Table of Contents
Throughout this century the major production of copper (as it relates to Copper Smelting) and most other nonferrous metals has been through pyrometallurgical methods, treating sulfide ores by various batch processes. The predominant copper process is reverberatory furnace smelting of sulfide concentrates to produce matte and slag, followed by treatment of the matte in converters to form blister copper. The offgases from the reverberatory furnace are usually not more than 2 percent SO2, and of varying SO2 content from the converters, precluding economic sulfur recovery in most cases.
Flash Copper Smelting to matte has been a process improvement resulting in higher SO2 concentration. In the Outokumpu flash smelting process developed at Outokumpu Oy in Finland, the furnace charge is blown into the furnace through a specially designed burner with air preheated to approximately 850° C. The heat from the reactions supplies a large part of the total heat requirements. An SO2 gas concentration of about 13 percent is obtained. The International Nickel Company’s flash smelting process at Sudbury, Ontario, utilizes commercial oxygen (95 percent O2) to produce a 45-percent copper matte and an SO2 gas consistently over 70 percent concentration. The latter is known as an autogenous (self-smelting) process since no extraneous fuel is used to smelt the remainder of the charge to matte and slag. In both of these flash smelting processes, the matte is treated in converters.
Two recent processes, Noranda and Worcra, utilize flash smelting and conversion to blister copper in a single unit. Both use air or oxygen-enriched air and extraneous fuel to supply heat requirements. The Noranda process involves the following basic steps :
- Concentrates and flux are introduced in one end of the reactor which is similar in cross section to a Pierce-Smith converter;
- smelting takes place at the feed end;
- matte and slag flows are controlled as they move slowly to tapping ports ;
- oxidizing gas is introduced into the matte to oxidize the FeS;
- continued injection of the gas into the resulting white metal oxidizes the Cu2S to metallic copper; and
- metallic copper is tapped periodically after settling periods.
The Worcra process, invented by Dr. Howard K. Worner, differs significantly from the Noranda process, as follows:
- The slag flow is countercurrent whereas it is concurrent in the Noranda system;
