An aqueous oxidation treatment process developed by the Bureau of Mines has been shown to be effective for treating carbonaccous gold ores to improve gold recovery. Ozone, chlorine, sodium hypochlorite (NaOCl), and calcium hypochlorite [Ca(OCl)2] were all shown to be effective reagents for oxidizing the carbonaceous material in the ore. However, generation of oxidizing conditions in the ore pulp by electrolysis of brine was the most efficient and offered the lowest operating cost. During the course of development of the oxidation sequence for carbonaceous gold ores, it was observed that most of the mercury present in the carbonaceous ore was dissolved. Investigation of this phenomenon showed that the very small amount of HgS mineral present in the ore was oxidized to a soluble salt during electrolysis. The effectiveness of electrooxidation in extracting the mercury in the parts-per-million range from carbonaceous gold ores indicated that a sequence based on electrolysis of cinnabar ore, slurried with brine, would be effective in extraction of mercury from low-grade sources.
Many geologists and mill operators think that extensive deposits of low-grade mercury (1 to 2 lb/ton) exist in Nevada. This thought is confirmed by a University of Nevada study. Mercury recovery from these materials by retorting and furnacing techniques has generally been considered questionable from the economic standpoint, because of either untenable operating costs or excessive mercury losses in off gases.
Hydrometallurgical processes for treating mercury ores are not new. The use of aqueous chlorine solutions for leaching mercury was reported by Glaeser in a patent as early as 1927; however, no commercial use of the process was ever made. In the most recent patent, which appeared in 1969, by Parks and Baker, a hypochlorite system was used to leach mercury from the ore, and the dissolved mercury was recovered from solution by activated carbon. Although much of the chemistry involved is similar, electrolytic oxidation differs from hypochlorite addition in the following ways: (1) The chloride concentration is substantially higher, which facilitates formation of the stable and highly soluble HgCl4-² species, and (2) comparable data show that electrooxidation is more efficient than the addition of an equivalent amount of hypochlorite for dissolving mercury, which indicates that considerable oxidation of the mineral occurs in the proximity of the anode.
Results and Discussion
Preliminary electrooxidation experiments were conducted on a variety of ores from the Clear Lake Region in California and the Tonopah district, the Midas region, and the Winnemucca and Reno areas of Nevada to evaluate the oxidation system. This investigation represented a wide range of host rock, varying from clay to hard opalite. The grade of the ore ranged from 0.6 to 20 lb mercury/ton. Extractions of 90 to 99 percent were obtained on all of these ores with 10 to 50 kwhr/ton.
Important parameters in the electrooxidation process include temperature, salt concentration, current density, type of electrodes, electrode spacing, treatment rate (amp/ton of ore), and particle size of ore. Ore from the Goldbanks district, Nevada, was selected as a standard, and optimum operating conditions were studied for this ore. The ore occurs in volcanic rock and is opalitic in nature. The cinnabar is disseminated through the opalite rock and
coats fragments and line fractions in the brecciated chalcedonic rock. The ore sample treated contained 2.5 lb mercury/ton.
