Table of Contents
For many years, we conducted a research program on electrolytic preparation of rare-earth metals. Cerium, lanthanum, and neodymium, praseodymium, and didymium metals have been electrowon from their respective oxides dissolved in fluoride melts.
Commercial production of mischmetal, a mixed rare-earth metal consisting essentially of cerium, lanthanum, neodymium, and praseodymium, is presently limited to electrolysis of molten mixed rare-earth chlorides. Drawbacks of this method include the preparation of feed material as a chloride and the production of chlorine as a byproduct, which in some cases can lead to an air pollution problem.
To utilize a cheaper feed material as well as to avoid the emission of chlorine, we investigated electrowinning mischmetal from a treated bastnasite concentrate.
Equipment
Two cells were used in this investigation. One cell, 3 inches in diameter, was used to investigate the performance of the electrolyte over a prolonged period of operation and to determine the optimum electrolyte composition. The other cell, 12 inches in diameter, was used for demonstrating electrowinning and tapping of misch metal. The smaller cell consisted of a graphite crucible 3 inches in inside diameter and 4 inches deep, as the electrolyte container; a 0.2-inch-diameter molybdenum rod as the cathode; and a 1.5-inch-diameter hollow graphite tube as the anode, through which the treated bastnasite concentrate was added to the cell. The anode had a closed bottom and ¼-inch slots in the lower section of the wall. A molybdenum crucible was placed at the bottom of the cell to collect: the misch metal product. A selenium rectifier rated at 100 amperes supplied the direct current for electrolysis. The cell was externally heated.
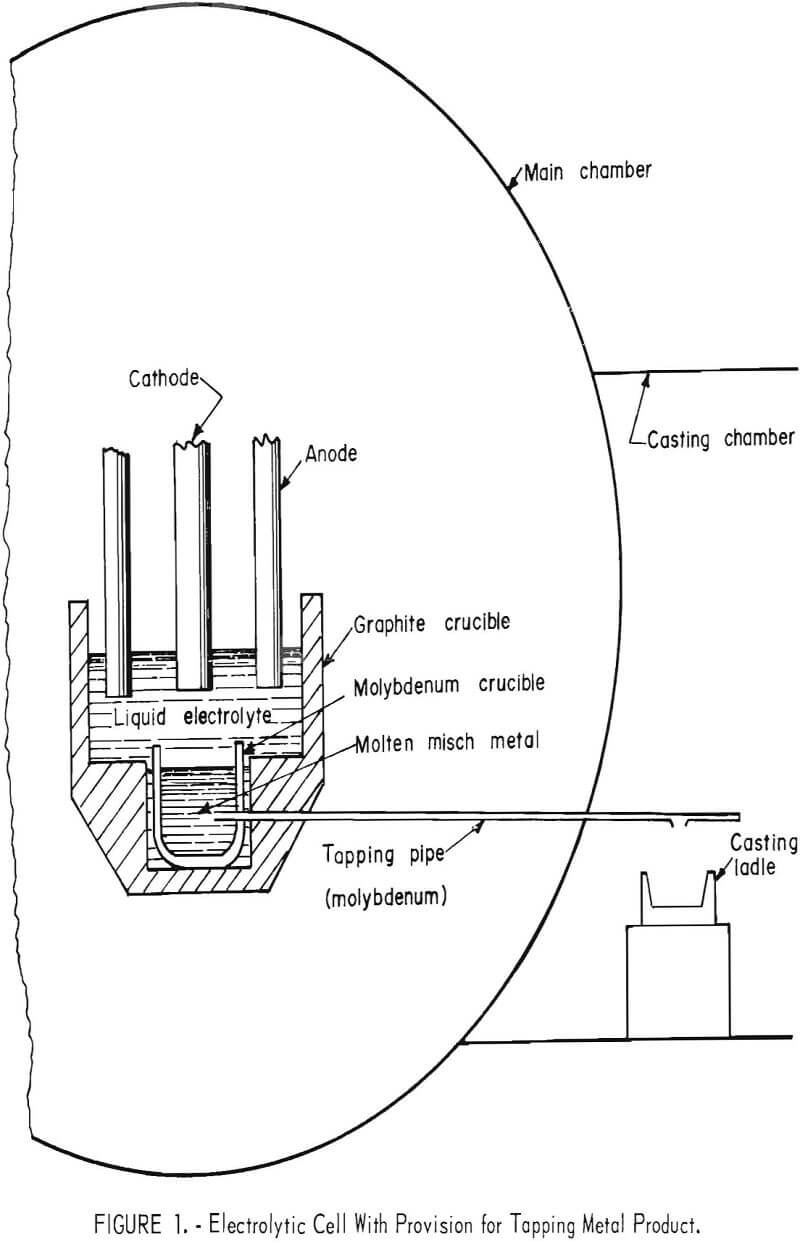
The larger cell was 12 inches in inside diameter with eight 2-inch-diameter graphite-rod anodes surrounding a 2-inch-diameter molybdenum-rod cathode. The anode-cathode spacing was 2-½ inches. Feed material was mechanically added to the cell through a 1-inch-diameter tube positioned between two anodes. A molybdenum crucible was placed below the cathode to collect the misch metal product. A molybdenum pipe was connected to the product-containing crucible and extended — 30 inches into a casting chamber, where the molten misch metal was collected in a mold. A schematic drawing of the cell is shown in figure 1.
