Table of Contents
- Hydrogen Sulphide in Precipitating Copper
- Analysis of Matte and FeS
- Sponge Iron Copper Precipitation Method
- Sulphur Dioxide Copper Precipitation
- Reduction of Cuprous Chloride
- Precipitation with Sponge Iron
- Reduction with Coke in the Presence of Limestone
- Electrolytic Reduction
- How to Precipitate Copper from Solution
In a leaching process, having obtained the copper in solution, the choice of the precipitation method is influenced by the following factors:
- Availability of precipitant.
- Adaptability to the leaching process.
- Final product desired.
- Regeneration of leaching solutions.
- Fouling of solutions.
There are three general classes of copper precipitation methods:
1. The copper precipitation by use of iron, scrap or sponge.
2. Electrolytic deposition.
3. The use of some gas or reagent by which the copper is obtained, usually in the form of an intermediate precipitate which requires further treatment. For example, the precipitation of the copper as Cu2Cl2 from chloride solutions; with SO2 gas.
The use of iron is adapted to almost any process. It has the great advantage of extreme simplicity, and the recovery of the copper and any values present in a highly concentrated, easily treatable form. The disadvantages are the cost in isolated places, the uncertainty of the scrap-iron market, and the fouling of the leaching solutions. The latter is a serious factor, when a reagent such as salt is used in them, which makes necessary their re-use.
Where applicable, electrolytic deposition is very attractive. The copper is obtained in a form directly marketable, there is a regeneration of acid and no fouling of solutions. Unfortunately, chloride solutions do not lend themselves to electrolysis. An entirely satisfactory anode material has not yet been discovered, although magnetite, apart from being rather brittle, seems to answer the requirements fairly well.
The use of a method of the third class is involved in many special leaching processes, and is highly attractive theoretically. Such methods usually involve regeneration of leaching solutions, no fouling of solutions, and the use of some cheap reagent, as lime, or a by-product of the process itself, or of some process such as smelting. Some method of this kind is undoubtedly that of the future where electrolytic deposition is not applicable.
The copper solution from the 80-ton leaching plant, in which the sand-treatment method now being installed on a large scale was developed, averaged Cu, 1.91; Fe2O3+Al2O3 3.88; NaCl, 8.3 per cent.; Ag, 0.634 oz. The copper content of this solution cannot be increased much economically. The application of this solution to successive lots of roasted tailing, from which about 10 lb. of copper per ton can be recovered, would necessitate excessive washing of the tailing. The copper can be precipitated from this solution by means of scrap iron, the cement copper going directly to the blast furnaces or converters. The use of sponge iron is more attractive than the use of scrap iron, however. It can be made from calcines which contain about 50 per cent. iron. This makes the process independent of the scrap-iron market. But the use of iron necessitates the discard of about one-fourth of the solution each time to stop the accumulation of iron sulphate, and this leads to the loss of much valuable salt.
Owing to its high chlorine content the solution is not adapted to electrolysis. With a view to getting something better than scrap iron, experiments have been and are being conducted on precipitation by sulphuretted hydrogen made from matte (also from calcium sulphate), sponge iron from calcined concentrates, and sulphur dioxide from roasting-furnace gases.
In the following discussion we shall attempt to explain the operation, difficulties, advantages and disadvantages, and the results obtained with each of these methods.
Hydrogen Sulphide in Precipitating Copper
Hydrogen sulphide was used as the precipitant in the work done on the experimental plant operated.
The precipitation tank, 10 by 10 by 7 ft., was equipped with an agitator and a system of lead pipes in the bottom for gas distribution. The generator was a lead-lined iron drum 2 ft. in diameter and 4 ft. high. It was built to stand 90 lb. pressure and fitted with air and steam connections. The iron sulphide was put in through a handhole in the top. The acid was introduced through an iron pipe by gravity. The gas passed through a lead main to the distributing pipes. When action ceased a plug was removed from the bottom of the tank, 90-lb. air applied and the residue blown out.
The copper sulphide was filtered in a small hand-made wooden filter press, the inside dimensions of the frames of which were 12 by 12 by 1 5/8 in. Ordinary filter paper backed up with heavy canvas was used. The copper sulphide was allowed to settle over night, the clear solution drawn off and the thickened residue drawn into a pressure cylinder, from which it was forced with 90-lb. air through a ½-in. lead pipe to the press.
The absorption of the H2S gas was very inefficient. The gas entered under considerable pressure and escaped in big bubbles through a depth of 3½ ft. of solution. Some of the iron sulphide, which was the commercial kind, was of very poor quality. It was found that when ground to 4 mesh the rate of evolution of the gas was about right.
A charge was 400 lb. of water, 100 lb. of 4-mesh iron sulphide, and 130 lb. of 66° Baume acid which was slowly added. The acid strength on starting was about 24.5 per cent, and averaged 7.5 per cent, at the end; 2.4 lb. acid per pound of copper was required for the generation of the H2S. The efficiency of the acid used in the generation was about 62 per cent. The efficiency of the FeS in the laboratory was 80.2 per cent., but in the plant only 54 per cent, was realized, due to poor absorption of the gas.
About 100 lb. of matte was made by fusing in proper proportions a heavy pyrite ore, an oxidized iron ore, and lime rock, in graphite crucibles. This matte decomposed readily and was 97.2 per cent, as efficient as the commercial FeS.
Analysis of Matte and FeS
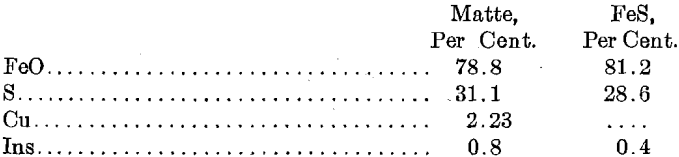
The filtration gave some trouble, due to defects in the press. When the press was working well the cakes were of about the consistency of cheese and assayed: Cu, 58; S, 29.0 per cent.; Ag, 69.5; Au, 0.04 oz. The cakes carried 48 per cent, moisture.
