Table of Contents
- Description of Samples Tested
- Laboratory Batch Dewatering Tests of Talc Slurry
- Initial Testing of Flocculants
- Dewatering of Talc Slurry with Hercofloc 1027
- Continuous Dewatering Tests of Talc Slurry
- Trommel Dewatering Tests
- Static Screen and Belt Dewatering Tests
- Enviro-Clear Dewatering Tests
- Laboratory Clarification Tests on Talc Waste Water
- Clarification of Talc Waste Water by Sand Filtration
Talc, a hydrated magnesium silicate, is a major constituent of soapstone, which is used in the manufacture of thermal and electrical insulators. Talc also finds applications as filler for use in the paper rubber, and textile industries, in the preparation of soap, cosmetics, lubricating and special polishing agents, as well as in the paint and ceramic industries. Domestic production of talc in 1980 was well over 1.4 million tons. In processing talc, ultrafine waste materials, which respond poorly to conventional separation and dewatering techniques, are often produced. The waste materials usually are impounded in a series of disposal ponds, but even after flowing through as many as nine ponds, the water is still too turbid for reuse or discharge into streams. The Bureau of Mines investigated a method of flocculation and dewatering that allows for disposal of the solid talc waste in land-fills and reuse of the water.
The dewatering technique was initially developed by the Bureau for treating phosphatic clay wastes produced during the mining and beneficiation of phosphate in Florida. It consists of mixing the clay wastes with a flocculant and dewatering the resulting agglomerated mass, first on a static screen to remove most of the water, and then on a rotary screen for further dewatering. In this report, a variation of this dewatering technique is applied to talc waste material.
Description of Samples Tested
Two types of talc waste samples were investigated talc slurries generated from a beneficiation plant with solids ranging from 3.5 to 9.7 pct; and talc water samples taken from a settling pond with solids contents of about 0.03 pct.
Examination of the solids by X-ray diffraction identified talc and chlorite as major components, magnesite and dolomite as major-to-minor components, and kaolinite as a minor component.
Laboratory Batch Dewatering Tests of Talc Slurry
Test Procedure
Batch dewatering tests were conducted by adding dilute solutions of polymer to a 400-ml beaker containing 200 ml of talc slurry. The polymer was added to the slurry from a buret at a rate of about 1 drop per second. The mixture was continuously stirred with a magnetic stirring bar 1.5 in long, turning at approximately 500 r/min to create a vortex about 1 in deep. Generally, upon addition of polymer, there was immediate visual evidence of flocculation, but polymer addition was continued until the flocs agglomerated and moved in the beaker as one coherent mass, as shown in figure 1B. At this point the supernatant water was decanted until the solid mass could be handled. Then it was hand-squeezed by the operator until no more water could be released. Turbidity measurements were made on the released water after 5-min, 1-hr, and 4-hr settling intervals. The solids content of the dewatered mass was determined by weighing the wet sample and reweighing the sample after drying. The buret was read to determine the amount of polymer used in order to calculate the dosage, expressed in pound of polymer per ton of dry solids treated.