The initial melting of the electrolyte in the 12-inch-diameter cell was accomplished by applying alternating current to a graphite resistor positioned between the cathode and two anodes. When the electrolyte reached the desired temperature of 950° C, the resistor was removed., and the direct current used in the electrolysis supplied sufficient heat to maintain the cell at this temperature. The direct current was furnished by a silicon rectifier rated at 1,000 amperes.
Both cells were insulated by refractory bricks and installed in a steel chamber, 4 feet in diameter and 4 feet long, equipped with glove, view, and access ports. The gas tight chamber was evacuated and backfilled with helium four times before starting each operation. The electrolyte temperature was determined with an optical pyrometer and was also monitored by a Chromel-Alumel thermocouple inserted into the bottom of the graphite crucible.
Materials
In the preliminary electrowinning experiments, both commercial-grade and treated bastnasite concentrates were tested as feed materials. When the sulfur content of the materials was >0.30 percent, the misch metal produced was not well coalesced, and a layer of black and brown salts settled out in the electrolyte. This problem was finally overcome by using feed material that had been given the following treatment:
- The bastnasite concentrate was leached with dilute hydrochloric acid to remove CaCO3 (calcium carbonate) and SrCO3 (strontium carbonate).
- The residual was roasted with Na2CO3 (sodium carbonate) at 700° C to convert the impurity barite to BaCO3 (barium carbonate) and to decompose the carbonate.
- The material was leached in water to remove Na2SO4 (sodium sulfate) and dried at 400° C.
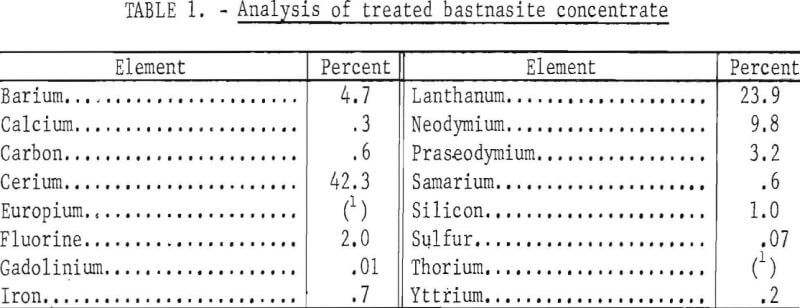
The treated bastnasite concentrate, which was used in all experiments in this report, was essentially a mixture of oxides with a small amount of fluorides. Analysis is given in table 1.
The electrolyte was composed of REF3 (mixed rare-earth fluoride), LiF (lithium fluoride), and BaF2 (barium fluoride). The mixed rare-earth fluoride was produced from the treated bastnasite concentrate by hydrofluorination. Oxygen content in the fluoride was 0.64 percent and the sulfur content was only 0.04 percent.
Both the lithium fluoride and barium fluoride were technical-grade commercial products. The lithium fluoride was listed by the manufacturer as 99.6-percent pure, containing from 0.4 to 0.6 percent H2O. The barium fluoride was listed as 98.2-percent pure, containing 0.2 percent BaSiF6 (barium fluosilicate), 0.25 percent BaCO3, and 0.1 percent BaSO4 (barium sulfate). All the electrolyte components were dried at 300° C for 24 hours before they were used.
Experimental Procedures and Results
Only a very limited number of salts are stable in the presence of rare-earth metals and form an electrolyte capable of dissolving rare-earth oxides. Therefore, the selection of salts to compose the electrolyte is, for all practical purposes, restricted to lithium fluoride, barium fluoride, and rare-earth fluorides. The temperature of operation must be high enough to allow a complete coalescence of the metal product and to maintain the electrolyte sufficiently fluid in order to obtain a satisfactory electrolysis. On the other hand, the electrolyte temperature should not be higher than necessary. From experiences in electrowinning of cerium and lanthanum metals with similar electrolytes, an operating temperature of 950° C was selected. This temperature was later proved experimentally to be satisfactory and was used throughout the investigation.