With a generator having an agitating device, and a properly designed absorber, there is no doubt but that a high efficiency could be obtained by the use of this process. The reaction between CuSO4 and the H2S liberates 1.55 lb. acid per pound of copper precipitated, and the reduction of the ferric salts also liberates some acid.
The advantages of this process are:
- Regeneration of acid.
- No fouling of solutions.
- A product which is high grade and can be readily worked into the smelter process.
- The matte used in generating the H2S is enriched by removal of its iron and sulphur.
Another method of generating H2S which was considered, but not experimented with in connection with the present problem, consists of an adaptation of the Chance process, which is most successfully used for working up the so-called “soda waste,” resulting from the manufacture of soda by the LeBlanc process. “Soda Waste” is largely composed of calcium sulphide (CaS). This is finely ground and made into a thin mud with water and is then decomposed with carbon dioxide, the reaction being as follows: CaS + CO2 + H2O = CaCO3 + H2S.
Experiments were made on this line by one of the writers several years ago and gave quite satisfactory results. The calcium sulphide (CaS) was made by reducing gypsum (CaSO4) with coal in a shaft furnace similar to a lime kiln. The gypsum and coal were formed into bricks with water. It was found that a very fair quality of calcium sulphide could be made in this manner, a yield of about 75 per cent, of the theoretical being obtained.
The calcium sulphide was decomposed by means of gases taken from the reduction furnace. These averaged about 14 to 16 per cent. CO2 and answered the purpose very well. The calcium sulphide absorbs the CO2 with avidity. No heat is required. A train of five cylinders, 1 ft. in diameter by 3 ft. tall, constructed of galvanized iron and connected by pipes and valves in such a way that any cylinder could be made the first (or last) of the series, was used for the decomposition of the CaS. The CO2 gas was forced through the cylinders by means of a small compressor. The gas on leaving the last CaS cylinder was conducted to a similar series of wooden cylinders containing copper solution, no intermediate pumping of the gas being required. The absorption of the H2S was perfect. No odor whatever was perceptible at the outlet of the last cylinder. The conversion of the calcium sulphide into calcium carbonate by the carbon dioxide gas was also perfect. No odor of H2S could be detected when the residue from the tanks was dissolved in dilute hydrochloric acid.
Sponge Iron Copper Precipitation Method
A sample of a concentrate high in iron sulphide was roasted to a calcine assaying: SiO2, 4.3; FeO, 72.2; S, 4.2; Cu, 6.2; Fe, 56.2 per cent.
Two parts of this calcine were mixed with one part of fine Diamondville coal, sealed in a graphite crucible and heated to about 1,700° F. for a couple of hours. The residue had a copper equivalent of 1.77. (The “copper equivalent” is the grams of sponge iron required to precipitate 1 g. of copper from a 1 per cent, copper solution prepared from crystallized copper sulphate.) A series of crucible tests followed, which gave sufficiently good results to warrant the collection and roasting of about 125 tons of the special high iron sulphide for further experiment.
The roasting was done in a 16-ft. six-hearth MacDougall furnace at an average rate of 16.3 tons per day. Oil burners were used on the lower hearths to maintain the temperature so that the roast could be carried below 4 per cent, in sulphur

A retort furnace was built near the leaching plant and operated during October and November, 1913. The equipment consisted of a three-retort coal-fired furnace, an elevation of which is shown in Fig. 1, and three air-tight cars for cooling the sponge. The retorts, similar to those used in gas making, were 8 5/8 ft. long, 15 in. deep, and 2 ft. wide inside, had 3 in. thick walls, and cast-iron fronts with gas connections. The fine reduced iron reoxidized so easily that air-tight cars were necessary for cooling.
The charge consisted of a mixture of 30 parts ground Diamondville coal and 70 parts calcine. The first lot was prepared by grinding the coal, mixing with the calcine and grinding the mixture so that 96 per cent, passed 40 mesh. Later it was found unnecessary to grind the calcine. The coal was ground in a Hardinge mill so that 90 per cent, passed 60 mesh.
The first charge, only one muffle used, was left in for 24 hr. and gave a copper equivalent of 2.3. This was continued for a couple of weeks. The temperature was raised as fast as possible and the pyrometer reading was at 1,600° for at least 4 hr. before discharging. The pyrometer rod was not stuck down in the mixture, but was above it. The results from these charges were uniformly good, the copper equivalent varying from 2.2 to 3.0 g. iron per gram of copper. No special precautions were taken at any time in charging or discharging. The samples of material were taken with a pipe sampler from a car of cooled material. The charge was always dampened to prevent too much dusting. The charges were about 450 to 500 lb. of original mixture. These gave on an average, 275 to 300 lb. of “iron.” One charge during this time was rabbled every half hour for 9 hr. and the temperature worked up as fast as possible. It was discharged at a temperature of 1,500° F. and gave a copper equivalent of 2.7 g. iron per gram of copper.
At the end of the first two weeks it was deemed advisable to try to get more material through the furnace, and a scheme was worked out to give each charge 8 hr. and work them off faster. This proved unsatisfactory. It was not possible to obtain more than 1,300° temperature and the resulting iron was very poor. About this time the muffles began to crack and let air in. This naturally resulted in the production of a very poor grade of iron.
The best iron which was made under the foregoing conditions was a black, dusty material. It contained considerable coke and was quite light. The coarser calcine gave as good results as the ground material. None of the pyrometer readings was over 1,620°. In all cases the pyrome-
ter tube was over and not buried in the mixture. Toward the end of the experiments, the muffles had quite a cake in the lower corners and on the bottom, which seemed to be more or less fused to them, and was impossible to clean out.