- air or oxygen-enriched air is blown through the slag at high pressure to contact the matte bath for conversion to copper, whereas Noranda uses tuyeres;
- copper flow is essentially continuous from a Weir, whereas in the Noranda system copper is tapped periodically after settling periods; and
- slag is cleaned by passing through the low oxygen potential feed zone whereas reducing gas tuyeres are used in the Noranda furnace.
The process described in this report, continuous oxygen smelting (COS), differs from the previously described processes principally as follows:
- It is a continuous process utilizing commercial oxygen to produce blister copper in a single unit;
- a very high, over 80-percent-concentration (low-flue-volume) SO2 gas is produced; and
- the converter oxygen is introduced below the surface of the matte by means of an immersed probe.
Copper Smelting Process
In the autogenous system described by this report, sulfide concentrate and silica flux are blown into the furnace through a specially designed burner with oxygen and is flash smelted to about 50-percent copper matte. The matte flows down an inclined crucible, countercurrent to the slag flow, to the converting zone at one end of the furnace. Oxygen is introduced by means of a water-cooled probe immersed into the matte bath where conversion to blister copper proceeds.
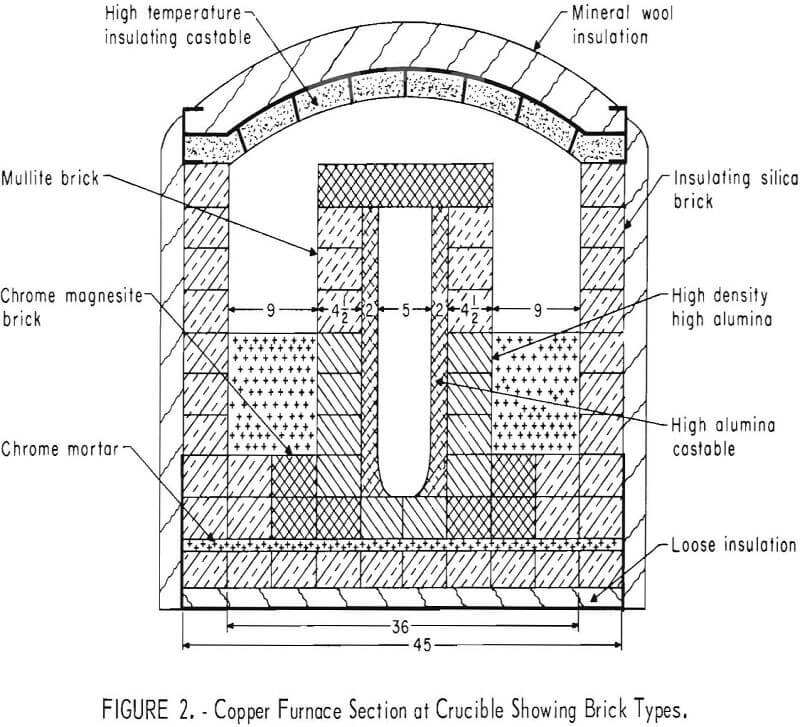
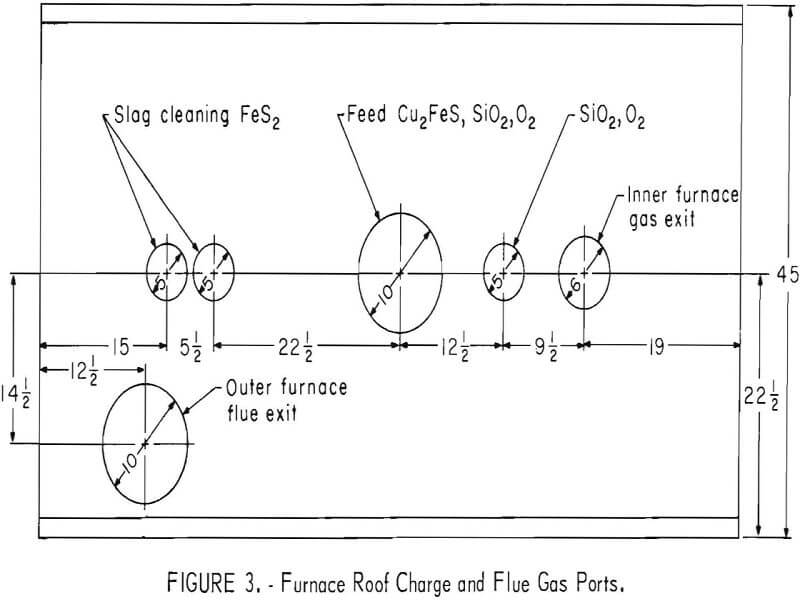
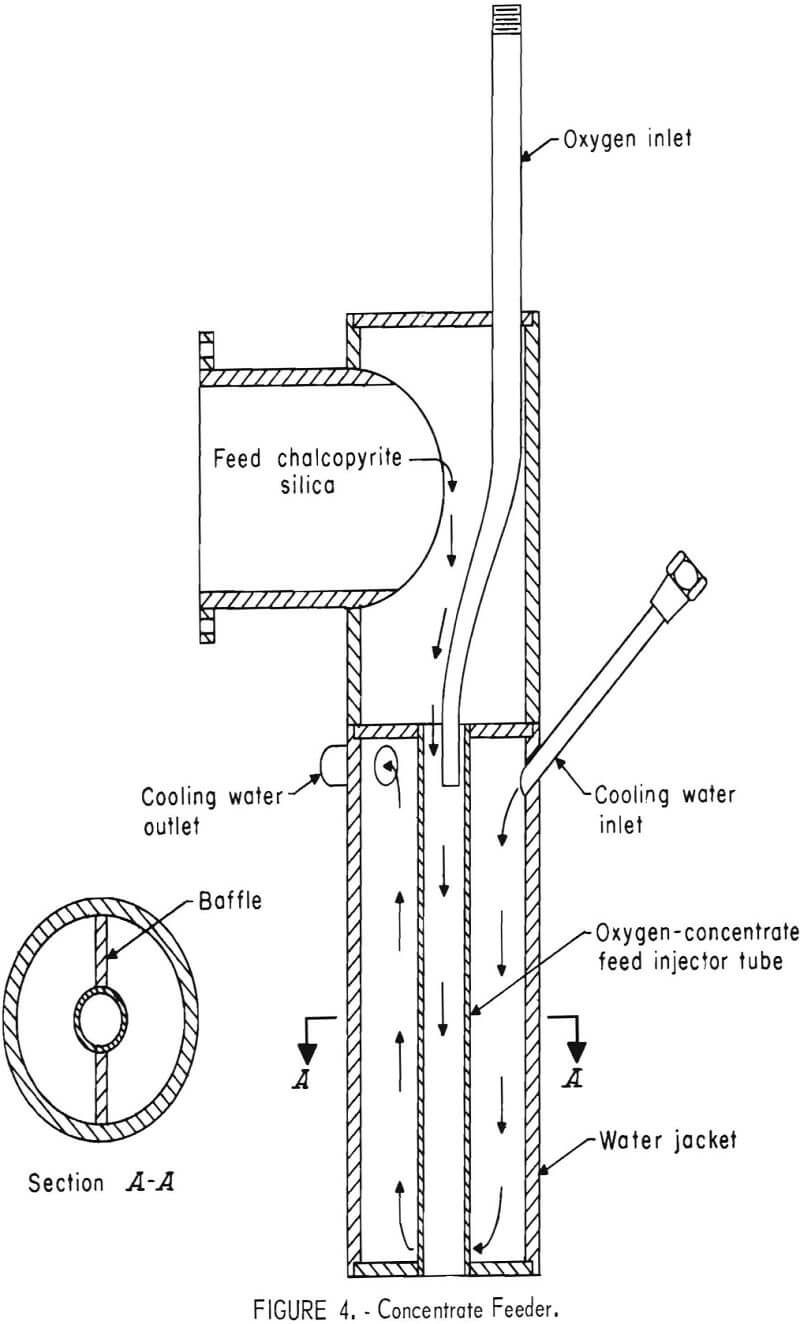
The ratio of heat loss to input is inherently large in a small furnace and since it is desirable to maintain an atmosphere undiluted by outside contaminants, with only the products of reaction present, a double furnace was designed, as shown in figures 1 and 2. The outer furnace serves as a thermal barrier. A long flame from a hydrocarbon burner passes hot gases around the outer furnace. These gases discharge through a water-cooled flue into a water scrubber. Gases from the inner furnace pass out another water-cooled flue, through the roof, and also into a water scrubber. Figure 3 shows the positions in the roof where the various products are introduced into the furnace and where the gas exits. Chalcopyrite concentrate, dried and screened to remove large lumps, along with silica flux, is fed by screw from a hopper into the concentrate feeder (fig. 4). The feed is mixed with oxygen in the water-cooled section of the concentrate feeder and is blown into the furnace at position one (fig. 1) where a combination of flash roasting and smelting occurs.

