Table of Contents
- Introduction to Electrowinning Molybdenum
- Electrowinning Molybdenum Experimental Procedure
- Character of Metal Deposited
- Electrolyte Composition
- Effect of Temperature on Current Efficiency and Yield
- Effect of Current Density on Current Efficiency and Yield
- Effect of Current Density on Codeposition and Separation of Molybdenum and Tungsten
Laboratory results of fused salt-bath electrolysis indicate that recovery of crystalline tungsten metal from scheelite may be commercially feasible; that purity of the product, although not extremely high, can be controlled to a considerable degree in accordance with varying requirements of cost, product specifications, and purity of raw materials.
The selective deposition of molybdenum and tungsten from a single electrolyte was demonstrated as a technique for removal and recovery of molybdenum from scheelite ores.
Research studies are continuing on the evaluation of various materials of construction, on larger scale or continuous operation, and to delineate the factors of process control.
During the investigation of fused-bath electrolysis, it was observed that under controlled conditions molybdenum and tungsten can be deposited selectively. At a low voltage, molybdenum deposits first. In treating a scheelite containing 68 percent WO3 and 3 percent molybdenum, using a stationary graphite cathode, 82 percent of the molybdenum and 12 percent of the tungsten were deposited on the cathode. Of this amount, 50 percent of the molybdenum and 1.47 percent of the tungsten were deposited during the first hour of electrolysis. In another experiment, using a rotating cathode to overcome local depletion of the molybdenum, 98 percent of the molybdenum and 2 percent of the tungsten were deposited during 1.5 hours of electrolysis. This phenomenon offers a means of controlling the molybdenum content of electrolytically deposited tungsten without chemical purification of the scheelite before reduction.
Electrolyte reagents are relatively inexpensive. Approximately 67 percent of the reagents are water-soluble and can be recovered from the spent electrolyte. Power consumption is small, averaging 1 to 2 kilowatt hours per pound of tungsten produced. A systematic research program has been initiated to delineate the parameters and establish key factors of the electrolytic process for the direct production of tungsten from the mineral scheelite.
Introduction to Electrowinning Molybdenum
The principal tungsten minerals of commercial importance are wolframite (Fe, Mn)WO4 and scheelite (CaWO4). Present techniques for the commercial production of tungsten metal involve expensive, complex chemical purification to produce tungsten trioxide (WO3) before reduction to metal. A usual method of chemical treatment involves sodium carbonate decomposition of the ore in pressure digesters to produce sodium tungstate, which subsequently is precipitated with calcium to yield synthetic scheelite (CaWO4). Calcium tungstate is dissolved in hydrochloric acid to produce a tungstic acid, which is precipitated and ignited to tungsten trioxide. This product is reduced with hydrogen to produce tungsten metal.
The purpose of this preliminary investigation was to determine the feasibility of electrowinning tungsten directly from impure tungsten compounds, particularly scheelite, in a fused alkali phosphate, or borate bath without chemical purification of the starting material. Past investigations achieved satisfactory results on a laboratory scale in electrowinning tungsten from pure WO3, and wolframite and scheelite by a similar technique It is
believed, however, that the separation of molybdenum by this technique has not been investigated previously.
Electrowinning Molybdenum Experimental Procedure
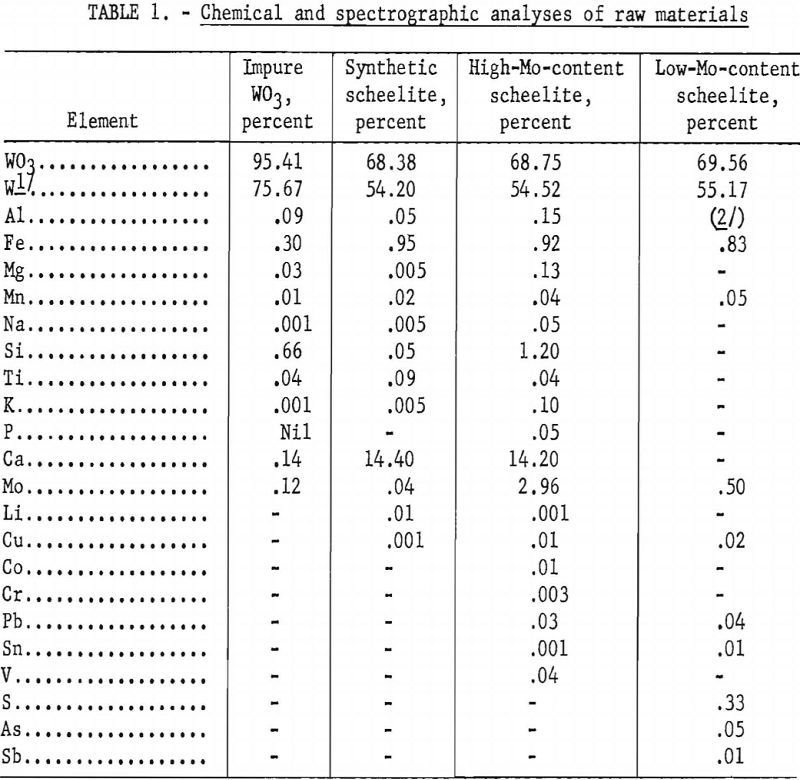
The electrolysis unit has basically an externally heated graphite or silicon nitride cell with graphite, tungsten, or cold-rolled steel electrodes. Two shop-constructed furnace were used in the investigation. Initial experiments were conducted in 2-½-inch-diameter, 7-inch-deep graphite cells, using an ore-electrolyte charge of 650 grams. The crucible was machined from a 6- inch-diameter AGX graphite commercial electrode. A graphite block was placed in the bottom of the furnace, which served as a cell rest and conductor for the direct-current power source.