It was early recognized that while a good sponge iron could be made in retorts, the fuel efficiency was low and the life of the retorts limited. So it was decided to try reduction in a specially rebuilt MacDougall furnace. The furnace was so equipped that hot partly roasted calcine from another furnace was fed to the first hearth by a steel conveyor. The roasting was completed and the calcine preheated to 1,700° by oil burners on the third hearth. The three lower hearths were equipped with oil-fired muffle floors. Fine coal was fed in on the fourth hearth. The plan was to obtain a mixture of the preheated calcine and fine coal on the lower hearths under strongly reducing conditions such that the reduction would be accomplished. The oil burners directed into the lower hearths worked all right, but those into the muffles, due to the necessarily restricted size of the muffle, localized the flame too much, melting the bricks in the direct path of the flame and depositing carbon on the other portions.
The oil burners were removed and coal firing was tried. Two fire boxes were installed on the third floor, the fine coal being fed on the fourth floor. The addition of this cold coal lowered the temperature to such an extent that the calcine did not reduce, and on increasing the temperature the coal burned off. It seemed impossible to get a high temperature and a reducing atmosphere at the same time on the lower hearths. It was then decided to add another fire box. At first it was connected to the fifth hearth muffle only, but finally to both the fourth and fifth hearth muffles. While increasing the heat in these hearths, the muffles were poor conductors of heat and the piers and floors above burned out. Gas analyses made on the fifth and sixth hearths showed CO2, 15; O2, 2.7; CO, 0.7 per cent., the temperature in the fifth hearth being 1,240° F. The best gas sample showed only 2.0 per cent. CO on the fifth hearth and this for only a short time.
Fine coke was next tried and had the same effect of cooling the calcine. Though added on the third hearth, it practically all dropped down to the fourth hearth before mixing with the calcine. By laboratory experiments it was shown that coke did not have the same reducing action on calcines as coal or charcoal under the same conditions and its use was discontinued. No appreciable reduction was obtained during this period. The product made by the furnace was magnetic, but showed no signs of metallic iron and would not precipitate any copper.
While using two fire boxes the coal burned was 4,000 lb. per 24 hr. When the three were in operation it required 7,500 lb. per 24 hr. The special iron concentrates fed to MacDougall No. 62 at a rate of 26 tons per 24 hr. averaged: Insol., 7.1; FeO, 47.6; and S, 46 per cent. They were roasted to 5 per cent. S.
Owing to the cooling effect of adding the coal dust to the calcine in the furnace, feeding a mixture of coal dust and calcine thoroughly mixed was tried. A mixture of two-thirds calcine and one-third coal dust by weight was fed into the top of the furnace. No difficulty was experienced in obtaining a high heat on the three upper hearths, but the lower hearths were cold. No reduction was obtained, due, probably, to unavoidable leakage of air.
In January, 1914, it was decided to start a new series of experiments. A series of laboratory experiments had been made in December using Diamondville coal, Sunnyside coke, and charcoal as reducing agents. Equally good results were obtained from the coal and charcoal, but practically no reduction from the coke at the temperatures used. The significance of these results was not appreciated at the time, as will be explained later.
The retort furnace was remodeled, a scrap cast-iron hydraulic cylinder 8½ ft. long and 18 in. in diameter, with a hinged door, being installed. This retort was operated for a couple of weeks with a mixture of 30 parts fine Diamondville coal and 70 parts calcine, with indifferent results.
In the meantime it happened that a crucible run was made, in the laboratory, using lumps of coal about thumb size instead of fine coal. To our surprise, excellent results were obtained, A new line of experiments was started in which calcine was heated and then mixed with coarse coal. A charge of calcine was heated to 1,500° in the retort and coarse coal was charged and rabbled in. The results were poor because of the difficulty of rabbling the coarse coal down into the fine calcine. However, some reduction was obtained.
The great disadvantage of the retort method is the low fuel efficiency. This was especially true when Diamondville coal, 38 per cent, volatile combustible matter, was used. The gas formed carried off the heat as rapidly as applied and the temperature could not be raised above 1,000° F. until it was driven off. It was known that efficient reduction did not take place under 1,500° F., after all the gas was driven off; also that while blast-furnace coke was not a reducer at this temperature, charcoal was. So it was suggested that the coke resulting from the coal must be the reducer and that the hardness and character of the coke must be factors in its efficiency.
Diamondville coal assayed in 1912; volatile combustible matter, 38.38; fixed carbon, 45.28; ash, 10.01 per cent. It is not a coking coal, in the commercial sense, coke resulting from it being very soft and friable.
A charge of fist-size Diamondville coal was coked in the retort at 1,400° F. A charge of coke was ground, mixed with calcine, and reduced in the retort with indifferent success. The next day the remaining coke, uncrushed, was charged with calcine into the retort. Conditions seemed to be just right this day and an excellent product resulted. The residue was a gray spongy mass mixed with coarse unconsumed coke and had a copper equivalent of 1.4. It was now decided to preheat coke and calcine under efficient conditions and mix them in an air-tight container.
The retort was shut down and a series of 40 crucible tests made in the laboratory to determine conditions of time, temperature, per cent, of reducer, hardness of coke, possibility of mixing hot, and other details. The results of these experiments showed that if 15 parts of Diamondville coke and 85 parts of calcine could be preheated to 1,800° F., mixed, and maintained at this temperature for an hour, the resulting residue would have a copper equivalent of 1.5.
A double crucible furnace to hold two No. 50 graphite crucibles was built. Oil burners were used for heating. The mixer was a sheet-iron shell 2 ft. deep and 20 in. in diameter lined with 4½ in. of fire brick. The mixer was provided with an air-tight cover and three tuyeres, and placed on trunnions so it could be dumped. Coke was burned in the mixer to preheat it, air being supplied through the tuyeres. When the charge was ready the coke was dumped from the mixer and the tuyeres closed.