Laboratory experiments were conducted in the following manner:
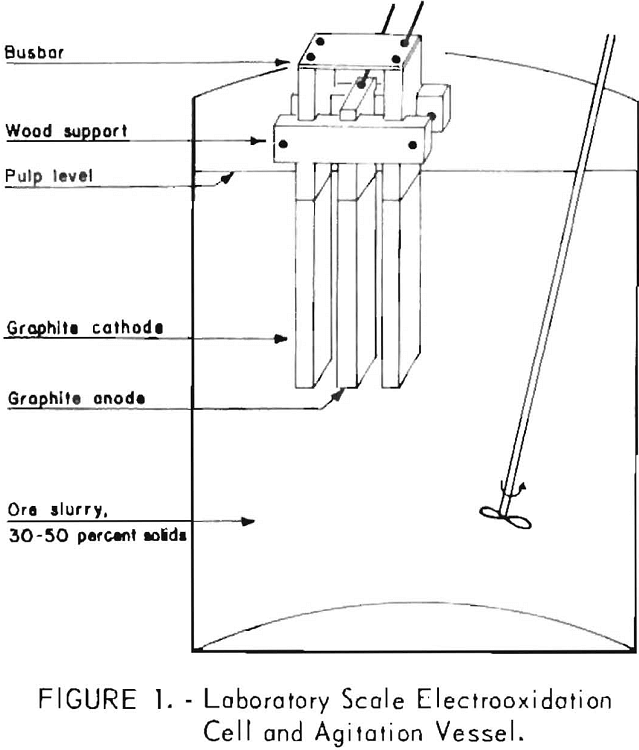
(1) 1,350 grams ( 3 pounds) of ore, 300 grams of NaCl, and 2,500 ml of water were slurried together in a 4-liter beaker; (2) the temperature was held constant; (3) the electrodes were immersed in the ore slurry; and (4) the direct current was adjusted to the desired amperage. Figure 1 shows the reaction system. (The electrolytic cell dimensions are out of proportion so that the anode-cathode relationship can be shown.) The following conditions were chosen for initial runs, based on preliminary experiments and experience gained from development of the carbonaceous gold process: Ore size, 100 percent minus 35 mesh; treatment rate, 5 amperes; current density, 0.3 amp/in²; pulp density, -35 percent; salt concentration, 10 percent; reaction temperature, 30° C; and graphite-graphite electrodes. The possible cell reactions and overall oxidation and solution reactions are presented below. The hypochlorite ion is produced in the proximity of the anode, with equivalent production of hydroxyl ions and hydrogen gas at the cathode. The hypochlorite or oxidizing species then decomposes with the following oxidizing reaction:
OCl- → O + Cl-…………………………………………………………………………….(1)
HgS + 4O → HgSO4…………………………………………………………………….(2)
and
3HgSO4 + 2HgO → HgSO4 · 2HgO + 2H2SO4…………………………….(3)
These two products have limited solubility in relatively neutral aqueous solutions. However, the tetrachloro mercury complex is very soluble in brine solutions. Therefore, the following reaction is proposed:
HgSO4 + 4NaCl → Na2HgCl4 + Na2SO4…………………………………………..(4)
or
HgSO4 · 2H2O + 12NaCl + 2H2O → 3Na2HgCl4 + Na2SO4 + 4NaOH……………………..(5)
If some direct oxidation of cinnabar takes place at the anode, it could be represented by the following equation:
HgS + 4O= — 8ε → HgSO4…………………………………………..(6)
Reactions 3 and 5 are included because the basic mercuric sulfate is known to exist in the oxidation sequence of cinnabar.
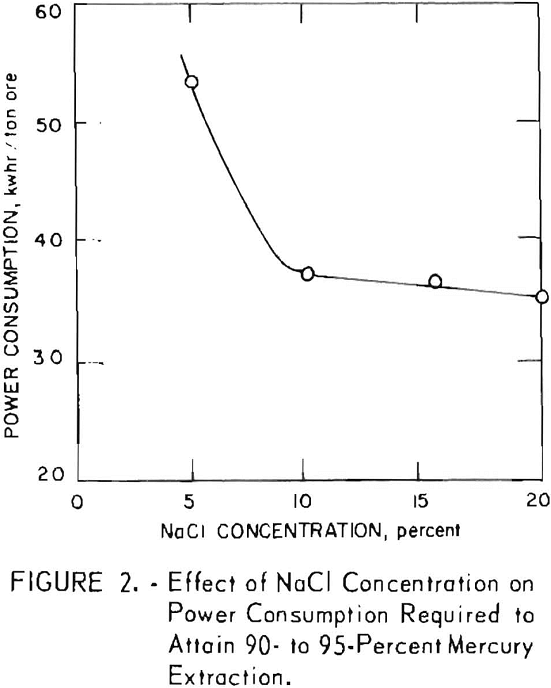
The effect of salt concentration on the power consumption required to attain 90- to 95-percent mercury extraction was the first parameter investigated (fig. 2). Salt concentrations of 5, 10, 16, and 20 percent were studied. Figure 2 shows a sharp decrease in required power for 90- to 95-percent extraction if the salt concentration is increased from 5 to 10 percent. The curve levels off between 10 and 20 percent salt. The data indicate that 10 percent salt is an adequate concentration to obtain high conductivity with the graphite-graphite electrode system. Because salt consumption is an important economic factor, selection of the salt concentration to be used in a commercial operation depends on several factor such as the price of salt versus the cost of power at the millsite, the amenability of the particular ore to low-cost liquid-solid separation and washing schemes, and the attendant salt loss in the tails. For example, if operations were being conducted on an ore that filtered poorly and was located in close proximity to low-cost salt, a counter-current decantation washing circuit would be favored. Conversely, if the filtering and washing characteristics of the ore were good, comparatively high prices could be paid for both power and salt because high salt concentrations
could be used to reduce power consumption and the salt could easily be recovered for recycle.