The matte, about 45 to 55 percent copper and the remainder iron silicate slag, is formed in a very fast, almost instantaneous reaction; hence the name “flash smelting.” The matte sinks through the lighter slag layer and flows down the
inclined crucible. Conversion to copper proceeds in a zone around position two. Ferrous sulfide is oxidized preferentially since the affinity of oxygen is greater for sulfur and iron than it is for copper. The system is in a dynamic state with copper, white metal, and matte coexisting, and new matte being continuously formed in zone 1. The relative amounts of each is a function of the converting and feed rates. Oxygen is introduced directly into the matte bath at position two by an immersed probe. The probe provides for much better gas liquid contact and results in at least 50-percent better oxygen efficiency, according to comparative tests. Cleaner slag is produced as well, since the copper content of the slag is directly related to its oxygen potential. Silica is added close by the oxygen probe to obtain dispersion and good contact with the new ferrous oxide formed. It is generally believed that, without silica immediately available to form silicates, the ferrous oxide will further oxidize to magnetite and build up in the cooler portions of the furnace. Unfortunately, even with a large silica deficiency, the high temperature and availability of ferrous sulfide prevents magnetite deposition in the converting zone where it is desired to offset refractory loss. A cooler wall to effect magnetite deposition can be attained in the experimental furnace by providing a larger converting zone, or in a commercial furnace by water-jacketed walls.
Slag formed in the smelting and converting reactions flows to the slag well. Slag cleaning occurs as a result of contact with lower grade matte in passing through zone 1, by the roast reaction of copper sulfide and copper oxides by the reduction of copper oxide by sulfur and ferrous sulfide, and by the reduction of magnetite.
Since there is a small amount of molten material in relation to the heat conducting surface inherent to a small furnace, a carbon arc is used to prevent freezing and to maintain slag and matte flow out of the furnace. The stationary electrode is a graphite rod inserted into each taphole. Movable electrodes contact the melt in the respective wells. The slag is heated by a combination of resistance heating and arcing, whereas the copper, owing to its high conductivity, is heated only by arcing at the surface. Thus the wells are easily kept open and continuous flow of the slag product is maintained. Some matte results from slag cleaning and from the settling of entrained matte which can be intermittently tapped and returned to the converting section of the furnace.
Injection of oxygen through tuyeres into the matte was discontinued owing to the rapid erosion of the refractory at the tuyere inlet, and thus the extreme difficulty in keeping the tuyere open.
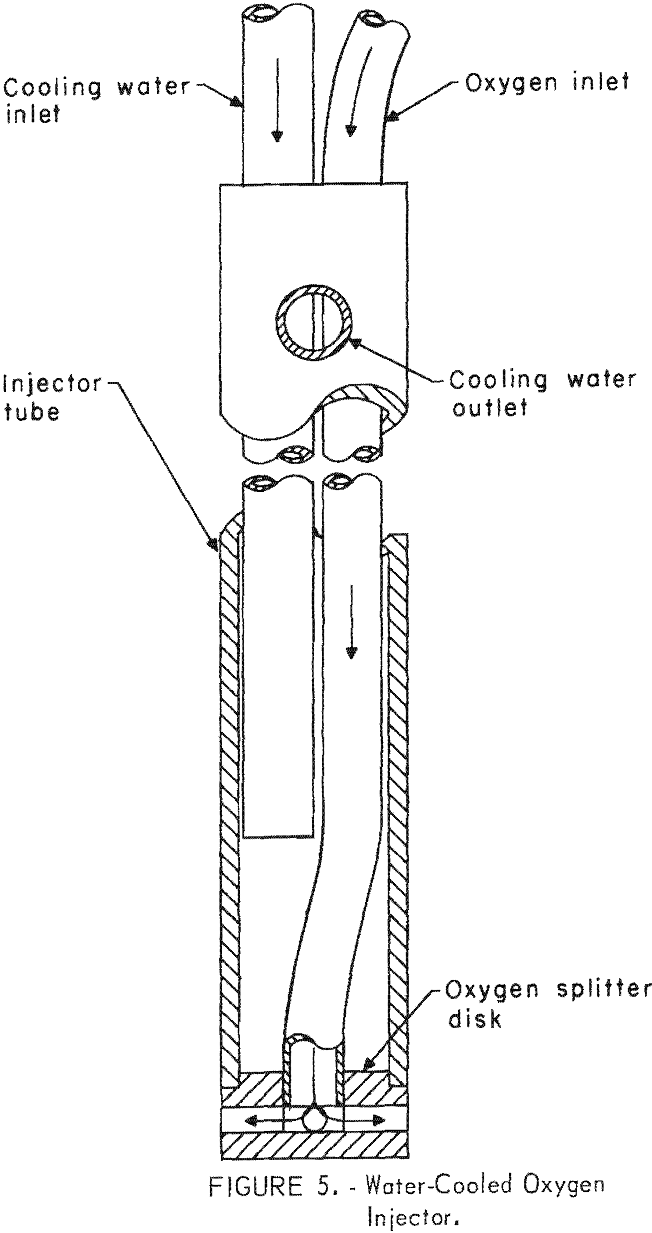
A water-cooled probe, shown in figure 5, was designed to inject oxygen directly into the matte, in contrast to blowing a high velocity jet through the slag, as is done in the Worcra process. This procedure leads to the advantage of improved gas-matte contact, increased rate of reaction, and total utilization of the oxygen. Other advantages are that heat is concentrated in the matte, where needed, rather than above the slag, therefore minimizing refractory consumption above the slag level. Also, since the oxygen does not pass through the slag, the slag will remain at a lower oxygen potential thus lowering its copper content. Oxygen is delivered to the base of the probe by a copper tube, where it is split as desired according to the number and position of the holes in the oxygen splitter disk, and enters the bath at right angles to the probe. This design aids in oxygen distribution and enables positioning the probe near the bottom.