Sustained Performance of Electrolyte System
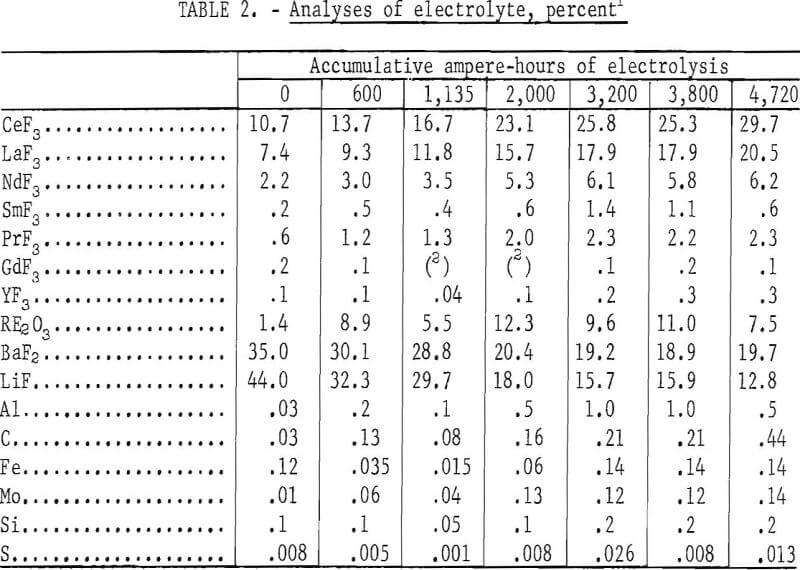
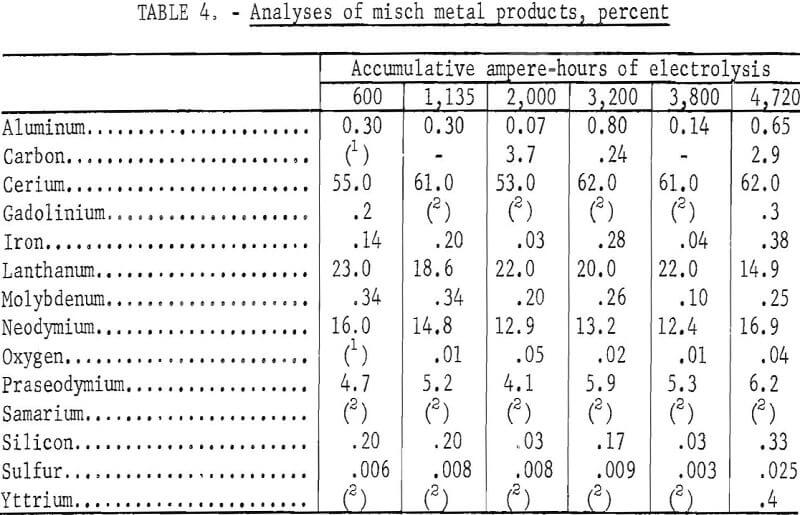
Because the cell feed was a relatively impure mixture, a series of experiments was made to determine whether the electrolyte can maintain a reasonably uniform performance over a prolonged period of operation. Two pounds of the same starting electrolyte was used in 38 electrodepositions over 4,720 ampere-hours of electrolysis. The electrolyte was initially composed of 21 weight-percent REF, 35 weight-percent BaF3, and 44 weight-percent LiF. The detailed
analysis is given in table 2.
The electrolyses were performed in the 3-inch-diameter cell previously described. The current was 40 amperes; average applied voltage was 6.0 volts; cathode current density was 64 amp/in²; and anode current density was 8.5 amp/in². Treated bastnasite concentrate was added continuously as feed material. After each electrodeposition of 124 ampere-hours, the electrolyte with the misch metal product was poured into an iron mold and cooled. The metal product was recovered, and the electrolyte was crushed, mixed, and sampled. Makeup salts with the same composition as the initial electrolyte were added to restore the electrolyte to its original volume before each electrodeposition. The total makeup electrolyte used in the entire series was 4 pounds, equivalent to a turnover of twice the original electrolyte.