Experiments are presently being conducted with a different electrolysis cell which incorporates a lead dioxide (PbO2) anode-iron cathode electrode system. Data obtained with this cell indicate that effective oxidation is generated with half the salt concentration required for graphite-graphite electrodes. Successful development of this system will add considerable flexibility to the process in that salt and power costs will contribute much less to overall operating costs.
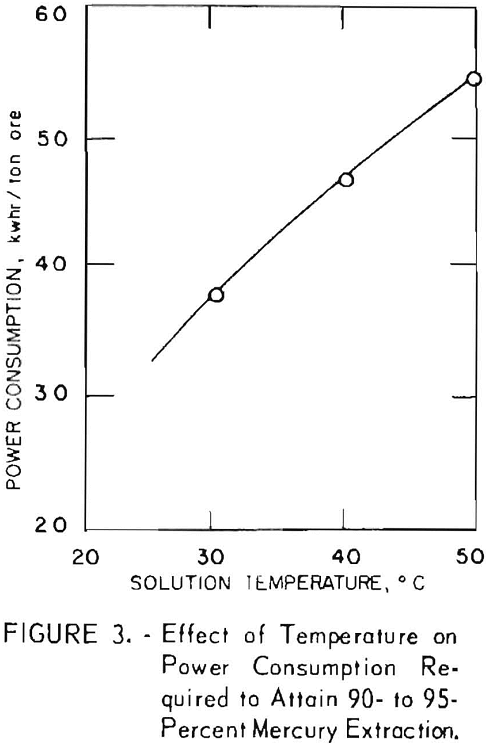
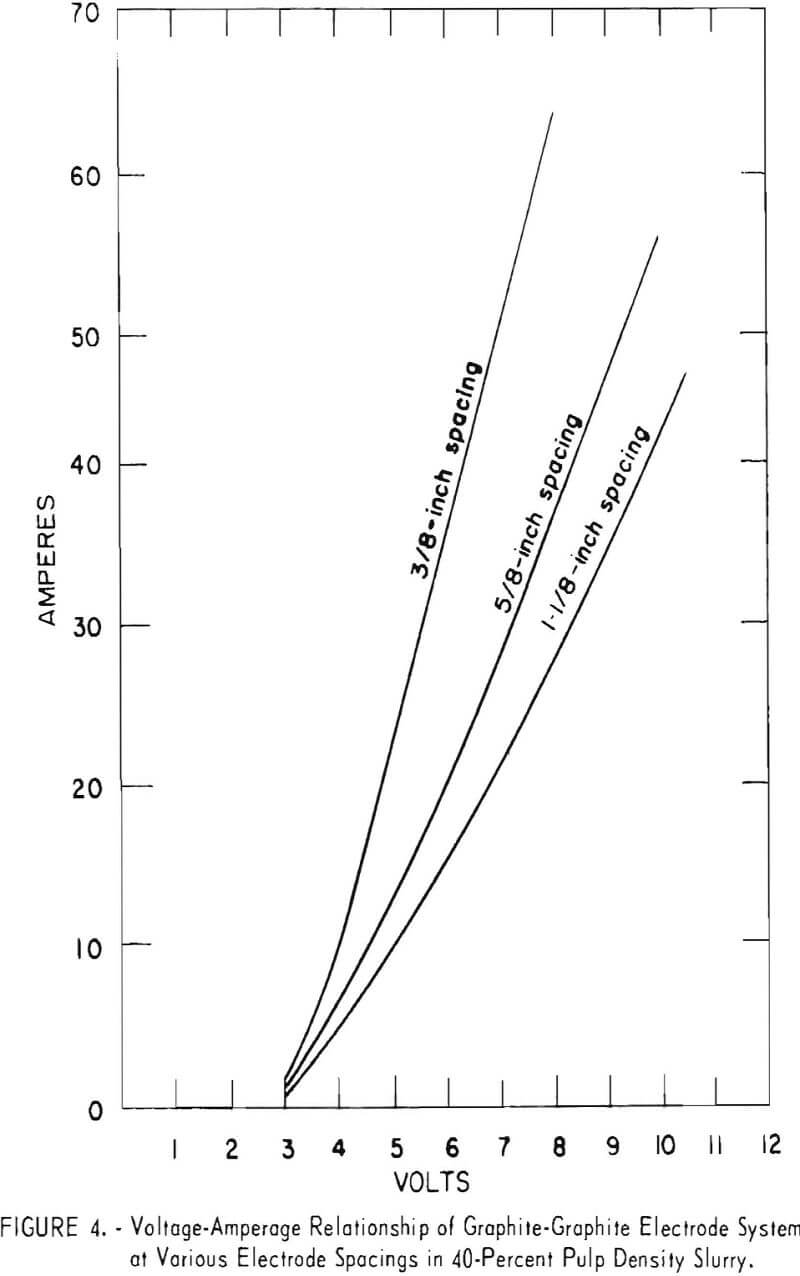
The effect of temperature on mercury extraction was determined at 30°, 40°, and 50° C. Figure 3 shows a sharp increase in power consumption as the temperature increases. The optimum temperature appears to be 30° C; moreover, a temperature of 30° C can be sustained in the system under the power conditions without external temperature control. Operation at temperatures much lower than 30° C would be impractical, because the heat generated during electrolysis would require cooling of the pulp. As temperature is a function of the conductivity of the electrolyte and the power required to accomplish the oxidation, both the electrode spacing and salt concentration are critical in maintaining desired temperature. Generally speaking, electrode spacing should be as close as is consistent with good pulp flow between the electrodes. The effect of increasing electrode spacing on voltage-amperage relationships is shown in figure 4. Spacings of 3/8, 5/8, and 1-1/8 inches, a pulp