Heat Balance Using Pure Oxygen Versus Air
A simple heat balance has been prepared in table 1 to illustrate the large amount of heat available in oxygen smelting of chalcopyrite concentrates. An estimated 24 percent of the heat produced is made for furnace losses including water cooling.

If, for example, air had been used in place of 95-percent pure commercial oxygen, the 0.435 kg oxygen per kilogram of chalcopyrite would be diluted with 1.43 kg nitrogen which would extract 1.43/28 x 9,790 = 501 kcal; thus, total losses would be 258 kcal greater than that produced. Consequently, the use of air rather than oxygen would require extraneous fuel.
Copper Smelting Furnace Construction and Refractories Used
The furnace is built in three integral sections comprising the bottom and inner furnace, the four outside walls, and the roof, providing ease of assembly and disassembly and relief from different expansion characteristics of these sections. The roof and sidewalls can be lifted separately in one piece for easy access to the inner furnace. Figure 2 shows the different brick types used. A standard mineral wool insulation covers the entire exterior. The arched roof is made of high-temperature castable refractory over a metal plate to form the underside. The walls are insulating silica brick set in an angle iron frame and supported by I-beam uprights and channel backstays. The various ports in the roof between the inner furnace and outer furnace are

formed with a magnesia ram mix. The bottom is brick laid into a metal pan, with the inner furnace built integrally with the bottom. High density, high alumina brick surround the crucible, which provides maximum resistance of any refractories tested to the corrosive matte.
Test Procedure and Discussion of Results
The experimental continuous oxygen smelting (COS) furnace, shown in figures 6 and 7, is preheated prior to charging by three propane gas burners located in the slag end of the outer furnace, through the slag cleaning (FeS2) roof port, and through the matte taphole. An inside furnace temperature of 1,200° to 1,300° C is attained, with an outer furnace temperature of about 400° to 700° C measured at the flue outlet. The outside wall of the inner furnace becomes hottest around the matte crucible, reaching 800° to 1,000° C. About 100 pounds of matte are premelted to seal the copper well. Since the matte must be at least 1,250° C before insertion of the water-cooled probe, it is heated initially to 1,400° C before being transferred manually in pots to the COS furnace. An arc is struck on the matte surface immediately after charging to prevent freezing in the well. The 8 kw of power required initially is gradually decreased to 3 to 4 kw.

After charging the matte, the feed is immediately begun. The feed consists of copper concentrate with added silica flux. Assays of the concentrate and mineral composition are shown in tables 2 and 3.
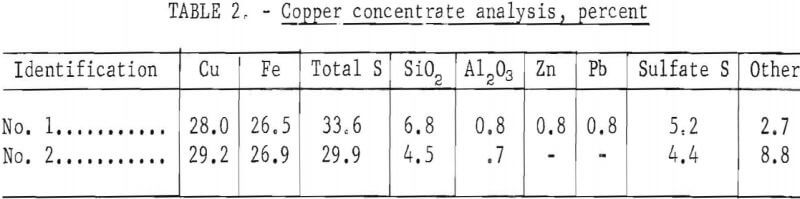

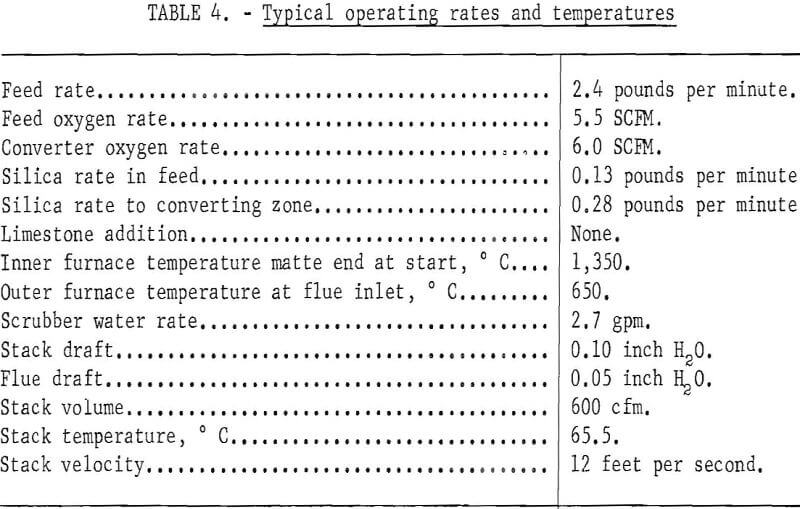
Conversion is begun after smelting about 1 hour in order to build up a pool of matte and slag and to heat the matte to about 1,250° C. The inner inside-furnace burner is removed and the outside-furnace burner is adjusted down according to heat requirements. The pool level in the crucible is allowed to build up to obtain slag flow and is held at this level. It was found that it is not necessary to seal the slag well as long as the residual slag in the well is premelted by the electric arc. Slag flowing over the crucible lip into the slag well is about 1,250° to 1,300° C. Typical operating rates are shown in table 4.