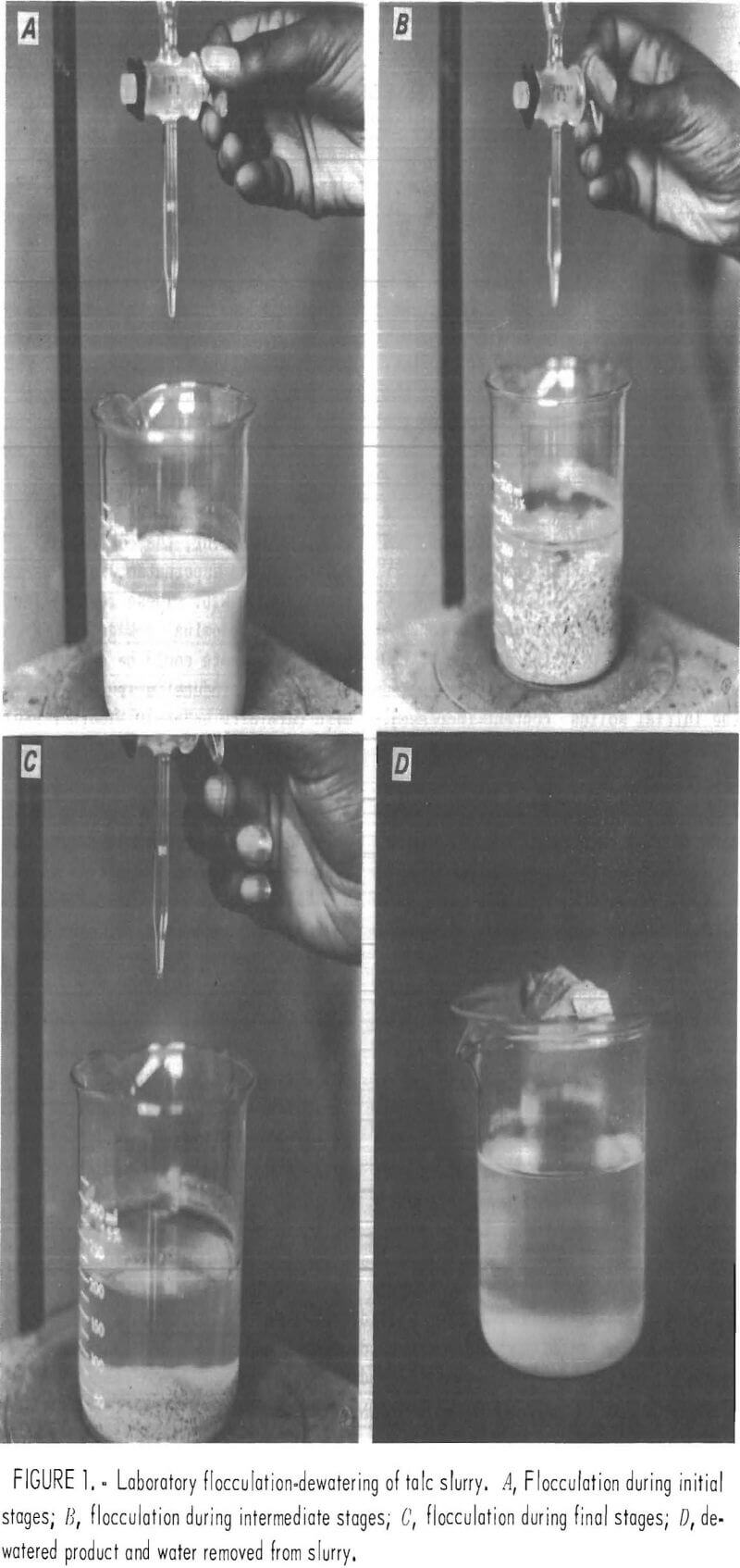
Initial Testing of Flocculants
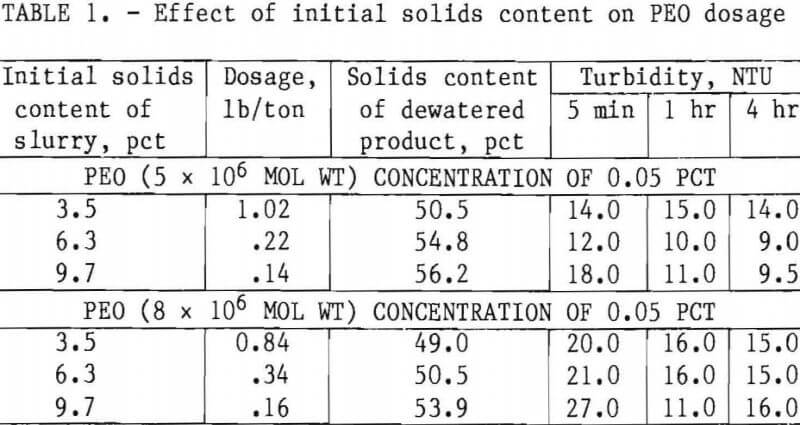
The investigation consisted of evaluating 18 flocculants effective in dewatering other industrial clay wastes that have colloidal systems similar to those of talc wastes. Of these flocculants tested, polyethylene oxide (PEO) and Hercofloc 1027 (a nonionic polyacrylamide) were found effective. Table 1 shows the results of dewatering tests using two polymers of PEO as flocculants on slurries of 3.5, 6.3, and 9.7 pct solids. In these tests, 0.05-pct solutions of the polymers were used to flocculate the slurries. This concentration was selected because in research done earlier on phosphatic clay waste, it effected optimum dosage, maximum dewatering, and highest quality of supernatant water.
