained in the leach material. Copper, iron, and aluminum compounds are both formed and redissolved at different depths within the leach dumps, depending upon environmental conditions prevalant at a given time and site. The chemical and physical reactions taking place within the dumps are numerous and complex. Under a given set of conditions, it is possible that as much copper is being removed from solution as is being dissolved.
The chief problem in copper precipitation is maximum extraction of copper with relatively low iron consumption. Iron consumption is high and the cement copper product is contaminated with impurities when the pregnant liquors contain excessive ferric iron and acid and low amounts of copper. A low-quality cement copper is obtained also when the precipitant Is heavy metal and is unsuitable with respect to freedom from acid-insoluble impurities.
Improper plant design causes materials-handling problems. Mechanical and chemical problems, such as seal and bearing failures in pumps and acid attack on reactive surfaces, are common to nearly all operations.
Production of copper by use of the various leaching methods is increasing continually and accounts for a larger part of the total copper production in the Western United States. Many of the major copper producing companies currently are improving and expanding their leaching operations. Other companies are planning to increase their outputs by leaching in the near future.
Significant amounts of aluminum, uranium, and other metals are present in the pregnant liquors produced by leaching. Several companies and the Bureau of Mines are conducting research to find means of profitably recovering these metals as byproducts following the removal of copper from solution by precipitation.