density of 40 percent, and a salt concentration of 10 percent were used for the experiments. As expected, the resistance drop across the electrodes increased directly with an increase in electrode spacing. Generally, the use of lower pulp density enables the use of closer electrode spacing, which in turn allows the use of lower salt concentrations. However, exploitation of these favorable effects is limited by lower concentrations of the oxidation agent and consequent slowing of reaction rates, difficulty in suspending solid particles in the agitation system, and increased complexity in solid-liquid separation.
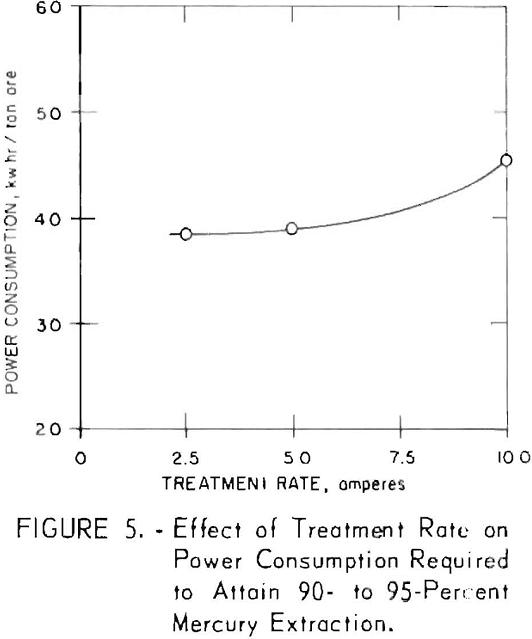
The effect of treatment rate (amperes) on the mercury extraction, obtained at a total treatment of 17.5 amp hr, was determined in a series of experiments in which the ore was treated with current loads of 2.5, 5, and 10 amperes (fig. 5). The treatment rate of this particular ore appears to be a 5-ampere load, which corresponds to 3.5 hours of treatment time. If the treatment time is doubled to 7 hours (2.5 amperes), no change in the power consumption per ton is observed; but if the treatment time is halved (10 amperes) the power required for 90- to 95-percent extraction is increased nearly 20 per cent. Because the treatment rate affects the sizing and capital cost of almost all the plant equipment, the increased cost of rectification and operation for higher treatment rates should be carefully balanced against the cost of smaller agitators and supplemental equipment.