Charges of calcine and coke were heated in the crucibles to between 1,700° and 1,800° F. and dumped together into the mixer. There was too much cooling in this manipulation and poor results were obtained. Putting the coke in the mixer and heating it by burning a part of it, using air through the tuyeres, was tried. This failed, due to the difficulty of getting the coke heated to a uniform temperature throughout. However, a copper equivalent of 3.2 was obtained from a picked sample.
It was then decided to build two small hand-rotated, oil-fired, brick-lined cylinders for preheating, from which the charges could be very quickly drawn into the mixer. The soft Diamondville coke was heated in one cylinder and the calcine in the other cylinder to 1,800° F. The charges were so small, however, that when they were transferred to the mixer they lost about 300° F. in temperature. The resultant mixture, 1,500° F. at the start, cooled rapidly and very poor results were obtained.
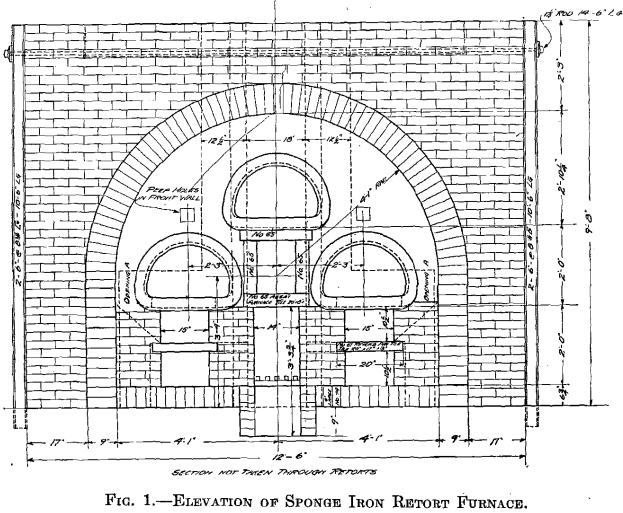
Heating a mixture of the coke and calcine in one cylinder to 1,800° and then quickly sealing the ends, gave a product with a copper equivalent of 2.35. This was so promising that a larger furnace of the Bruckner type was made by lining a section of a White Howell furnace, 5 ft. in diameter and 12 ft. long, with 4½ in. of fire brick about the middle and a 30-in. brick wall in each end, with a 12-in. opening in one end for an oil burner and an 18-in. opening in the other for a stack connection. A 6-in. charging and discharging door was put in one side midway between the ends, and a power drive arranged which gave 1.28 rev. per minute. This gave a furnace 4 ft. 3 in. in diameter inside and 7 ft. long, with a capacity of 2,000 lb. of charge. Fig. 2 is a photograph of this furnace, including the oil burner.
The sheet-iron box around the middle of the furnace is stationary and makes a reasonably air-tight space in which the sponge iron can be discharged without excessive oxidation. The discharging door revolves inside the box and the iron falls into a rapid stream of water in a launder below, where it is quenched and washed into a collecting box. Complete data on this furnace have not been obtained. However, the results obtained so far are satisfactory and no difficulties are apparent which will prevent the development of the process to a commercial basis.
The furnace is operated as follows:
1,400 lb. calcine are charged and heated with a fuel-oil flame to about 1,300° F. This requires about 1¼ hr. In a commercial plant the calcine would be drawn hot directly from the MacDougall hoppers to the furnace. About 600 lb. of coal are then shoveled in through the front, in small lots. The furnace continues to revolve and in about ¾ hr. after starting to charge the coal the hydrocarbons are burned off. The oil flame is again started. In 1¾ to 2 hr. the charge is up to 1,680° to 1,700° and reduction is complete. The discharging door is removed and the charge quenched.
We are making sponge iron with a copper equivalent of 1.5 on samples screened through a 14-mesh screen. The coal used amounts to 40 per cent, of the weight of the calcine. It is probable that this percentage
can be reduced. Coke to the extent of 25 per cent, of the weight of the coal is recovered by screening the product discharged from the furnace. This coke can undoubtedly be used again. The quenching works well, the quenched product averaging 1.7 copper equivalent. The best results are obtained at a temperature of 1,680° to 1,700° F. If the temperature is carried much above 1,700° F. there is a tendency for the charge to nodulize. The fuel consumption for heating, 20 gal. fuel oil to a ton charge, is very reasonable when it is considered that the process is intermittent, the scale is small, and the apparatus is still in the experimental stage. We expect to present a paper later giving a full account of this process.
Sulphur Dioxide Copper Precipitation
When cupric copper in solution is reduced with SO2, in the presence of chlorides, there is a precipitation of cuprous chloride, the amount of
precipitation depending on the solubility of the cuprous chloride in the solution. The reaction is, 2CuCl2 + SO2 + 2H2O = Cu2Cl2 + H2SO4 + 2HCl.
According to this reaction the equivalent of 1.54 lb. of H2SO4 is regenerated per pound of copper reduced. Actually, from our solutions, 2 to 2.5 lb. of acid per pound of copper reduced are regenerated, due to reduction of ferric salts and a catalytic action of the cuprous chloride. Either pure SO2, gaseous or liquid, or a gas containing 10 per cent. SO2 by volume, can be used for the reduction. A gas containing less than 8 per cent. SO2 by volume does not give satisfactory results. The most satisfactory conditions for the reduction and precipitation of the copper, so far as we have determined them, are saturation of the cold solutions by passing the SO2 gas through at 15 lb. pressure per square inch, heating to boiling under 20 lb. pressure per square inch and then cooling to 60° to 70° F. The
cuprous chloride separates as a heavy white crystalline precipitate which settles readily.