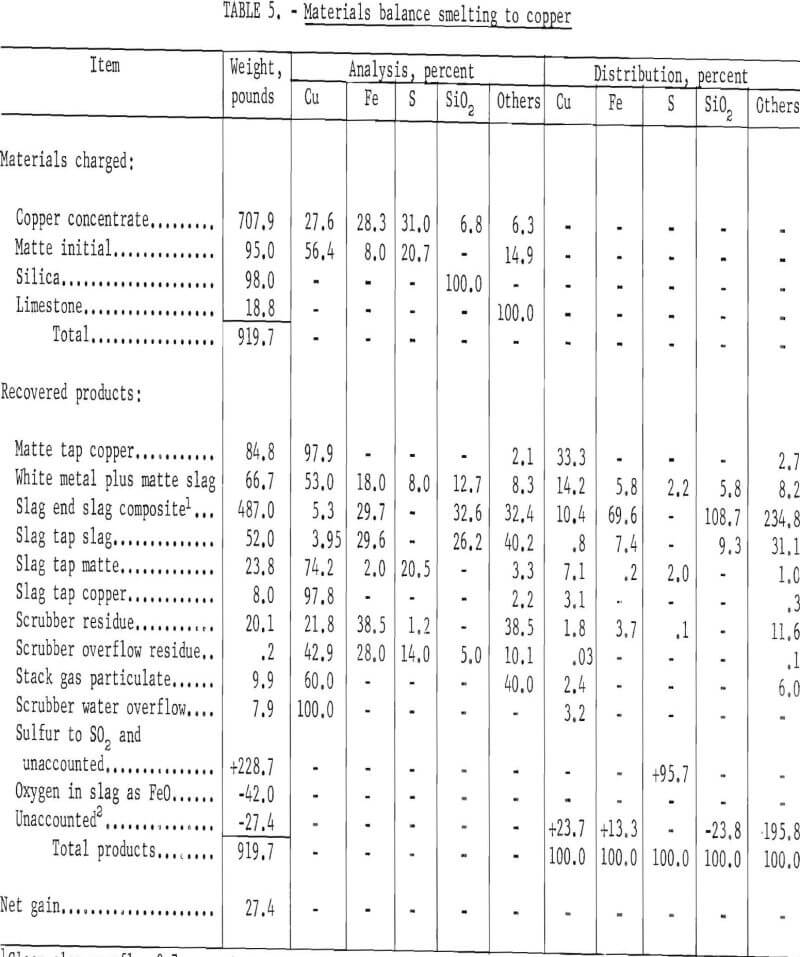
Materials charged and products obtained in two typical tests are presented in table 5, in which conversion proceeded to copper, and in table 6, in which the reaction was terminated prior to conversion to copper. Matte produced in slag cleaning is returned to the furnace, as well as flue accretions, scrubber residue, stack particulate, and other cleanup materials using conventional apparatus and processes. Copper in solution would not be present to this extent in a commercial operation since soluble sulfates would be removed prior to gas scrubbing. It can be seen in table 7 that when conversion proceeds to copper, the resulting slags are higher, as would be expected. These high slag values can be reduced by the addition of reductants such as pyrite, pyrrhotite. or copper sulfide concentrate to the cleaning section of the furnace. This phase of testing was not started, rather the resulting slags were treated by flotation. The slag composite is higher than the clean slag, owing to its containing matte from slag cleaning that ran out with the slag since no provision was made to tap it separately. The low recovery of metallic copper was typical, owing to copper losses, in this case 24.4 percent as shown in the unaccounted column. Copper is left in crucible recessions and cracks and is lost in matte splatter during conversion.
Tests to date have been conducted on a one-shift basis owing to the difficulty of sustaining an extended run in the small furnace. Attempts should be made to run continuously for at least 24 hours at a time, as capability is increased.
Of first consideration is the balance between conversion, feed, and oxygen. Only when this is accomplished can important variables, such as copper in slag, return matte, SO2 concentration, stack losses, and fluxing rates, be adequately determined. The data collected thus far are only an indication of what can be expected in continuous oxygen smelting. More importantly, the tests show that continuous oxygen smelting is possible within a single unit.
