Table of Contents
The broad general distinction between primary and secondary batteries lies in the fact that in the former the current is produced by the consumption of one or both of the elements composing the cell, while in the latter the elements retain their form as such, and merely pass from one state of chemical combination to another.
Much has been said in condemnation and but little in praise of storage-batteries; yet, notwithstanding the severe criticisms passed upon them, the skepticism with which electricians have viewed them, and the ignorance with which they have been handled, they continue to push their way into use. Their manufacture has now become a well-defined and established industry.
Speaking of this subject, Prof. W. E. Ayrton, in a recent lecture, says: “Secondary batteries have caused much heart burning, for their uses, from the apparent fickleness of their complex chemical action, are yet but imperfectly understood. But we have at length been taught what is good and what is bad treatment for them, and after years of brave, persevering application on the part of the Electrical Power Storage Company, that forlorn hope, the secondary battery, has become one of the most useful tools of electrical engineers.”
The evolution of the storage-battery to its present form (which we must admit is not by any means perfect) has been painfully slow and tedious. A history of the various attempts and failures, even up to the time of Plante, in 1859, would fill several volumes, and would be entirely beyond the scope of this paper. It will suffice, therefore, to say that the germ of the secondary battery dates from the discovery by Gautherot, in 1801, that the plates of a voltameter give a reverse current. It was thought at first that the plates had been charged in a manner somewhat similar to the charging of a Leyden jar; but this idea was, after a time, demolished by the chemists, who showed that a definite chemical reaction had taken place, and that the return current was due to the chemical action of the new substances produced by the original decomposition.
Gaston Plante, who made the most exhaustive researches in secondary batteries, constructed a secondary pile which is really the parent of all modern accumulators. A number of attempts have been made since that time to store the electrical energy of a dynamo, the most notable of which are those of Messrs. Thomson & Houston, who patented and afterwards exhibited at the Franklin Institute, in Philadelphia, a gravity form of the Daniell cell, in which the zinc was reduced from its solution and the copper re-dissolved by the action of charging the cell from a dynamo or other source of electricity. C. B. Brush, of electric light fame, M. Faure and M. Julien are the last in the field with their respective inventions.
THEORY
The standard Julien accumulator is that known as type “B.” It consists of nineteen plates, nine positive and ten negative, weighing 25 pounds net, i.e., without the box containing them or the acid solution. These add to the weight from 5 to 7 pounds. The following are the principal details :
Floor space, 6½ x 5¾ inches.
Height, 8 inches.
Net weight (of plates), 25 pounds.
Total weight, 32 pounds.
Quantity of acid solution, about 1¾ quarts.
Strength of acid solution, 23° Beaume.
Specific gravity, 1.185.
Electro-motive force, 2 volts.
Internal resistance, .005 ohm.
Charging currents, up to 20 amperes.
Discharging currents, up to 30 amperes.
Current capacity of each accumulator 150 ampere-hours, at a 10-ampere rate.
Energy capacity, 300-volt ampere-hours or watt-hours.
Each plate consists of a grid about 6 inches square and 1/8 inch thick, cast in iron moulds. It is composed of an alloy of lead (95 per cent.), antimony and mercury. The square holes or interstices are filled with litharge and red lead for the negative and positive plates respectively. These materials, mechanically applied to the grid or support-plate, which is in reality nothing but a conductor, constitute what is known as the active matter. They become more or less sulphated when placed in the electrolyte, and the active matter is changed by the action of the charging current, as explained below, into reduced or spongy lead and peroxide.
Of course, it is hardly necessary to say that there is no actual storage of electricity in an accumulator; it is simply a convenient reservoir for storing the active ingredients necessary to generate a current of electricity. In fact, a charged battery becomes a primary battery, and the current derived from it is based on identical principles.
The reaction which takes place when a battery is discharged can best be examined by referring first to a primary battery with a simple zinc-copper couple. Mr. John T. Sprague has pointed out that the old explanation of the working of a battery by the decomposition of water is not the correct one.
When a positive element like zinc is opposed to a negative element in dilute sulphuric acid, the following reaction occurs: On uniting the poles the molecules of sulphuric acid become polarized, then the acid radical is turned towards the electro-positive element, zinc, and the basic ion towards the electro-negative element. Substitution then takes place, and the sulphuric radical unites with the zinc to form a molecule of zinc sulphate, and an interchange of molecules takes place along the line until the negative pole is reached, where hydrogen (two atoms) is liberated.