Three and one-half tons of the 2 per cent. Cu solution carrying 8.5 per cent. NaCl have been precipitated in 500-lb. lots. An average of 80 per cent, of the copper and 100 per cent, of the silver was precipitated, giving a tail solution carrying 0.4 per cent. Cu and 4.0 percent. H2SO4, the cuprous chloride being soluble in this 8.5 per cent. NaCl solution to the extent of 0.4 per cent. Cu.
The first experiments were made in a lead-lined iron auto-clave, holding about 700 cc. The solution was saturated under 10 lb. pressure per square inch with pure SO2 gas, obtained from cans of liquid SO2, transferred to the auto-clave, and heated to boiling under 10 lb. pressure per square inch. On a leaching-plant solution carrying 1.5 per cent. Cu a 71 per cent, precipitation was made.
The copper solution used in the following experimental work contained: Cu, 2.0; Fe2O3 + Al2O3, 4.6; NaCl, 4.8 to 8.5; H2SO4, 0.5 per cent.; Ag, 0.45 oz.
A lead-lined pressure tank 1 ft. in diameter and 2 ft. deep, with a lead heating coil, was used. The copper solution from the 2,000-ton sands-leaching plant will have about this analysis and carry 8.5 per cent. NaCl. The solution was saturated under pressure, which was maintained during the heating stage. At first pure SO2 was used. Later 10 per cent. SO2 gas made from pure SO2 and air was tried. After the details of manipulation were worked out a 90 per cent, precipitation was made with no trouble. Following are the data of one of these runs:
Volume solution, liters…………………………………26.5
Per cent. Cu in head solution………………………….2.0
Per cent. NaCl in head solution………………………4.8
Per cent. Cu in tail solution……………………………0.2
Per cent, acid in head solution……………………….0.6
Percent, acid in tail solution…………………………..4.0
Per cent. SO2 in gas……………………………………..10.0
Saturation time in hours………………………………..3.0
Heating time in hours……………………………………4.0
Final temperature in degrees F…………………….196.0
Saturation pressure in pounds……………………….15.0
Heating pressure in pounds…………………………..20.0
Per cent, precipitation…………………………………..90.0
Note.—Later by a change of steam connections the heating was accomplished in ½ hr.
Experiments were made on the operation of a MacDougall roaster to give a 10 per cent. gas. The furnace was run hot on a heavy feed of undried fine concentrates. The gas, obtained without difficulty at 10 per cent., was drawn from the second hearth by a small compressor, and compressed to 90 lb. in a 5-cu. ft. oxygen cylinder. This gas gave as good results as the mixture of pure gas and air.
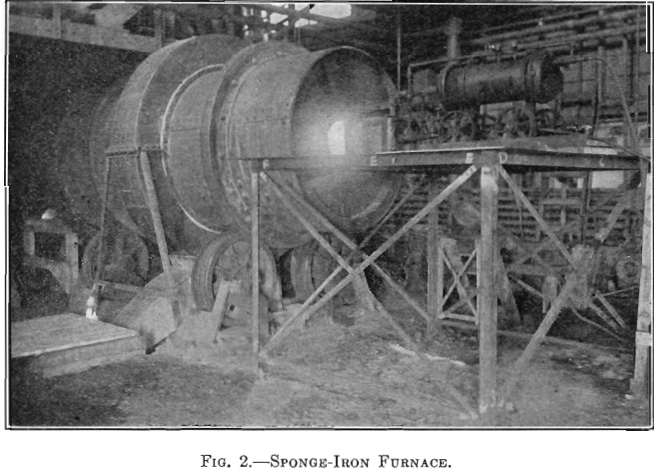
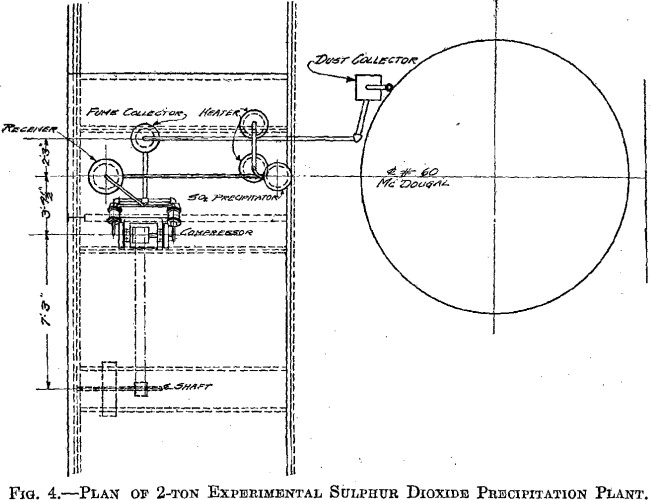
The success of the experiments mentioned warranted a trial of the method on a larger scale, 500 lb. to the charge. The installation shown in Fig. 3 was put in at the MacDougall building. It consists of a water- jacketed cooling pipe from the furnace, a dust catcher, a bag box, a small compressor, a receiver, the absorber, and two heaters. The general plan is shown in Fig. 4. A section of the absorber and one of the heaters is shown in Fig. 5. The absorber is made of sections of lead-lined 1-ft, diameter pipe with perforated diaphragms between sections. It holds about 500 lb. of solution. The heaters are lead-lined cylinders 4 ft. long and 1 ft. in diameter. A woolen bag is used to remove the fine dust and fume. The residue in the bag consists of fine dust, elemental sulphur arsenic, and sulphuric acid, and is very corrosive, the bags lasting only about 6 hr.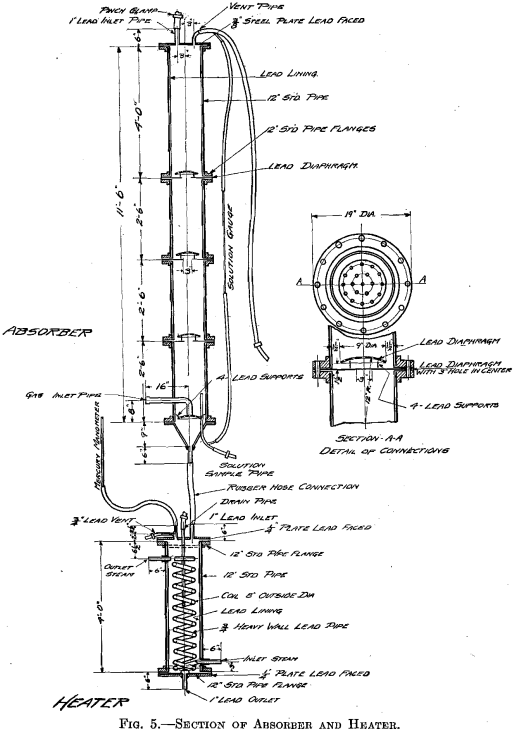