Erosion of the small furnace is excessive because of the close proximity of the oxygen jets to the wall; however, it slows as the crucible erodes out. By using low temperatures on the outer furnace, an equilibrium with magnetite deposition might be approached to protect the wall. Should the present furnace prove unsatisfactory to perform extended tests, a furnace with a larger diameter conversion area would need to be designed to continue tests on a scale of 2 to 5 pounds per minute. A furnace large enough to smelt sufficient concentrate to overcome heat loss in an autogenous system would be on the order of 25 tons per day (35 pounds per minute), according to estimates based on calculations and our experience at the Albany Metallurgy Research Center. This was about the size of the final furnace built by International Nickel Company for pilot-plant testing of their flash smelting process.
Theoretically, almost 100-percent pure sulfur dioxide can be produced smelting a sulfide concentrate with pure oxygen. The highest value analyzed in our tests was 81.7 percent owing to atmospheric dilution into the system. Seventy-five percent sulfur dioxide is regularly produced by flash smelting copper concentrate, using pure oxygen, at the Sudbury, Ontario, smelter of the International Nickel Company. In this plant (the only flash smelting process in the Western Hemisphere) copper concentrate is smelted to about 45-percent matte. Commercial oxygen produced on site is used, and pure liquid sulfur dioxide is recovered.

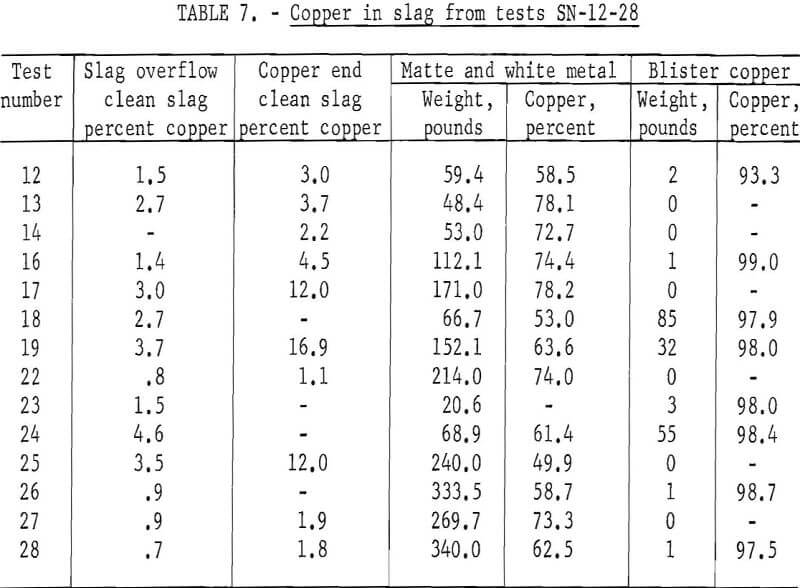
Tests were made jetting the oxygen through the slag, from about 6 inches above the slag at 40 psig, for comparison with the immersed probe. Conversion proceeded best in the first 30 minutes before much slag cover developed, then diminished rapidly as the slag built up to operating level. Using higher pressures would, of course, increase the jet penetration through the slag into the matte. Results of these tests are contained in table 8. The oxygen efficiency in tests in which the jet was used is only about one-third that of the immersed probe. The apparent oxygen efficiency of over 100 percent is likely, owing to the breakdown of magnetite contained with the initial matte and to the reaction with atmospheric oxygen.
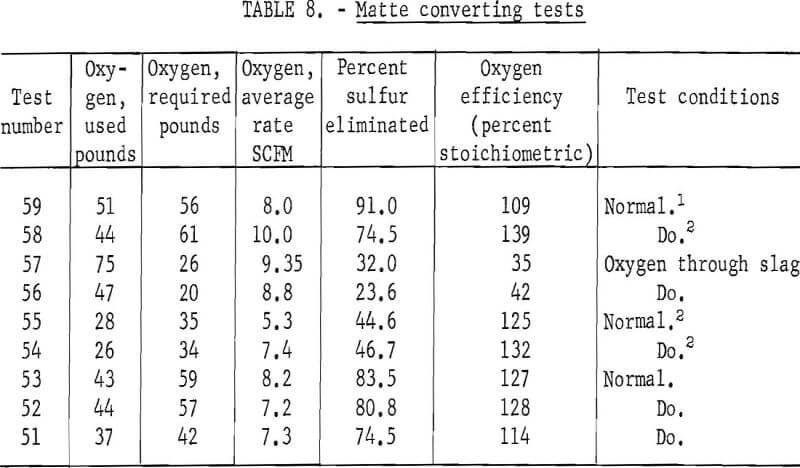
Although water and matte react violently, there is a built-in safety factor in that when a leak develops there is rapid cooling and a buildup of accretion around the leak which seals it. This has occurred on two occasions, with oxygen stoppage due to the skull formation being the only indication that a leak developed. In a commercial operation, a nonreactive cooling liquid might be used in place of water.
Slag Composition, Losses, and Forms of Copper
Analytical Tests
Slags from the COS furnace have varied widely from 20 to 40 percent silica and 35 to 60 percent ferrous oxide. Slags of about 30 percent silica and 55 percent ferrous oxide were of low viscosity, of reasonable liquidus temperature, and relatively low copper content. A typical slag analysis is as follows:
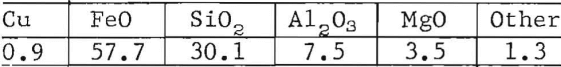
Limestone was discontinued because the use of silica alone to flux the iron facilitated control. The resulting slags appeared equally as fluid and less foaming occurred. More work is needed to identify an ideal slag composition and to establish an optimum.