This can be expressed graphically as follows:
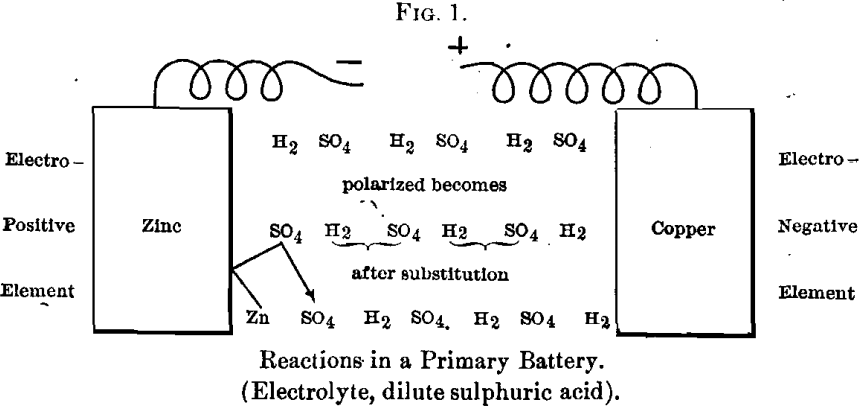
Let it be supposed that at the electro-negative element, where the hydrogen is liberated, there is some substance that it can combine with or displace, then the following reaction takes place, as in the ordinary Daniell cell:
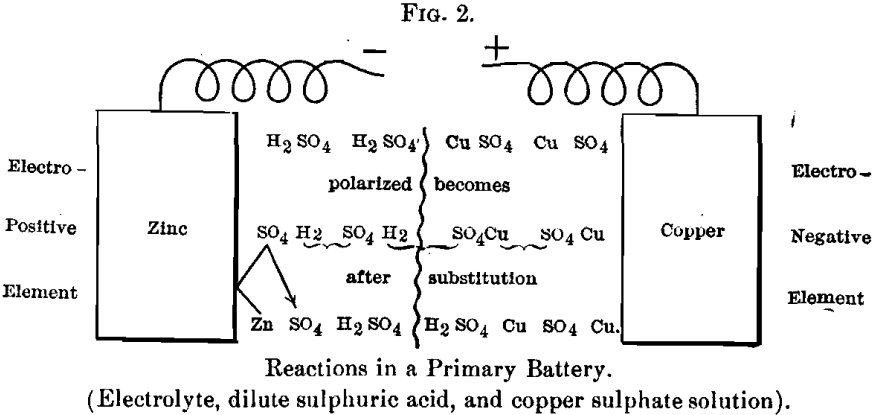
The first action is the polarization of the molecules; the second a general substitution all along the line, forming zinc sulphate at one end and depositing metallic copper at the other, one atom of copper being equivalent to two atoms of hydrogen displaced in the former reaction.
With the knowledge thus gained, we can now study the discharge of a secondary battery :
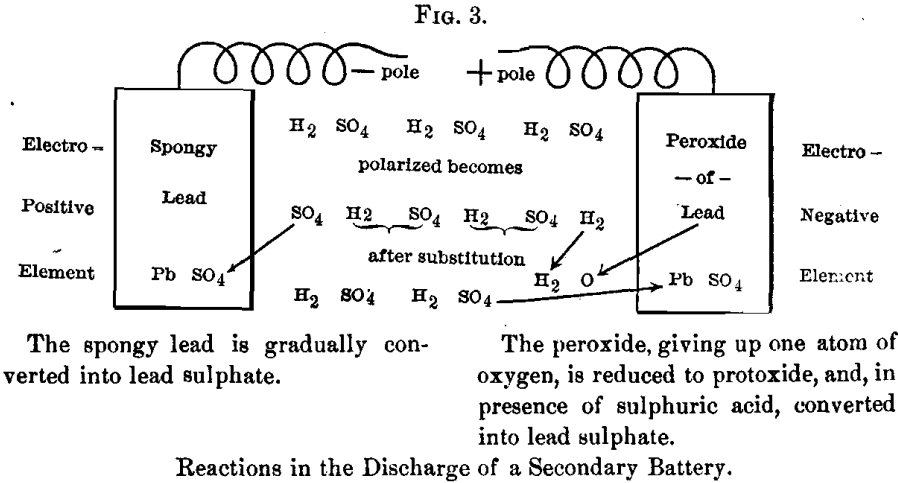
In this case the polarization occurs as above, and the substitution then takes place, forming a sulphate of lead on one pole and liberated hydrogen at the other. But here there is a substance, peroxide of lead, which yields up its oxygen to the liberated hydrogen and forms a molecule of water. The oxide of lead formed by the reduction of the peroxide is, of course, instantly changed into sulphate of lead by the sulphuric acid. The action of the discharge is therefore to gradually sulphate both poles of the battery. Sulphate of lead, being insoluble in sulphuric acid and water, remains in the grids or support- plates in place of lead and peroxide.