The data show that when using a concentration of 0.05 pct, the dosage of PEO required to dewater the slurry decreased as the initial solids content increased. Maximum dewatering (highest percent solids of dewatered product) was achieved at higher initial solids contents and lower dosages without any significant change in water quality.
Although the dosage required to dewater talc slurry with PEO was nominal and the solids content of the dewatered product was very high, the dewatered product was dry and brittle, and fell apart on standard dewatering equipment.
Dewatering of Talc Slurry with Hercofloc 1027
Various concentrations of Hercofloc 1027 were investigated to flocculate and dewater 3.5- 6.3-, and 9,7-pct-solids slurries. Table 2 shows the results of dewatering tests on the 3.5-pct-solids slurry.
The data show that in the experiments on the 3.5-pct slurry, the Hercofloc 1027 dosage decreased from 2.34 to 0.29 lb/ton as the concentration was decreased from 0.25 to 0.005 pct. The solids content of the dewatered product was about 68 pct for the three higher dosages and about 49 pct for the two lower dosages. After settling for 1 hr, the corresponding turbidity of the supernatant water decreased from 17 to 6 NTU. These results indicate that with a nominal dosage of Hercofloc 1027, talc waste could be effectively dewatered to produce a supernatant water with turbidity under 10 NTU that could be discharged into streams.
A similar series of dewatering tests was conducted on waste containing 6.3 pct solids to determine the effect of initial solids content on the dosage requirement and settling capabilities. The data show that decreasing the Hercofloc 1027 concentration from 0.25 to 0.005 pct reduced the dosage requirement from 0.60 to 0.27 lb/ton. The percent solids of the dewatered product decreased from 72.0 to 55.3. The turbidity of the supernatant water after settling for 1 hr decreased from 27.0 to 15.5 NTU.
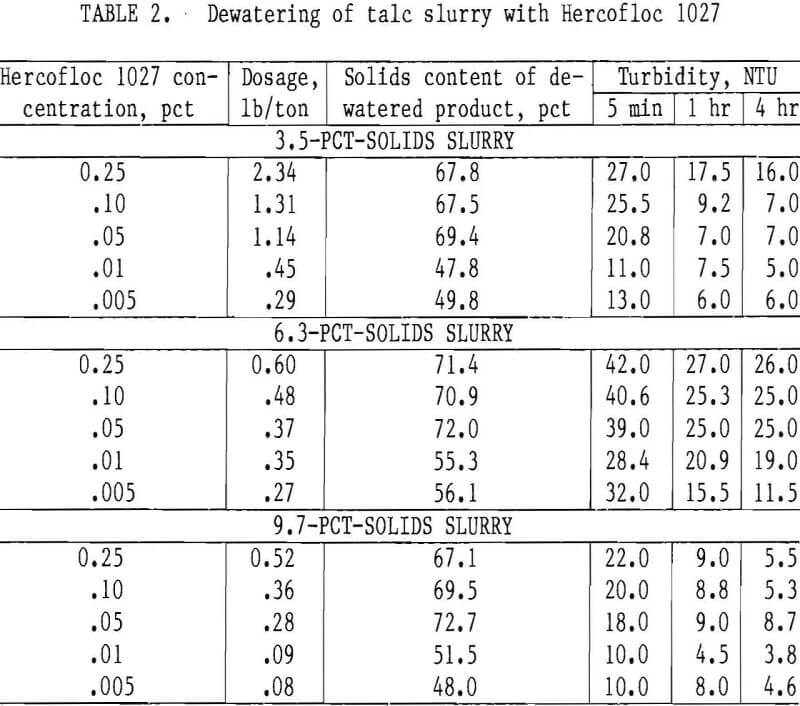
Although the results obtained from tests of 3.5- and 6.3-pct-solids slurries were similar, there was a marked reduction in the dosage requirements for the 6.3-pct-solids slurry, especially at higher flocculant concentrations. For example, at 0.25 pct only 0.60 lb/ton was required to dewater the 6.3-pct-solids slurry to 71.4 pct solids, but at the same concentration, 2.34 lb/ton was required to dewater the 3.5-pct-solids slurry to 67.8 pct solids.