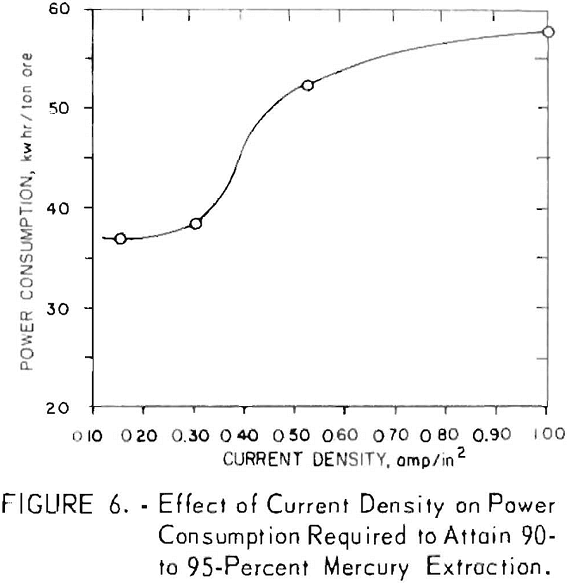
Current density is a critical operating parameter in that excessive current densities tend to increase the temperature and decrease the efficiency of the desired chemical reactions at the electrode-electrolyte interface. Figure 6 shows the effect of increasing the current density from 0.15 to 1.0 amp/in² on the power required to give 90- to 95-percent mercury extraction at
a constant temperature of 30° C. The data show that current densities of <0.40 amp/in² should be used for maximum efficiency; however, the tonnage of a mill and the agitator size required are important considerations in projecting the practical number of cells that can be employed.
Grinding is an important part of any hydrometallurgical process because it is necessary to release the mineral from the host rock so that it can come in contact with the. reactants. The effect of particle size on mercury extraction was investigated using several ores with different host rocks. A coarse grind of 100 percent minus 35 mesh was shown to be sufficient for all ores tested. However, excessive grinding resulted in an 80 percent minus 200-mesh pulp and caused a decrease in mercury extraction. The decline in mercury extraction with fine grinding was attributed to a loss in oxidizing efficiency caused by consumption of oxidant by other minerals in the gangue material. A grind of 100 percent minus 35 mesh should not be construed as a standard grind required for the process, because each ore would have to be tested individually to determine the optimum particle size for reaction.
Precipitation of the mercury from leach solutions was investigated in the laboratory, with active metals such as zinc, iron, and aluminum. Zinc was the most effective for precipitation, followed by iron and finally aluminum which gave poor results under all conditions investigated. A pH of 2 to 3 gave the best results in the precipitation step. Because the ore buffered the recycle barren solutions only a small amount of brine was required to maintain an electrolysis pH of 7 to 8. Iron does not amalgamate with mercury; consequently, experiments with iron as the precipitant resulted in recovery of part of the mercury as finite droplets and the remainder as a black iron-mercury sludge containing 30 to 50 wight-percent mercury. The only problem encountered with iron was that it became passive with time and a zinc scavenging step was required to obtain barrens in the 1- to 10-ppm range. The effect of pH and the amount of zinc in contact with solutions on mercury recovery is shown in table 1.
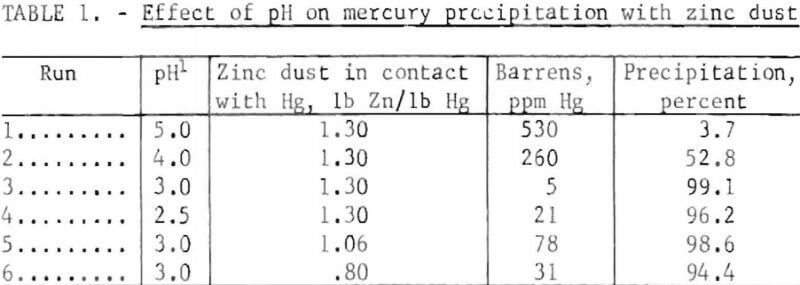
The data indicate that near optimum pH for precipitation appears to be close to 3.0 with a ratio of 1.3 lb zinc/lb mercury in contact with the pregnant solution. Mercury recovery was 99.1 percent under these conditions, and zinc consumption per pound of mercury recovered was 0.5 pound. Since amalgamation with zinc starts immediately on contact with the pregnant solution, vigorous stirring is important.