The absorber is charged with about 500 lb. of 2 per cent, copper solution. The SO2 gas is forced in under the lowest diaphragm. A pressure of 15 lb. per square inch is maintained by regulation of the vent. The gas has been passed through at rates varying from 0.8 to 6.1 cu. ft. per minute. The absorption of the SO2 gas is almost complete for the first hour, and then gradually decreases to about 60 per cent. The solution changes from the blue cupric color to a greenish-brown color, and some Cu2Cl2 is precipitated. Samples are taken every hour, and after 2.5 to 3 hr. a small portion boiled in an Erlenmeyer flask and cooled has a characteristic appearance when the reduction is completed, which we have learned from experience indicates that the charge is ready for heating. The charge is drawn off in 125-lb. lots to the heaters. The heating time averages 35 to 40 min. The pressure is maintained at 20 lb. After 35 to 40 min. the pressure is gradually reduced and the charge allowed to boil several minutes. The four heats from one absorber charge are drawn into a barrel, and after cooling over night the supernatant solution is sampled for copper.
The first 18 runs were made on a solution running 2 per cent. Cu and 4.8 per cent. NaCl. An average precipitation of 90 per cent, of the copper was obtained. Then it was decided to increase the salt content of the solution to 8.5 per cent., the percentage which will be carried in the solutions from the 2,000-ton sands-leaching plant.
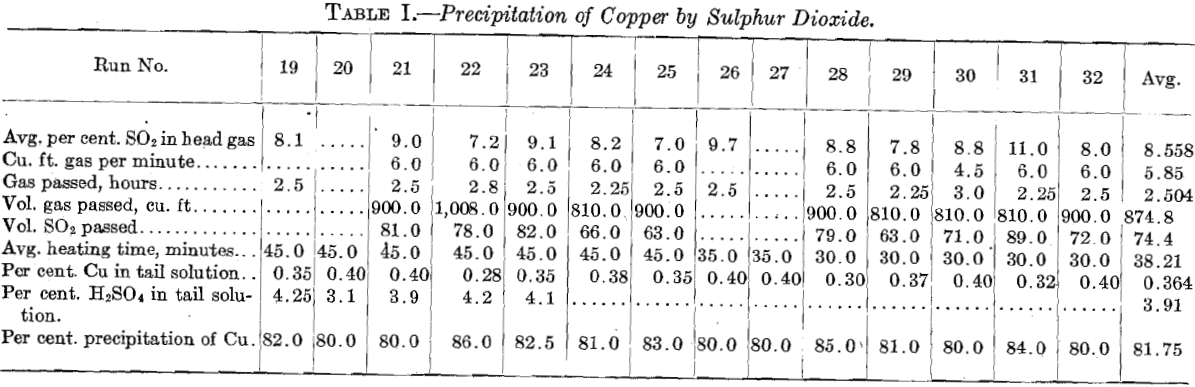
Table I shows the data of 14 runs. In all these runs, 500 lb. of copper solution assaying 2 per cent. Cu, 8.5 per cent. NaCl, and 0.5 per cent. H2SO4 was used. The saturation pressure was 15 lb. and the heating pressure 20 lb. per square inch. The absorption of the SO2 gas varied from 60 to 75 per cent. The average copper content of the tail solutions was 0.36 and the average precipitation 81.7 per cent.
A series of runs were made with varying salt percentages. Other conditions were about the same as those given in the table. Extra salt was added to the regular leaching plant solution to bring it up to the desired salt content.
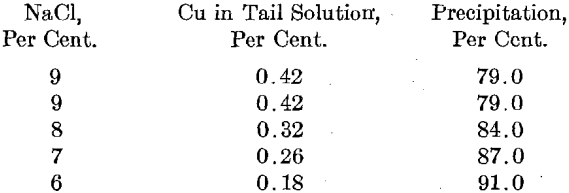
The best precipitation ever obtained was on a laboratory experiment. The tail solution carried 0.14 per cent. Cu and the precipitation was 93.0 per cent.
The operation of this plant was highly satisfactory. The only trouble experienced with the plant proper was the failure of the heater linings.
The heaters were originally lined with old 6-lb. lead, and both failed. The first one which failed, after relining with new 8-lb. lead, served for 48 heats and then showed no appreciable deterioration.
Reduction of Cuprous Chloride
There are three methods of reduction of the cuprous chloride which have been worked out to some extent and which seem capable of development to a commercial basis.
- Precipitation with sponge iron.
- Reduction with coke with limestone present to flux and hold the chlorine.
- Electrolytic reduction.
Precipitation with Sponge Iron
Sponge iron, when mixed with cuprous chloride 40 to 50 per cent, solids, gives a rapid and complete precipitation, with the evolution of considerable heat. The reaction is, Cu2Cl2 + Fe = 2Cu + FeCl2. It is evident from this reaction that only one-half as much iron is required, to precipitate the copper from cuprous chloride as from a cupric salt. A sponge iron with a copper equivalent of 2.30 on a copper sulphate solution gave a copper equivalent of 1.20 on cuprous chloride. The cement copper from the precipitation of copper from cuprous chloride with sponge iron is rather granular, settles readily and washes easily. A sample made from sponge iron with a 1.20 copper equivalent on cuprous copper assayed 56 per cent. Cu and 0.1 per cent. Cl.