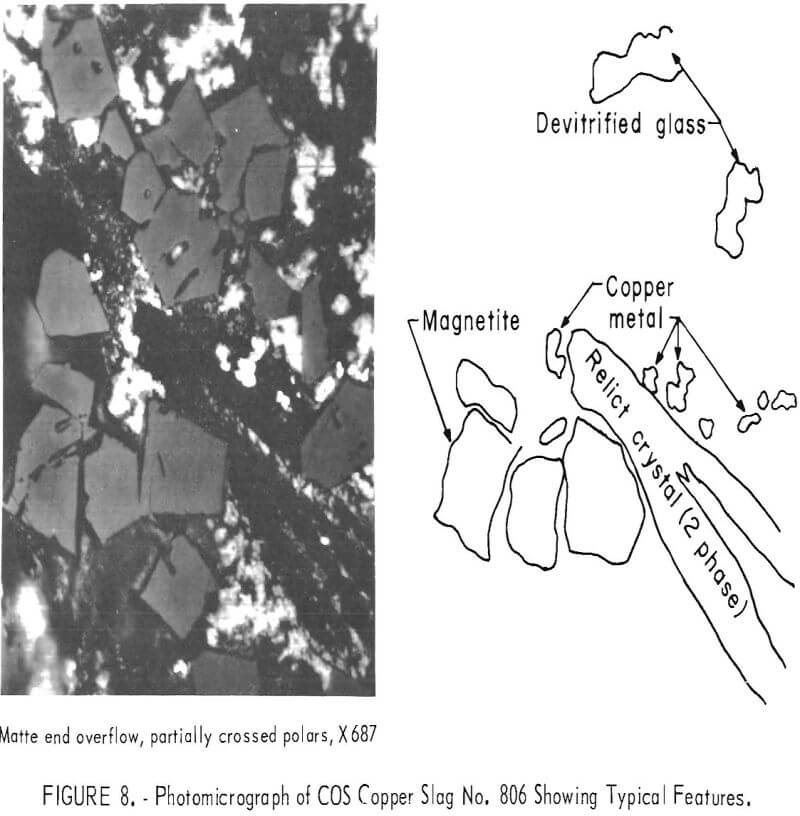
Microprobe, X-ray diffraction, and photomicrograph studies identified the primary copper phase in these slags as metallic copper of micron size, often locked or associated with magnetite and ferrites. Some copper was present as entrained sulfides, exsolution growth, and copper spinel, and when using chrome-magnesite refractory, precipitates of copper oxide in the center of large chromite grains. A photomicrograph of air-cooled slag in figure 8 shows the typical features of finely dispersed metallic copper, with blocky magnetite crystals and long, slender silicate crystals. The copper crystals are from 1 to 80 microns in size. Figure 9 shows electron-beam scans from the microprobe examination of slag from the copper end when using chrome-magnesite refractory. The long needle, fayalite-type silicate crystals are combined with magnesium. Magnesium from refractory erosion produces s more difficult slag than alumina contaminants from the refractory now in use.
Induction Furnace Tests
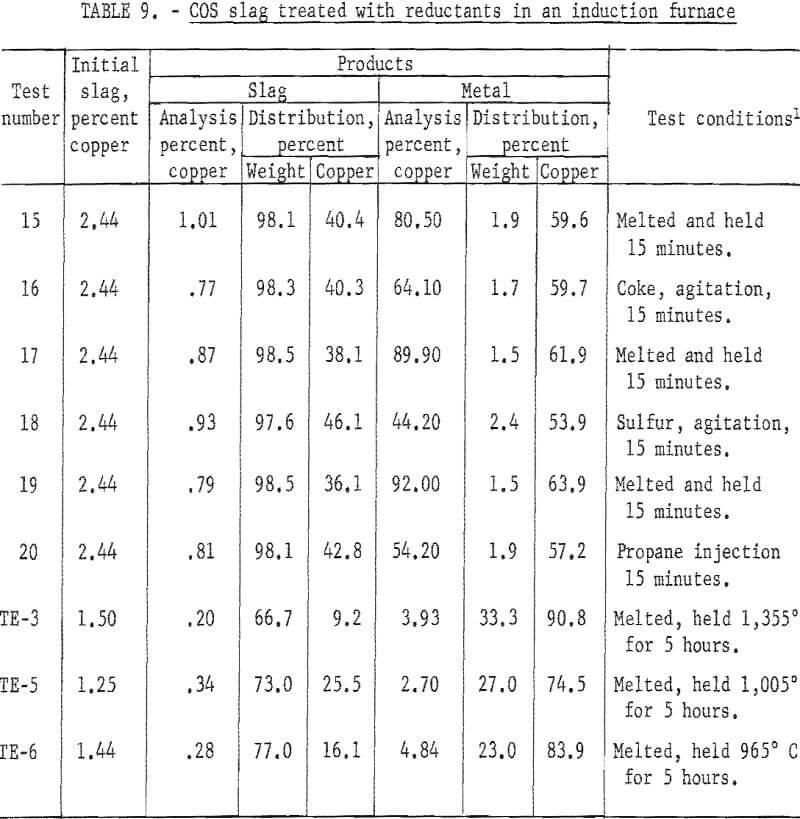
A small induction furnace was used to determine the effect on copper content of converter slags by injection of natural gas, coke, and sulfur (table 9). Coke causes heavy foaming owing to the evolution of CO and CO2. There is difficulty obtaining good contact with propane and sulfur since they volatilize so rapidly. The control tests, in which the slag was melted and held 15 minutes, were as effective in lowering the copper content as those in which reductants were added, since there was reduction from the carbon crucible.

