Table of Contents
Galvanic contact, occurring between electrically conducting minerals in flotation pulps, may affect the nature of mineral surfaces and influence their floatabilities. Rest potential and galvanic current measurements were made on an electrode combination of pyrite and pyrrhotite under nitrogen, air and oxygen aeration conditions, and the results were correlated with their oxygen-consuming characteristics and flotation behaviors. The galvanic contact improved the floatabillty of pyrrhotite, but adversely affected that of pyrite.
Materials and Methods
Pyrite and pyrrhotite used in this investigation were, respectively, from Coahuila, New Mexico and Falconbridge Ltd.’s Strathcona mine, Ontario. For Hallimond tube flotation tests, +297µm (+48 mesh) fractions of those minerals were ground to 74/44 µm (200/325 mesh) in a porcelain mortar and pestle before use. The 74/44 µm pyrrhotite thus obtained was concentrated with a laboratory hand magnet and demagnetized. Since pyrrhotite is easily oxidized resulting in decreased floatability, each flotation sample of one gram was briefly cleaned with sulfuric acid solution of pH 2 and washed several times with distilled water immediately before its use. The freshly ground 74/44 µm pyrite sample was cleaned by shaking with distilled water five times. The collectors employed in the study were potassium n-butyl xan-thate (KBX) for pyrrhotite and potassium ethyl xanthate (KEX) for pyrite, both prepared in the usual manner (Foster, 1928).
Results
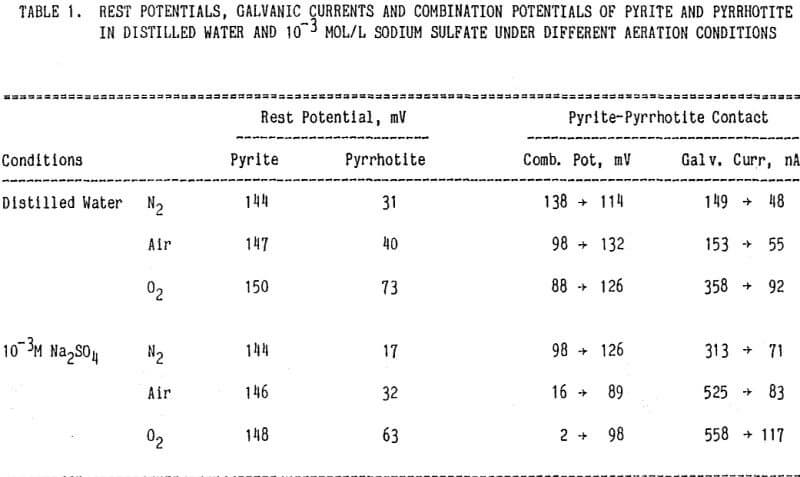
It is observed that the rest potentials of pyrrhotite were higher in oxygenated solutions than in deoxygenated solutions and that the rest potentials of pyrite were less strongly dependent on aeration conditions. Since pyrite is nobler than pyrrhotite, pyrite would act as a cathode whereas pyrrhotite would act as an anode when they are galvanically coupled. Galvanic currents were measured by short- circuiting the two electrodes as soon as they were immersed in solution. Galvanic currents decreased and combination potentials increased with time, except in deoxygenated distilled water.
Flotation tests were conducted to determine the effect of galvanic contact between pyrrhotite and pyrite. Concentration of the collectors, KBX and KEX, required to achieve full recovery was determined by a series of flotation tests. At natural pH, the recovery of pyrrhotite reached 92% with 7 x 10 -4 mol/L KBX and that of pyrite reached 93% with 4 x 10 -4 mol/L KEX, and these concentrations were used in subsequent tests.
The effect of galvanic contact between two minerals on the flotation was investigated by mixing 74/44 µm (200/325 mesh) fraction of one mineral and 297/210 µm (48/65 mesh) fraction of the other mineral in a 40 mL pyrex beaker. This beaker was placed in 600 mL of distilled water in a pyrex beaker, through which oxygen, air or nitrogen was bubbled at a rate of 330 mL/min. for 15, 30, 60, 90 or 120 minutes.
To investigate if the galvanic contact affected the copper activation behavior of pyrrhotite, two series of flotation tests were carried out as a function of copper sulfate concentration. In one series pyrrhotite particles were by themselves and in the other series pyrrhotite particles were in contact with pyrite in oxygenated water for one hour. The 40-mL beaker containing the mineral particles was removed from the oxygenated water, conditioned with copper sulfate by agitating the supernatant water using a glass T-stirrer while mineral particles remained settled at the bottom of the beaker in order to maintain the galvanic contact.
Initially, survey scans of the Auger spectra were obtained for the samples prepared in different ways. The survey scans showed strong peaks of carbon, oxygen, sulfur and iron. Depth profiles of elements were obtained by progressively etching away the surface layer with an argon ion sputter gun with an estimated etching rate of 1.2 nm/min.
Discussion
The oxidation of pyrrhotite adversely affects its floatability. In deoxygenated solutions, the recovery of pyrrhotite was shown to change little even after two hours of nitrogen aeration. Its floatability decreased, however, as the aeration time with air, and particularly with oxygen, increased. The decrease in the recovery of pyrrhotite may be attributed to the formation of a surface film such as iron hydroxide on the pyrrhotite. SEM photomicrographs showed numerous precipitates on the pyrrhotite surface and oxide, hydroxide or sulfate species of iron were identified by XPS. However, the recovery of pyrrhotite increased when pyrite was present in oxygenated solutions.
The flotation results showed that the galvanic contact with pyrrhotite decreased the floatability of pyrite. Some investigators indicated that the formation of dixanthogen at the surface of pyrite might be responsible for the flotation of pyrite.