To obtain additional data on the dewatering capabilities of talc wastes, the same experiments were conducted on a slurry of 9.7 pct solids. Test results followed the same general trend established by results of the more dilute slurries (table 2).
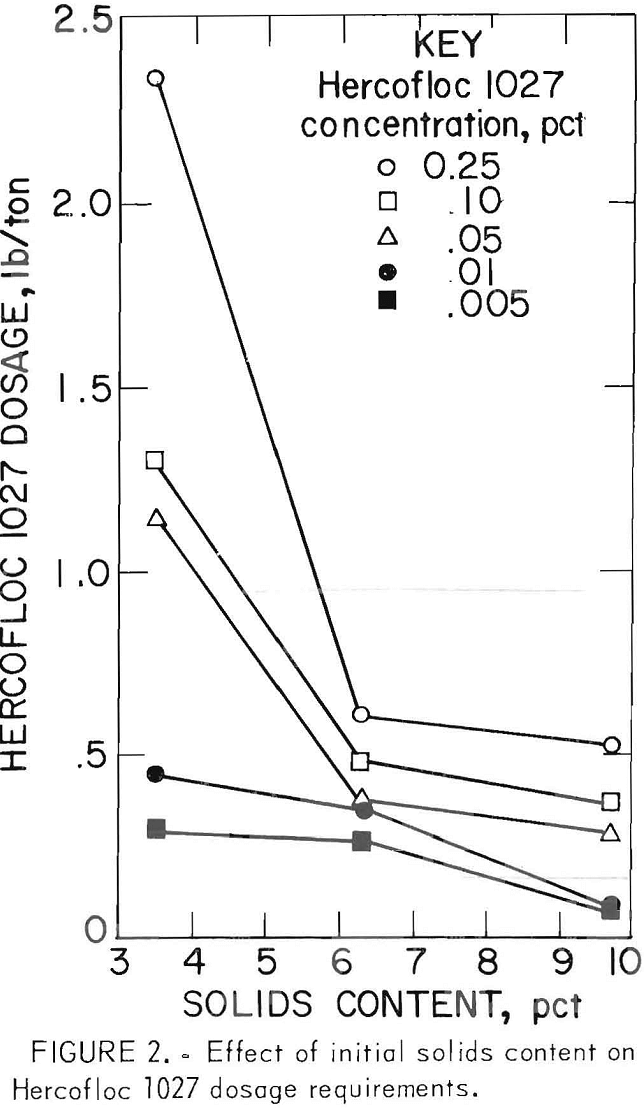
Data show that over the concentration range tested, Hercofloc 1027 was more effective in dewatering the 9.7-pct-solids slurry than it was in dewatering slurries of 3.5 and 6.3 pct. The dosage requirement was considerably less, ranging from 0.52 to 0.08 lb/ton, as the concentration was decreased from 0.25 to 0.005 pct. Also, the turbidity of the supernatant water was lower than that of previous tests, whereas the solids contents of the dewatered product was in the same range. These data indicate that dosage decreased as the initial solids content increased, shown graphically in figure 2.