Flotation Tests
Flotation tests on 11 COS slags treated in an induction furnace and two untreated slags (table 10) indicate the following:
- There is rapid growth of copper crystals at some temperature below its melting point.
- The best recovery was from the COS furnace overflow slag cooled at room temperature (72 percent recovery with 48 percent copper grade).
- The most pronounced effect upon copper recovery is cooling rate.
- The addition of lime to produce a more fluid slag did not result in better copper recovery by flotation.
- Holding near the melting point for long periods may not be desirable since this temperature range favors the formation of magnetite, copper spinel, and copper ferrites. Flotation results of slags held at this temperature were inferior to those of untreated furnace slags.
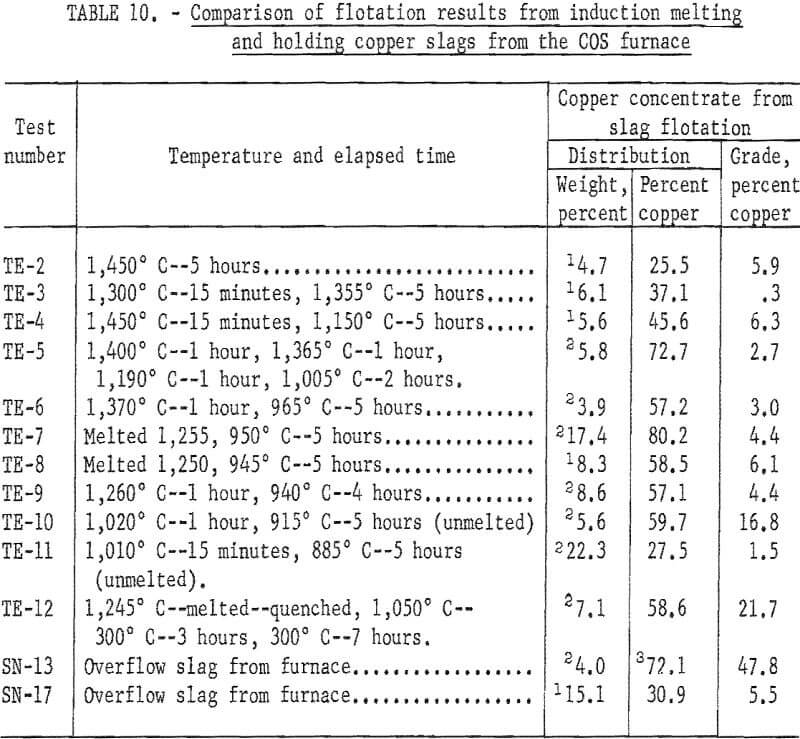
Microprobe examination of quenched slag, SN-17, table 10, shows that the major part of the copper, with the exception of entrained matte prills, is in solution or occurs as extremely fine micro precipitate, whereas naturally cooled slag. SN-13, table 10, contains copper particles of an estimated size distribution as follows: 20 to 40 microns, 50 percent; 10 to 20 microns, 30 percent; and less than 10 microns, 20 percent. It appears that the copper was in solution while molten, and remains as such when quenched. Crystal growth occurred by relatively slow cooling at room temperature. These differences in particle size account for the large difference in flotation results of the two slags.
Conclusions
Continuous oxygen smelting was demonstrated on a laboratory scale for several hours at a time. As capability is increased, longer runs are needed to adequately delineate process parameters. It is recommended that a furnace of about 5-pounds-per minute capacity, employing a heat sink similar to the present furnace, but with a larger conversion area and a longer crucible, be designed to continue tests on a laboratory scale. A furnace large enough to smelt sufficient material to compensate for heat losses in an autogenous system would need to be on the order of 25-tons-per-day capacity.
An immersed oxygen probe enables oxygen injection below the matte surface and away from the wall, it is more efficient than blowing through the slag and superior to tuyeres.
Copper in the slag is largely in the form of metallic copper and good recovery by flotation can be obtained by slow cooling the slag at ambient temperature to promote copper crystal growth. More study is needed to determine the optimum cooling cycle for maximum copper crystal growth.
Copper passing out of the furnace in the form of vapor and dust is not excessive and would be lessened in a larger furnace with greater distance between the flash smelting area and flue opening.
There is a high degree of conversion efficiency of the oxygen to sulfur dioxide.
Continuous oxygen smelting can be conducted in a totally enclosed unit with a smaller flue and gas-cleaning system, compared to present-day plants.
Since the sulfur dioxide in the flue gas is highly concentrated, economic recovery and superior cleaning of effluent can be expected.