The reaction in charging is more complicated and is not so well understood ; but the final result is to leave the plates in the same condition as mentioned above before discharging. The conditions in charging, moreover, are not identical with those of discharging. It has been found that when a current from an external source is passed through two platinum-plates in acid, polarization results, and oxygen is liberated at one pole and hydrogen at the other.
When a secondary battery is completely discharged the plates are practically similar, that is, they are both lead-plates, containing sulphate of lead. Hence, there is no difference of potential, and no current results from connecting their terminals. It is therefore immaterial (so far as the chemical action is concerned) to which pole is connected the positive pole of the external source, which may be either a battery or a dynamo. In either case the plates now act in a manner similar to the platinum-plates mentioned above, and oxygen is liberated at the pole through which the current enters and hydrogen at the opposite pole. The reaction at the positive pole is:
PbSO4 + O + H2O = PbO2 + H2SO4.
At the negative pole it is :
PbSO4 + H2 = Pb + H2SO4.
In other words, peroxide of lead is re-formed at one pole, spongy lead at the other, and sulphuric acid at both poles. The battery is then once more in condition to perform work.
The reaction can be represented graphically as follows (see Fig. 4), the current flowing in the opposite direction from that shown above.
That sulphuric acid is formed at both poles is proved by the specific gravity of the solution after charging. What has been done, then,
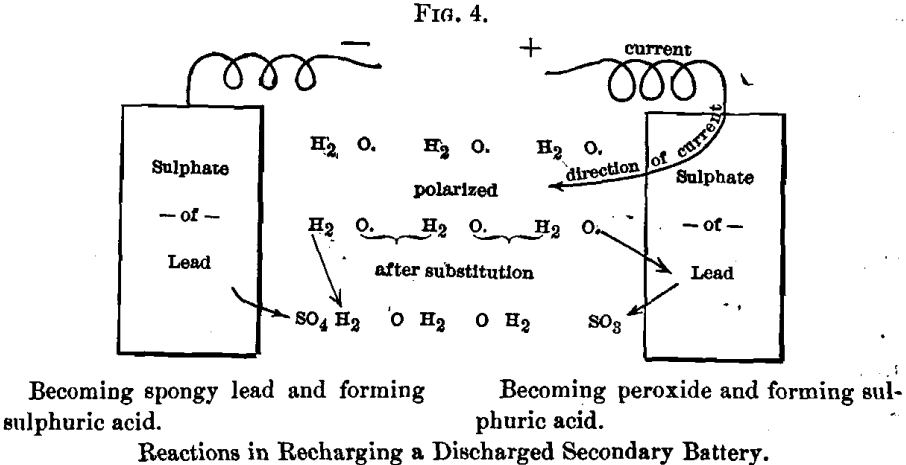
when a secondary battery is discharged, is to burn lead to sulphate. The amount of energy produced thereby can be determined quantitatively.
It will be necessary, before proceeding with the calculations, to go a little into molecular physics.
The mechanical equivalent of heat was the direct result of the true theory of combustion as established by the experiments of Lavoisier Priestley, Scheele and others.
The mechanical equivalent of electricity has been determined only within the last few years, and its determination is founded solely on a chemical basis.
Unit E. M. F., or one volt, acting through unit resistance, one ohm, gives a current of one ampere, which, continued for one second, yields the unit of energy, one coulomb, equal to 1/746 horse-power; and since one horse-power equals 550 foot-pounds of work per second, one coulomb equals 550/746 = 0.7373 foot-pounds in a second. Electric current is measured by the amount of chemical work performed in a given time.