Continuous Dewatering Tests of Talc Slurry
Following the laboratory evaluation, continuous tests were conducted using several laboratory-size trommels, a static screen and belt-roller apparatus, and an Enviro-Clear clarifier-thickener unit. Since Hercofloc 1027 proved to be most effective in laboratory experiments, it was used to flocculate the slurries in these continuous tests.
Trommel Dewatering Tests
Tests were run to evaluate the Bureau developed dewatering technique using a rotating trommel (fig. 3). A 6.3-pct- solids, as-received slurry, representing a typical waste generated from a talc beneficiation plant, was used in the investigation. The slurry was mixed with a
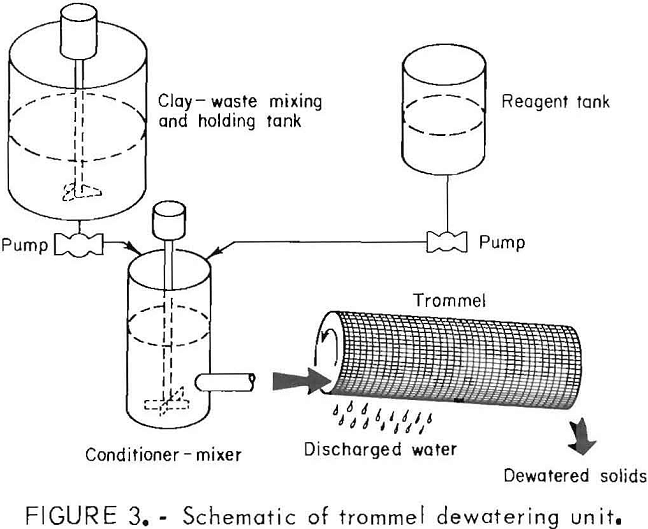
0.05-pct solution of Hercofloc 1027 in a cylindrical tank mixer of 4-3/16-in diameter and 6-in height, equipped with a pitched blade stirrer. Tests were conducted using a variety of conditions in regard to trommel size and mesh.
In these tests, the lowest dosage that could be obtained ranged from 2.4 to 3.0 lb/ton. The dewatered products ranged from 37 to 42 pct solids, and the underflow waters were very murky, containing as much as 2.7 pct solids.
Static Screen and Belt Dewatering Tests
In another series of continuous tests, a static screen was used as a primary dewatering device. The waste slurry was flocculated with Hercofloc 1027 in a conditioner-mixer and dewatered on static screens. In some tests, the material discharged from the static screen was further dewatered on a belt-roller device.
The static screens had openings of 0.020 and 0.010 in. Each screen measured 18 in wide and 4 ft long. The belt apparatus consisted of a 9-ft-long, 12-in- wide conveyor belt made of finely woven nylon material, and fitted around two 6- in-diameter rollers. The apparatus was equipped with three 4-in-diameter rollers that pressed the flocculated material against the belt, causing it to be dewatered. The rollers were powered by variable-speed motors. The discharge end of the belt was equipped with a metal scraper that liberated the dewatered material from the belt. A launder, constructed with an outlet port from which the belt underflow water was sampled, was fitted beneath the belt length.
In one test using a 0.020-in-opening static screen as the only dewatering device, the 6.3-pct-solids slurry was effectively dewatered using 1.19 lb/ton of 0.05-pct Hercofloc 1027. The static screen discharge material was 42.2 pct solids, but the water recovered from the screen contained 1.6 pct solids. To determine if the flocculated material could be further dewatered, another experiment was conducted under the same conditions but using a 0.010-in opening in the static screen. There was no appreciable difference in the solids content of the screen discharge, but the underflow water was much clearer, containing less than 0.01 pct solids.
To improve the quality of the underflow water and achieve maximum dewatering, a static screen with 0.010-in openings joined to a screen with 0.020-in openings was used with the belt apparatus in a dewatering sequence. The two static screens were each 45 in long and 3.5 in wide. The 6.3-pct-solids talc slurry mixed with 0.05-pct Hercofloc 1027 in a conditioner-mixer was discharged onto the screen from which it rolled onto the belt for further dewatering. Each section of screen was equipped with an underflow port from which water samples were collected and tested. The static screen and belt assembly are shown in figure 4.