A total of 3,275 grams of treated bastnasite concentrate was used, and a total of 1,225 grams of misch metal was recovered. Misch metal was produced and recovered even at the end of the series. The quantity of metal recovered in each electrodeposition varied; the variation was probably caused by the presence of undissolved RE2O3 (mixed rare earth oxide) in the electrolyte, which would interfere with the coalescence of the metal. Porter and Brown reported that the solubilities for CeO2 (ceric oxide) and La2O3 (lanthanum oxide) ranged from 2.0 to 2.3 weight-percent in a similar electrolyte system at 850° and 950° C.
The analyses of the electrolyte samples taken at various intervals are shown in table 2. Except for the increase in carbon, no significant buildup of impurities in the electrolyte was observed. The varying amounts of mixed rare-earth oxide in the electrolyte were caused by periodic overfeeding of the treated bastnasite concentrate. The increase of rare-earth fluorides was largely because of the introduction of fluorides with the concentrate. The presence of uncoalesced rare-earth metal in the electrolyte also made the rare-earth fluoride content appear higher because of the analytical methods used. Since barium was introduced with the concentrate and some LiF was lost by volatilization, the BaF2-to-LiF ratio was progressively increased. A constant electrolyte composition could be maintained by making adjustment in the amounts of LiF and BaF2 in the makeup salts.
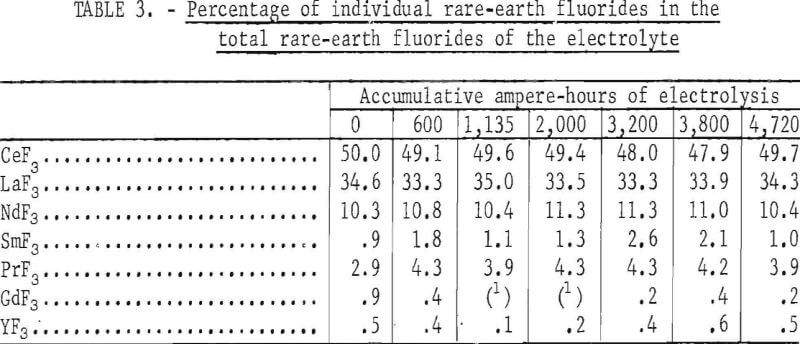
Table 3 shows the individual rare-earth fluorides as percentages of the total rare-earth fluoride content in the electrolyte. Notwithstanding the fact that the analytical results could be affected by the presence of uncoalesced metal, the proportion of the major individual rare-earth fluorides remained constant.
The analyses of the misch metal are shown in table 4. With the exception of carbon, the increase in impurities was not appreciable. The variations in individual rare-earth contents were small.
Determination of Optimum Electrolyte Composition
After the feasibility of sustaining the electrowinning of misch metal from treated bastnasite concentrate in the LiF-BaF2-REF3 electrolyte system was indicated, experiments were performed to determine the optimum electrolyte composition. From previous experiments, it was apparent that for an electrolytic cell to perform satisfactorily, the settling out of undissolved mixed rare-earth oxide or the separation of a second phase in the electrolyte system must be avoided. Therefore, the most desirable electrolyte composition would be one that offers the following results at the operation temperature:
- Allows the highest treated bastnasite concentrate feed rate with 100-percent utilization of the mixed rare-earth oxide.
- Carries the highest current.
- Permits no separation of a second phase in the electrolyte.
Electrolytes containing from 35 to 80 weight-percent of mixed rare-earth fluoride with the balance in equal parts of LiF and BaF2 were tested in the 3-inch-diameter cell. The experimental conditions were as follows: Electrolyte, 2.5 pounds; treated bastnasite concentrate, 180 grams (at a feed rate of ~ 1 g/min); duration, 3 hours; temperature, 950° C; voltage, from 7 to 9 volts; and current, 40 to 90 amperes.
The electrolyte containing 80 weight-percent mixed rare-earth fluoride showed separation of a rare-earth fluoride-rich phase. The electrolyte containing 50 weight-percent mixed rare-earth fluorides resulted in the greatest rare-earth oxide utilization and the highest current-carrying capacity.
Further experiments were made with electrolytes containing 50 weight-percent mixed rare-earth fluoride and varying amounts of LiF and BaF2. Experimental conditions were the same as those described earlier. The data are given in table 5.