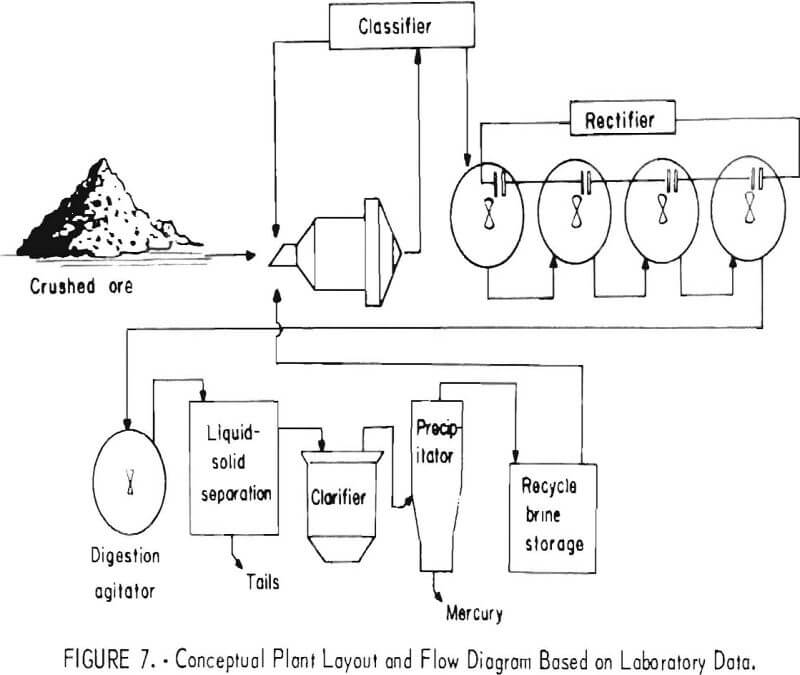
A process flow diagram, based on laboratory data, is shown in figure 7. The conceptual plant would consist of a crusher; a ball mill; four agitation tanks for electrolytic oxidation; a digestion tank; provision for solid-liquid separation such as a filter, centrifuge, or thickeners; a clarifier; and a mercury precipitator. This general flow scheme was utilized for additional experiments conducted in the 100- to 200-lb/hr pilot mill shown in figure 8. Mercury ore from a hot-spring deposit containing 1.4 lb mercury/ton was used


for initial pilot mill experiments. The ore was ground to 100 percent minus 20 mesh and oxidized with a power consumption of 12 kwhr/ton of ore. Figure 9 is a closeup view of an oxidation tank, showing the PbO2 anode-iron cathode electrolytic cell utilized for the experiments. Total mercury recovery was 96 percent. The ore was filtered, and the filter cake was washed with a displacement wash. Total salt loss in the filter cake was 26 lb/ton of ore. The pregnant solution was acidified with H2SO4 to a pH of 2,3 to 2.5 and recovered from leach solutions by two methods, including precipitation on detinned cans, followed by a scavenging precipitation on zinc shavings and precipitation with zinc dust. Barren electrolyte solutions were recycled to the ball mill. Precipitation on detinned cans closely paralleled laboratory experiments in which the iron was coated with mercury and became passive with time, thus requiring a zinc scavenging step to obtain low barrens. The use of a 30 percent excess of zinc dust for precipitation resulted in >99 percent (1 to 4 ppm barrens) mercury recovery as zinc amalgam. Distillation of the mercury for the zinc at 550° C resulted in complete recovery as metal. The precipitation procedure is being optimized at present.
Conclusions
The Bureau of Mines Reno Metallurgy Research Center has developed a hydrometallurgical process for low-grade mercury ores. Mercury recovery of 90 to 99 percent was obtained in the laboratory by electrooxidation of mercury ore pulps at 30° C with a 10-percent salt solution as the electrolyte. Power consumption ranged from 10 to 50 kwhr/ton ore. Operation of the process in a 100- to 200-lb/hr pilot mill substantiated laboratory data in demonstrating the feasibility of the process. Precipitation of mercury from solution in a sequential iron-zinc treatment was shown to be feasible; however, zinc was more effective as a precipitant in achieving low barren solutions. Pilot mill experiments are continuing with emphasis on evaluating a variety of different ores and optimizing the precipitation step. The use of PbO2 anode-iron cathode electrode systems that show promise for effectively oxidizing the cinnabar minerals at salt concentrations in the 4- to 5-percent range is also presently under investigation.