In the first test, the 6.3 pct- solids talc slurry was successfully dewatered with 1.74 lb/ton Hercofloc 1027 to 44.8 pct solids. The underflow from the first screen contained 1.1 pct solids, but the underflow from the second screen and from the belt contained no solids. Attempts to repeat this test using a lower flocculant dosage resulted in a much poorer separation with a lower solids product, and water underflow containing as much as 2.0 pct solids. Consequently, it appears that continuous dewatering with trommels, static screens, and static screen plus a

belt-roller device requires higher polymer dosages than do laboratory batch tests.
Enviro-Clear Dewatering Tests
To produce highly consolidated dewatered products using low polymer dosages, an Enviro-Clear clarifier-thickener laboratory unit was used to treat talc waste slurries containing 3.5, 6.3, and 9.7 pct solids. The apparatus consisted of a 3.5-in-diameter cylindrical vessel fitted with rotating rakes and a concentric feed pipe. The flocculated slurry was pumped into the center of a 5-in sludge bed. As the solids settled, the dewatered material was discharged from the bottom while the recovered water overflowed from a port near the top of the vessel. A schematic of the apparatus is shown in figure 5. Results of these tests are shown in table 3.
These data show that in experiments conducted on the 3.5-pct-solids slurry, Hercofloc 1027 dosage over a range of 0.21 to 0.03 lb/ton produced 4-hr turbidities of 2.2 to 15 NTU. The dewatered products ranged from 52.8 to 56.9 pct solids. Also, using the same dosage range of Hercofloc 1027, the
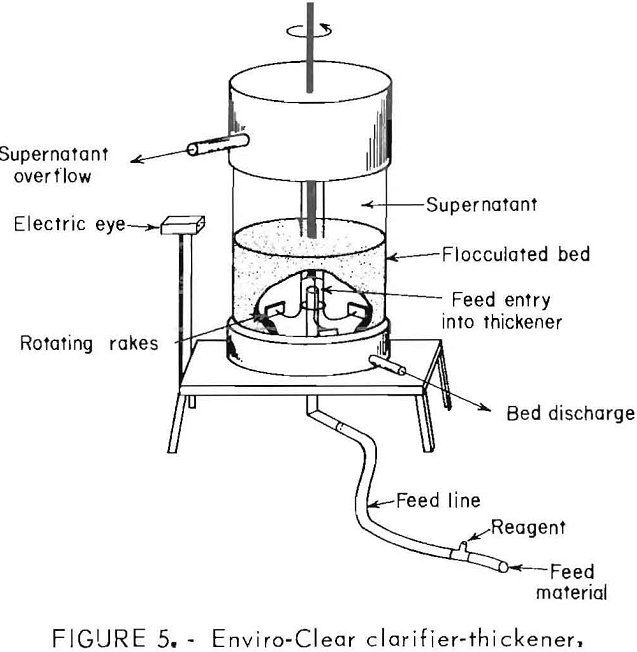
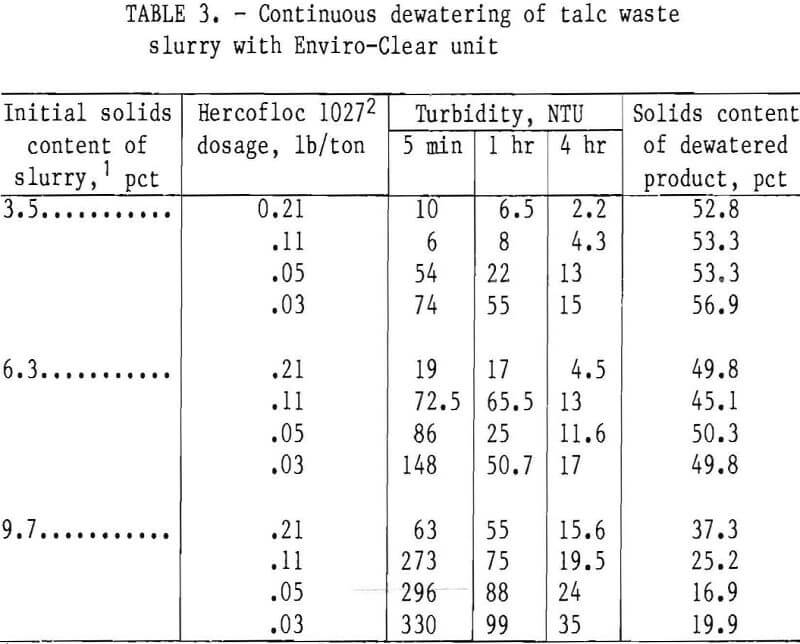