Taking hydrogen as the unit, the amounts of the other elements set free by the same electric current are proportional to the atomic weights of the various elements compared with hydrogen as one, due allowance being made for the valency of the element under consideration.
Mr. John T. Sprague,in his great work on electricity, has proposed the name “Chemic” as the unit of electric quantity which will set free from combination one-grain weight of hydrogen in ten hours. It is possible from the above to calculate the amount of electric energy to be derived from a given weight of zinc or lead in a battery in the same manner as the amount of energy, and consequently the amount of work, to be derived from the combustion of a pound of coal under a boiler is determined. The “ Chemic ” is equal to 4673 foot-pounds.
The amount of current which will release .00001022 gramme of hydrogen (equals 0.000158 grain) per second is called the ampere- second or coulomb, which is the purely arbitrary unit of current. It is a great misfortune for scientific workers that it is so, and a matter of such importance that it may yet be changed. It is for this reason that Sprague adopted the unit which he calls the Chemic, mentioned above.
If the ampere had been based on hydrogen as the unit (one gramme, one grain, one ton, or one anything), then the electric equivalents of all the other elements would have followed in the order of their combining weights.
Sprague says: “Let us consider the weight as taken in grains; then taking hydrogen, one grain and one valency, as our base, we have the unit of quantity as that necessary to set free from combination one-grain weight of hydrogen or one atom of any monad, or to do equivalent work in any given atom ; thus, iron 56 grains or zinc 65.2 (both dyads), would require two units of quantity to set them free, or would furnish two units themselves while dissolving. To convert this into current, time has to be taken into account, and for convenience of calculation I take ten hours.”
The energy derived from the substitution of lead for hydrogen in sulphuric acid in the above reactions is the source of the E. M. F., and the amount or actual weight of lead consumed represents the current which will be produced, measured in coulombs.
Now, the atomic weight of lead is 207. Lead being a dyad, its electrical equivalent would therefore be 103.5; and 103.5 x .00001022 = .00105800 gramme. In other words, the consumption of .00105800 gramme of lead in a battery furnishes a current of electricity of one ampere for one second, or one coulomb.
One gramme of lead, therefore, equals 944 coulombs (1/.001058 = 944).
In an accumulator consisting of nineteen plates, ten negatives and nine positives, we have in the ten negative plates about 5 pounds of spongy lead. Since 5 pounds = 2273 grammes of lead, 2273 x 944 (the number of coulombs per gramme) equals 2,145,712 coulombs. Dividing by 3600 (number of seconds in an hour) we have 596, say 600, ampere-hours at 1 volt pressure as the theoretical capacity of the accumulator, or about 8/10 of a horse-power hour. This corresponds fairly well with actual practice in the total discharge of an accumulator, as given below.
This is a matter of the first importance, inasmuch as it sets at rest forever the extravagant claims and pretensions of those who, having only a partial knowledge of the subject, are very apt to claim more for their ideas than can be realized in practice. For every pound of active matter in the form of spongy lead, 120-volt ampere-hours is the maximum output that could conceivably be obtained. If 60- volt ampere-hours is reached, the results are equal to the best practice.
I am not able to calculate the theoretical E. M. F., as I have not the data at hand for the purpose; but, given the calories or foot-pounds per grain equivalent for the energy generated by the combination of lead and oxygen and oxide of lead and sulphuric acid, and dividing this number by 4673, the energy in volts represented by this reaction is obtained.
4673 (the equivolt of Sprague) is the mechanical equivalent of electricity in foot-pounds, i.e., 4673 foot-pounds per volt equivalent, and he gives, therefore, the following definition :
“ The E. M. F. corresponding to any chemical action is equal to the product of the equivalent weight of the basic ion; and of the heat of combination of the two ions expressed as C. G. S. units of work.”
The above theory at least offers a simple and apparently intelligible explanation of the phenomena involved, and one also apparently in harmony with the facts. It will be of great aid in guiding our steps to further discoveries in the future.