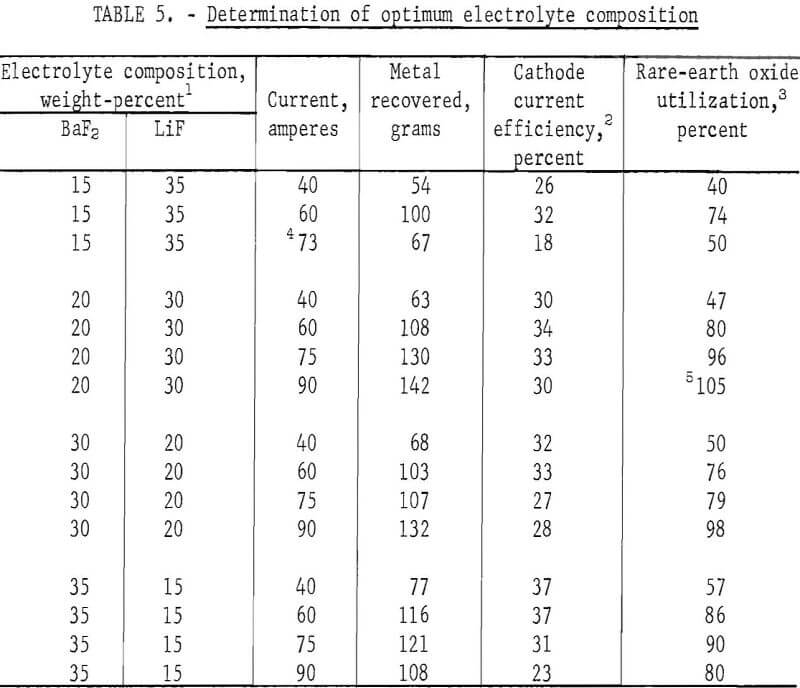
In another series of experiments with the same electrolyte composition, the treated bastnasite concentrate used was increased by 15 percent to 207 grams (at a feed rate of — 1.15 g/min). Settling of undissolved rare-earth oxide occurred, and utilization of the rare-earth oxide was appreciably <100 percent.
An electrolyte containing 50 percent REF3, 20 percent BaF2, and 30 percent LiF appeared to have the optimum composition. The highest utilization of rare earth was obtained at a current of 90 amperes and a concentrate feed rate of 1 g/min.
Electrowinning and Tapping of Misch Metal
The operation of electrowinning and tapping of misch metal was investigated in a 12-inch-diameter cell with provisions for tapping the metal product (fig. 1). The cell was rated at 1,000 amperes. One-hundred pounds of electrolyte containing 50 weight-percent REF3 , 20 weight-percent BaF2, and 30 weight-percent LiF was used. The cell was maintained at the operating temperature for 4 days; however, because of the work schedule, only three electrodepositions were performed, each lasting — 3 hours and 20 minutes. During the hours when electrolysis was not conducted, alternating current was passed through the electrodes to keep the electrolyte molten.
To tap the metal product after electrolysis, the cathode was raised out of the electrolyte, and an alternating current of 1,500 amperes at 9 volts was passed between the anodes and the molybdenum tapping pipe. Metal flowing from the pipe was collected in an iron mold in the casting chamber. Tapping was continued until all the metal had been drained and the electrolyte started to flow. The alternating current was then turned off, allowing the electrolyte to freeze in the tapping pipe.
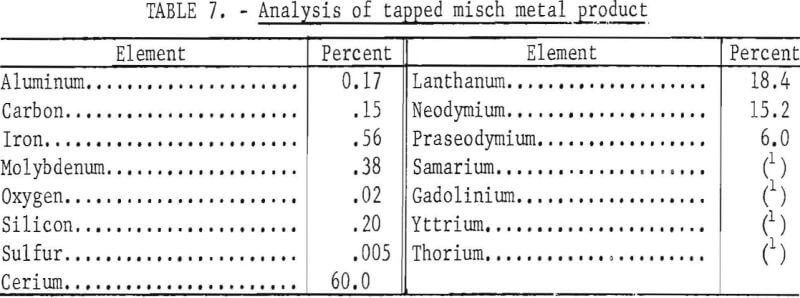
Table 6 lists the data for the operation, and table 7 shows the analysis of the tapped metal, which is depicted in figure 2.
The entire operation was conducted without difficulties. Settling of undissolved rare-earth oxide and separation of a second phase in the electrolyte were not observed. The metal product was well coalesced and tapped easily.

Conclusions
Misch metal was electrowon from a treated bastnasite concentrate at 950° C in a REFS-BaF2-LiF electrolyte. Experiments indicated that this electrolyte system would sustain prolonged use, providing periodic additions of salts were made to readjust the electrolyte composition. Satisfactory coalescence of the metal product depends on preventing the buildup of undissolved rare-earth oxide in the cell. The electrolyte composition-50 weight-percent REF3, 20 weight-percent BaF2, 30 weight-percent LiF allowed maximum utilization of rare-earth oxide at the highest current. The operation of electrowinning and tapping of the misch metal product was demonstrated in a 1,000- ampere cell.