6.3-pct-solids slurry was dewatered to products containing 45.1 to 50.3 pct solids. After the overflow waters had settled for 4 hr, turbidity ranged from 4.5 to 17 NTU. Results of similar tests run on the 9.7-pct-solids slurry followed the same general trend established by results on the more dilute slurries. However, data show that the 9.7-pct-solids slurry was less amenable to dewatering with the Enviro-Clear than were the slurries with lower solids contents. The turbidity was higher, ranging from 15.6 to 35 NTU, and the solids contents of the dewatered products were lower, decreasing from 37.3 to 16.9 pct. The reasons for the low-percent-solids product with the 9.7-pct- solids feed are unknown at this time.
Laboratory Clarification Tests on Talc Waste Water
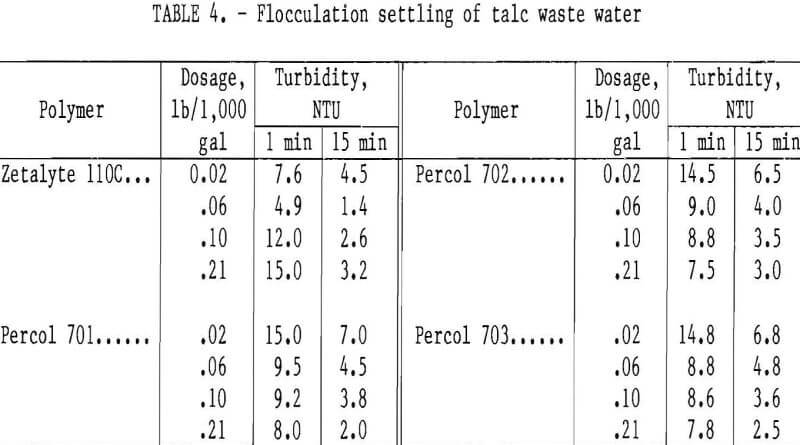
Experiments were conducted to investigate clarification of the talc waste water taken from a waste-holding pond at a talc beneficiation facility. Several laboratory flocculation settling tests were run on a sample that contained 0.03 pct solids and turbidity of 16 NTU. Several commercial polymers were used to flocculate the suspended solids in the water before allowing it to settle for predetermined intervals, after which the turbidity was measured. Laboratory batch tests were run on the water using 0.05-pct solutions of several commercial polymers, including PEO and Hercofloc 1027.
Of the polymers tested, cationic polymers Percol 701, 702, 703, and Zetalyte 110C were most effective. In tests using 0.02 lb of Percol 701, 702, 703, and Zetalyte 110C per 1,000 gal of waste water, turbidities after 15 min were 7.0, 6.5, and 6.8, and 4.5 NTU, respectively. These data indicate that for the limited conditions tested, Zetalyte 110C was superior to the Percol polymers in clarifying the waste water, as shown in table 4.