Referring to the chemical equations once more, it will be noted that the function of the electro-negative element, or so-called positive plate, is to take up or give out, as the case may be, the atoms of oxygen liberated during the charge and discharge, and on its ability to do this depends the proper working of the battery. Lead itself, under the ordinary conditions of temperature, etc., having a small affinity for the sulphuric acid radical, the action would cease altogether if the basic ion of the acid or hydrogen did not have a constant source of oxygen to combine with. It is, therefore, a prime essential, in order to keep a battery in good working order, that the positive plates should not be allowed to sulphate, since in such a case the basic sulphate of lead formed is so difficult to convert into peroxide that it frequently happens that the grid itself is oxidized, in preference to the sulphate, by the action of the charging-current. When this action once begins, it is only a question of a few weeks as to the life of the battery, because the cell then becomes simply a Plante cell, in which the current oxidizes the support-plate. As this oxidation increases with each successive charge and discharge, it is only a question of time when the whole grid or support-plate will be oxidized, and the internal resistance of the battery so increased by the absence of any metallic conductor for the current that little or no current flows.
It is its freedom from liability to this defect which constitutes the excellence of the Julien battery. The resistance to oxidation of the triple alloy of lead, antimony and mercury is such, under the ordinary working conditions of the battery, that with proper care the active matter actually wears away by attrition of the liquid, and leaves the grid a solid metallic conductor. I have seen such grids after two years of active service in car-lighting, and they appear to be just as good as when first cast, and I am engaged now in experiments as to refilling them for active service again. The importance of this freedom from oxidation has not been fully appreciated ; otherwise inventors and manufacturers of new storage-batteries would avoid a serious source of error.
Of course, if a battery is shamefully abused and neglected, it is bound to disintegrate, like any other piece of machinery or apparatus, no matter whose make or design it may be. The positive element or negative plate generally takes care of itself, and keeps in fairly good condition long after the positive plates have been ruined.
The actual capacity of a cell depends on a variety of conditions of size, shape, thickness, rate of discharge, etc. Various designs of plates have been made to secure maximum capacity. Other things being equal, the thinner the plate and the greater the surface the greater will be the capacity in ampere-hours per given weight. The strength of materials and the space occupied are the limitations in the design.
An ideal plate would be one of great surface and exceeding thinness; but if an attempt were made to produce such a plate it would buckle by its own weight and cause trouble. On the other hand, a thick plate implies an immense amount of dead weight in the conductor; and since the active matter is so thick, and consequently the inner portions are more or less protected from the outside, the chemical reactions cannot take place readily. As a consequence, much of the so-called active matter is really inactive.
We found, after making a great many types, that what we call our 19 “ B ” is about the best adapted for general work either in lighting or in traction. Of course, there are conditions where it is desirable to use heavier currents than can be obtained with safety from so small a cell, but in such cases I prefer to use a greater number of plates in a cell, or to discharge them in parallel.
The commercial capacity of our standard 19 “B” cell is 150 ampere-hours at 2 volts or 300-volt ampere-hours or watt-hours. This is just about 50 per cent, of its theoretical capacity. Under favorable conditions we have had a commercial yield of 200 ampere-hours, or 66 per cent, of the theoretical capacity. This must not be understood as referring to a given charge and discharge. Of course, in a cell yielding only 150 ampere-hours we do not charge more than that amount. This capacity is from 1/3 to ½ greater than that of the same weight of any other cell manufactured, unless it is of a very recent date, the official tests of which have not been published in the electrical journals.
Prof. Gerard, of the University of Liege, made the following test with 24 elements or cells of the Julien type:
Charge
Duration, 7 hours 33 minutes.
E. M. F. per cell, 2.35 volts.
Average current per kilo., 1.86 amperes.
Energy absorbed per kilo., 10,700 kilogram meters.
Ampere-hours per kilo., 14.
Discharge
Duration, 6 hours 48 minutes.
Final E. M. F. per cell, 1.89 volts.
Average current per kilo., 1.74 amperes.
Energy given out per kilo., 8600 kilogrammeters.
Ampere-hours per kilo., 11.83.
Commercial efficiency, 80 per cent.
On the subject of the capacity of the accumulators and their efficiency, Mr. Gerard says that to store one electric horse-power-hour (270,000 kilogrammeters) it requires:
180 kilos, of Plante’s accumulators.
60 kilos, of Faure’s accumulators, with an efficiency of only 50 per cent., according to the experiments made at the Conservatoire des Arts et Metiers in Paris.
According to the above, 270000/10700 or 25 kilos, of Julien’s accumulators give one electrical horse-power hour with an efficiency of 80 per cent.