The precipitation of the copper from solutions by the SO2 method followed by the treatment of the cuprous chloride with sponge iron makes an effective combination. Concentrates may be roasted in a suitable furnace to make the required SO2 gas, and the hot calcine drawn directly from the hoppers beneath the roasting furnace into the revolving furnace for reduction to sponge iron. In a suitable furnace the concentrates can be roasted to under 4 per cent. S and 10 per cent, gas produced. It would figure about as follows: 100 tons concentrates assaying, Cu, 5.3; insoluble, 6.4; FeO, 48.6; Fe, 37.8; S, 44.8 per cent., wall roast to a calcine assaying, Cu, 8.4; insoluble, 10.6; FeO, 62.6; S, 3.7 per cent. About 40 tons sulphur or 80 tons SO2 are available. About 60 per cent, of this SO2, or say 50 tons, can be absorbed in precipitation of the copper. The reaction is, 2CuCl2 + SO2 + 2H2O = Cu2Cl2 + H2SO2 2HCl. It is evident from this reaction that 50 tons SO2 will precipitate about 100 tons copper.
The original concentrates contain 37.8 tons iron, of which 80 per cent, is available, for precipitation of copper, in the form of a sponge iron with a copper equivalent of 1.5 to 1.6 on copper sulphate solution and 0.8 to 0.9 on cuprous copper. Cuprous copper requires 0.44 lb. pure iron per pound of copper for precipitation. 37.8 x 0.80 ÷ 0.44 = 68.7 tons copper precipitated. This gives a comfortable margin of SO2 for precipitation and any excess can be easily wasted. The above calculation is rather rough, because, as before mentioned, the complete data of the sponge iron process are not yet worked out, but it is probable that it is not far off.
The combination of the methods has these advantages:
- The conversion of a large part of the SO2 from the roasting process directly into sulphuric acid in the leaching solutions, accompanied by the precipitation of the copper in a concentrated form, from which it can be recovered by precipitation with the sponge iron resulting from the reduction of the calcine.
- The use of only one-half as much iron to precipitate the copper from the cuprous form as from the cupric, and hence the return of only one-half as much iron into the leaching solutions to foul them.
- The precipitation of the copper in a concentrated condition by a simple process, in a form easily washed and handled.
- The possibility of the treatment of the ferrous chloride which results in such a way that the valuable chlorides can be returned to the leaching solutions unaccompanied by iron.
Reduction with Coke in the Presence of Limestone
By this method the cuprous chloride is directly reduced to metallic copper, by a furnace process, with the production of a calcium chloride slag from which the chlorides maybe leached and returned to the leaching process. Cuprous chloride is very volatile at high temperatures. However, by having fine limestone about 10 to 15 per cent, in excess of the theoretical requirements, in intimate mixture with the coke and cuprous chloride, it is possible to accomplish reduction with a loss of not more than 5 or 6 per cent, of the copper.
A mixture of 100 parts of slightly moist cuprous chloride, 65 parts limestone, and 10 parts coke was melted in a No. 50 graphite crucible and the melt poured into a sand mold. The button assayed 97 per cent, and the slag 0.88 per cent. Cu; 93.1 per cent, of the copper was recovered in the button and 1.4 per cent, in the slag, making a total recovery of 94.5 per cent. The slag, which carried 86 per cent, of the original chlorine in the cuprous chloride, and assayed 35 per cent. Cl, was leached for 2 hr. with warm water; 56.6 per cent, was soluble and the residue assayed 1.6 per cent. Cl. This indicated a recovery of 98.7 per cent, of the chlorine in the slag and 84.9 per cent, of the original chlorine in the cuprous chloride. This recovery was probably low, due to loss of slag in cleaning up. The insoluble, consisting mostly of lime and carrying some copper, settled readily and could be worked up in the smelter process for its copper value without trouble.
Briquettes were made of a mixture of 100 parts moist cuprous chloride, 75 parts limestone, 10 parts coke, and 5 parts cement, by hand pressure, and with a testing machine at 2,500 to 5,000 lb. per square inch, in a die 3 in. in diameter. Excellent briquettes resulted in all cases, assaying about 26 per cent. Cu. A charge of briquettes was melted down in a small reverberatory about 12 in. wide and 20 in. long, using the flame of an oil burner for heating; 94.1 per cent, of the copper was recovered, 87.6
per cent, in a button and 6.5 per cent, in the slag. A charge of briquettes was also fused in a small cupola; 93.9 per cent, of the copper was accounted for, 90.8 per cent, in the button, which assayed 96.1 per cent. Cu, and 3.1; per cent, in the slag, which assayed 1.65 per cent. Cu.
In all cases there was considerable fume given off and the flame had a characteristic bluish color. It is very probable that with a suitable installation 95 per cent, of the copper could be recovered in metallic form and in the slag, and that 75 per cent, of the volatile copper could be caught in a Cottrell treater or a milk of lime absorption tower.