Clarification of Talc Waste Water by Sand Filtration
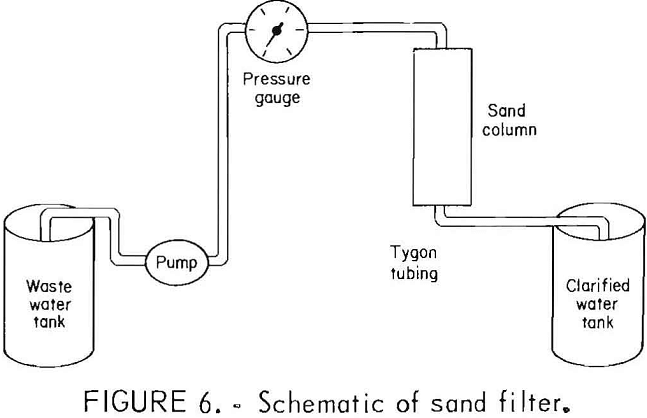
A sand filter was made by packing a 2-in diameter plastic column to a depth of 10 in with a minus 30- plus 40-mesh Ottawa sand. The talc waste water was pumped through the sand bed with a variable-speed pump. A pressure gauge was placed between the pump and the filter bed to monitor the pressure during operation. The apparatus is shown in figure 6. The turbidity of the filtered water was determined immediately after the run. In the first of three experiments conducted, the 0.03-pct-solids, 16- NTU water was pumped through the column at 260 ml/min, creating a head pressure of 3 lb/in². The turbidity of the filtered water was 0.50 NTU. In the second experiment, the flow rate was increased to 300 ml/min with a corresponding pressure of 3.3 lb/in². The turbidity of the filtered water was 0.53 NTU. In the third experiment, the flow rate was increased to 540 ml/min with a head pres-sure of 6.0 lb/in². The turbidity of the filtered water was 0.75 NTU.
Results indicate that sand filtration could be an ideal method for clarifying talc waste water because no prior treatment or flocculation of the solids is required. The possible need for frequent backflushing of the sand filter when used with waters of higher solids contents appeared to be the only potential problem with this system.
Conclusions
In laboratory batch tests, talc slurries of 3.5, 6.3, and 9.7 pct solids were effectively dewatered with Hercofloc
1027. Talc slurry of 3.5 pct solids was dewatered to 69.4 pct solids with 1.14 lb/ton Hercofloc 1027. The turbidity of the supernatant water after settling for 1 hr was 7.0 NTU. Similarly, talc slurries of 6.3 and 9.7 pct solids were dewatered to 72.0 and 72.7 pct solids with 0.37 and 0.28 lb/ton Hercofloc 1027; turbidities of the supernatant waters after settling for 1 hr were 25.0 and 9.0 NTU, respectively. This investigation revealed that slurries with higher solids contents were more amenable to dewatering than those with lower solids.
In continuous tests, a talc slurry of 6.3 pct solids, flocculated with 2.4 lb/ ton Hercofloc 1027 was dewatered to 42 pct solids on a rotating trommel. Also, a static screen was used to dewater 6.3- pct-solids slurry to 42.2 pct solids with 1.19 lb/ton Hercofloc 1027. In tests using a static screen-belt dewatering device, a 6.3-pct-solids slurry flocculated with 1.74 lb/ton Hercofloc 1027 was dewatered to 44.8 pct solids. The underflow water from the first screen contained a small amount of solids, but the underflow water from the second screen and from the belt contained no solids and was perfectly clear.
Talc slurries of 3.5, 6.3, and 9.7 pct solids flocculated with 0.21 lb/ton Hercofloc 1027 were dewatered to 52.8, 49.8, and 37.3 pct solids, respectively, with an Enviro-Clear clarifier-thickener; turbidities of the overflow waters after settling for 4 hr were 2.2, 4.5, and 15.6 NTU, respectively. This investigation revealed that the Enviro-Clear was superior to the static screen-belt dewatering technique because it required lower polymer dosage to achieve comparably dewatered products.
The turbidity of talc waste water with initial turbidity of 16 NTU was reduced to 4.5 NTU using 0.02 lb/1,000 gal Zetalyte 110C after allowing the water to settle for 15 min. Also, the turbidity of the water was decreased from the initial 16 NTU to a mere 0.5 NTU by pumping the untreated water through a sand filter of simple design.