The following diagrams of discharge of a Julien battery (Figs. 5 and 6) composed of 29 cells of 88 pounds each, used by the Edison Company in Paris, show its efficiency and regularity of action charged at a rate of 15 ampere-hours per 2 pounds, or 660 ampere-hours in all, and discharged at the rate of 13½ amperes per 2 pounds, or 594 ampere-hours in all, with an efficiency in ampere-hours of 90 per cent. It will be perceived that the E. M. F. of the battery was 2 volts after twenty-two hours of continuous discharge:
Fig. 7 shows the fall in potential of a Julien battery, discharged to 0.7 volt (open circuit), beginning at 2.2 volts and maintaining an E. M. F. of 2 volts for 16½ hours, at the rate of 10 amperes, the remaining 27½ hours of the discharge showing a gradual fall to 0.7.
Fig. 8 shows the curve of the amperes in discharging the same battery, beginning at 10 amperes, and remaining constant for 22½ hours, the current falling gradually to 0 on the 44th hour.
The total weight, including retaining jar and acid, was about 30 pounds.
The ten negative plates contained in the above element were carefully weighed, and the amount of spongy lead estimated to be 4.5 pounds. As only half of the active matter in each of the two outside plates of an element is available, the actual amount of spongy lead concerned in the discharge was 10 per cent, less, or 4.05 pounds.
Now, 4.05 pounds of spongy lead will yield, theoretically, 486-volt ampere-hours, and the amount actually yielded in the experiments shown by the above diagrams, was 480-volt ampere-hours. This practical confirmation of the theoretical calculation is most gratifying.
From the above, it is quite evident that we have all the necessary data at hand to calculate the cost of furnishing light or power by accumulators, provided we know the cost of the batteries and their life, or, what is the same thing, their cost of maintenance.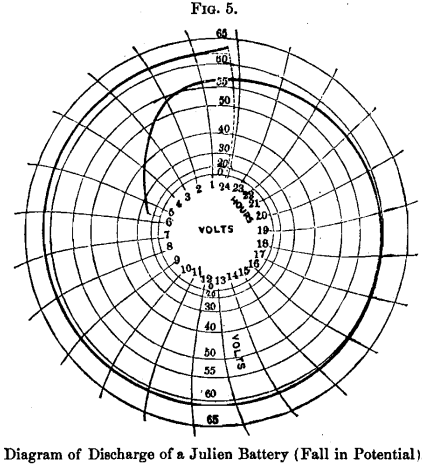
It will be unnecessary for me to go into calculations relating to the horse-power required to move so many hundred tons of ore or coal per day so many miles with a given load and in a given time. This would vary in every individual case, and if I simply point out the cost of generating an electrical horse-power and of maintaining it with batteries, it will be an easy matter to apply it to any special case.
The principal reason why there has been so little progress in the introduction and use of storage-batteries is, in my opinion, not that they were not able to stand up under the work required of them, but that their cost was enormous. We have, therefore, attacked the problem entirely from a commercial point of view, assuming that we had a first-class battery to start with, or at least the best that has ever yet been made; and I think the result of our labors in this direction are of the most gratifying nature.
We received from abroad the battery, but the crudeness, and consequently the costliness, of the methods of manufacture imported with it rendered it hopeless to ever expect to accomplish anything on a commercial scale. In the short space of two years we have so changed the process that we are able to make eight times the number of batteries with the same number of men. With the force formerly required to make 100 cells per day we can now make 800. 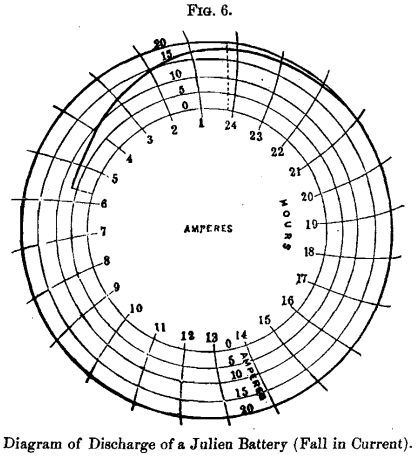 Here, then, we are met with the crucial question: how much will it cost to introduce this system, and after its introduction how much will it cost to maintain it?
Here, then, we are met with the crucial question: how much will it cost to introduce this system, and after its introduction how much will it cost to maintain it?