Electrolytic Reduction
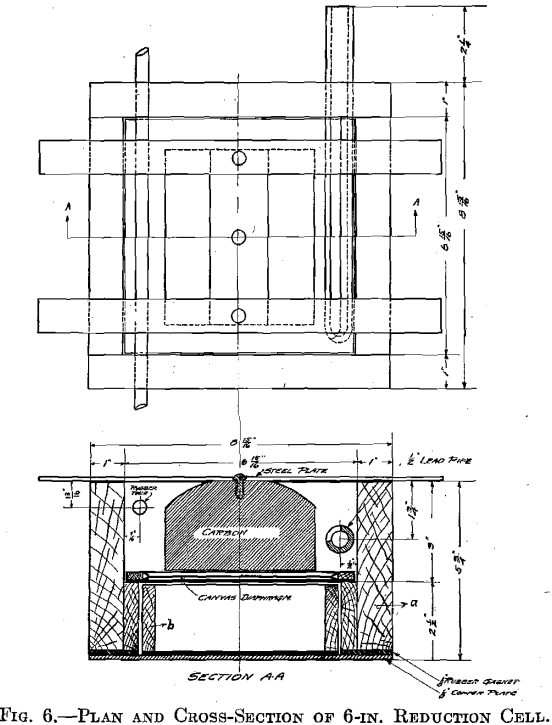
The reduction of the copper from cuprous chloride by electrolysis requires only one-half the current which would be required to precipitate it from the cupric form. However, it is difficult mechanically, because the cuprous chloride is a solid and the strongly oxidizing gases given off at the anode must not come in contact with it. This makes necessary the use of a horizontal diaphragm cell and a circulating depolarizing solution. The solution resulting from the SO2 precipitation after the cuprous chloride settles out is strongly reducing and makes an admirable depolarizing solution, which on oxidation gains the equivalent of about ¾ lb. of sulphuric acid per pound of copper reduced. This makes the combination, of this method with the SO2 precipitation method very attractive. The early experiments were made in a cell 6 in. square, shown in plan and section in Fig. 6. It consisted of a wooden box (a) with a copper-plate bottom; a tray (b) with wooden sides and a sheet-copper bottom; a canvas diaphragm; a graphitized carbon anode; and intake and outlet pipes for the depolarizing solution. The cuprous chloride, filtered and washed, was mixed with about 10 per cent. NaCl, which made it thin enough so it could be readily poured into the tray. The reducing solution was circulated until there was a noticeable smell of chlorine from about the anode. As the run approached completion there was considerable evolution of gas from under the diaphragm. Part of the copper was deposited firmly adhering to the starting sheet and part in a loose form. The data of one of these runs were:

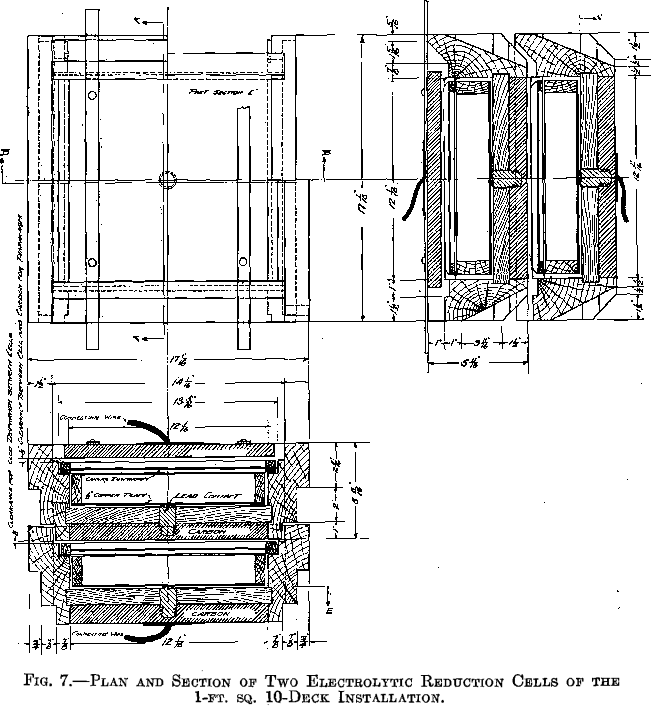
A combination of three cells similar to those shown in Fig. 7 was then tried. Graphitized carbon immersed electrodes were used, the anode of one cell serving as the cathode of the one above it. Each cell had its own circulating arrangements.
The data of the run were:
Inside dimensions of trays, inches……………………………………..1 by 4 by 4
Depth of cuprous chloride, inches…………………………………………..1.0
Total time of passing current, hours……………………………………..36.16
Average amperage………………………………………………………………..2.41
Average voltage…………………………………………………………………….7.46
Average voltage per cell…………………………………………………………2.49
Current density, amperes per square foot……………………………….21.72
Theoretical amount of copper deposited, grams………………………620.1
Copper deposited:
Adhering to starting sheet, grams…………………………………………….256.0
Loose, grams………………………………………………………………………….193.7
Assay of copper:
Adhering to starting sheet, per cent………………………………………….95.18
Loose, per cent……………………………………………………………………….94.04
Pure copper deposited, grams………………………………………………….425.8
Current efficiency, per cent……………………………………………………..68.66
Kw-hr. per pound of copper…………………………………………………….0.695
Volume depolarizing solution, liters………………………………………….18.74
Avg. acidity head depolarizing solution, per cent. H2SO4…………….4.07
Avg. acidity tail depolarizing solution, per cent. H2SO4………………5.64
It was then decided to try the method on a larger scale. Ten cells as shown in Fig. 7 were built and set in a frame so that a cell would slide out from over the one below it, exposing the tray of the lower cell for filling or removal. The cells were set on an incline of 3/8 in. to the foot, the overflow side being the high side. This made unnecessary the use of a dam on the overflow side to bring the solution in contact with the anode surface.
As yet the cell has not been worked out to an entirely satisfactory basis, but it seems capable of development.
How to Precipitate Copper from Solution
At present it seems advisable to precipitate the copper directly from the copper solutions of the 2,000-ton sands-leaching plant with sponge iron. The precipitation of the copper from such solutions with sponge iron is being worked out and promises to be simple. Until the leaching process is established on a definite operating basis it does not seem advisable to introduce a complicated precipitation method.
The acid plant now building will have ample capacity for the first 2,000-ton leaching unit. When the time comes to build further units, the installation of an SO2 precipitation plant will depend on considerations of first cost of acid and precipitation plants, cost of operation and other factors which undoubtedly will develop.