We have seen from the above that it requires 25 kilos, or about 55 pounds to generate 1 horse-power-hour; therefore, if the work to be done required 100 horse-power-hours, or 10 horse-power for ten hours, it would manifestly require 5500 pounds of battery. As each element of 19 “B” weighs about 25 pounds, this would mean 220 elements, and, at our present list-price of $8, would cost $1760. I have no hesitation in stating that we will be able to bring this price down one-half, owing to improved methods of manufacture.
The cost of maintenance per horse-power would be, allowing 2 cents per horsepower per hour, $2 per day or $600 per year of three hundred days; and assuming that the batteries only lasted one year before the positive plates had to be removed, the cost of renewals would be about $400, or, say, as high as $600. This would make the cost of a horse-power 4 cents instead of 2 cents. 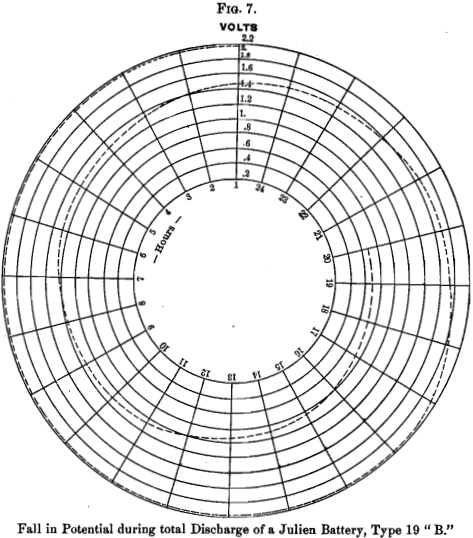 I venture the assurance that the price of batteries will so steadily decline, when once they are introduced in sufficient numbers, that the increased expense per horsepower-hour from using them will only be a fraction of a cent.
I venture the assurance that the price of batteries will so steadily decline, when once they are introduced in sufficient numbers, that the increased expense per horsepower-hour from using them will only be a fraction of a cent.
It may be of interest to some of our members who are engaged in mine-traction to know that the actual amount of power required to propel one of our 18-foot cars on the Fourth Avenue line in New York, from Eighty-fifth Street to the Post-Office and return, about 11½ miles, is 15 electrical horse-power-hours. At 2 cents, this is 30 cents, or 2 5/10 cents per car-mile.
The cars on which this result was obtained have two trucks with eight wheels, and two 15-horsepower motors, one on each truck. The gearing from the motor-shaft to the counter-shaft is by gears en echelon. The power is transferred by means of chains to the sprocket- wheels on the axles. 144 cells are used, coupled in four groups, so that the E. M. F. at the motor terminals can be 75, 150, 225, or 300 volts.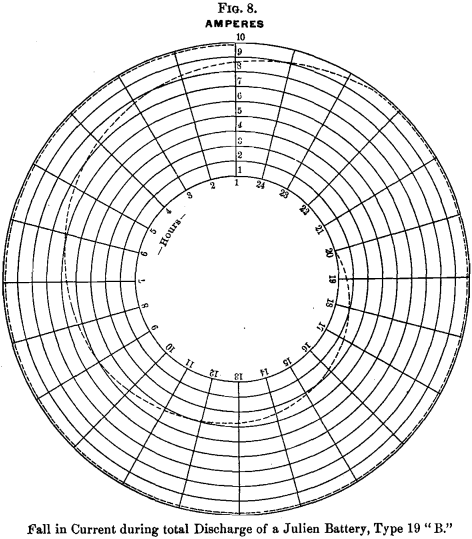 This allows four different speeds. After such a car has made a round-trip of 11½ miles, it requires, as I have said, 15 electrical horse-power-hours to replace the energy drawn from the batteries. We are able, however, to make two, or even three, round-trips, or about 36 miles in all, without changing the batteries. I am indebted for the above information to Mr. J. C. Chamberlain, Electrical Engineer and Superintendent of the Julien Electric Traction Company.
This allows four different speeds. After such a car has made a round-trip of 11½ miles, it requires, as I have said, 15 electrical horse-power-hours to replace the energy drawn from the batteries. We are able, however, to make two, or even three, round-trips, or about 36 miles in all, without changing the batteries. I am indebted for the above information to Mr. J. C. Chamberlain, Electrical Engineer and Superintendent of the Julien Electric Traction Company.