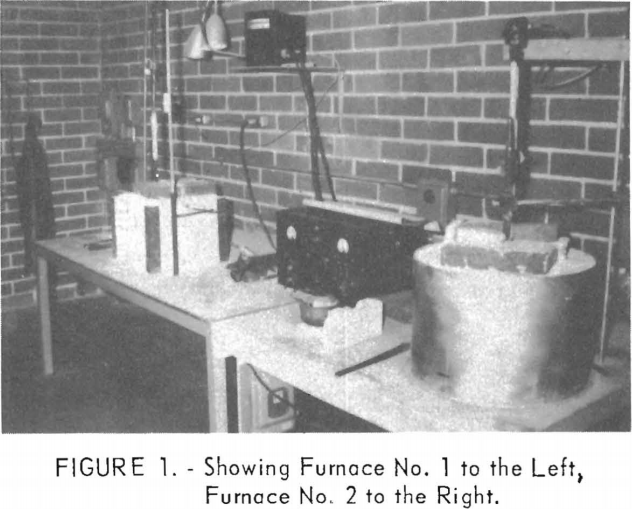
In some experiments the graphite crucible was used as the anode; in others, as the cathode. However, because the deposited metal was more easily removed and cleaned by using a 1-inch-diameter rod cathode, this technique was established towards the end of the investigations.
A typical electrolysis cycle involved preheating the cell to the approximate melting point of the ore-electrolyte mixture (750° C.). After charging the electrolyte mixture, the temperature was gradually increased to the selected temperature range of 900° to 1,050° C. After stabilizing at the selected temperature, the electrode was lowered into the bath, and electrolysis was started and continued until the desired current was passed through the cell usually 2.5 hours at a current density from 35 to 45 a./dm.². The electrochemical equivalent of tungsten is 1.14404 g./a.-hr. After completion of electrolysis, the centrally positioned 1-inch-diameter cathode was removed and the spent electrolyte in the cell was poured into a pot-type cast-iron mold. The electrolyte adhering to the cathode was permitted to drain from the deposited metal for a few minutes, then the cathode was water quenched. The electrolyte was mostly water-soluble and readily dissolved from the deposited metal. Similarly, metal entrapped in the solidified electrolyte (slag) was recovered by crushing and dissolving the electrolyte in water.
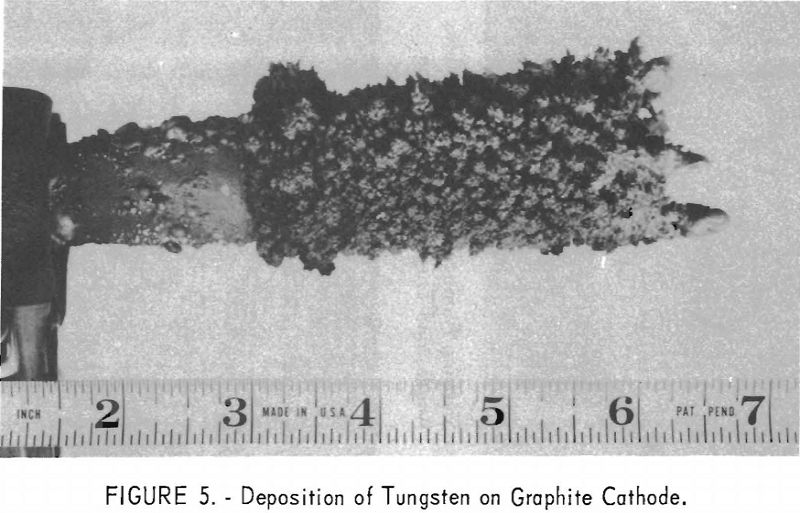
Graphite electrodes are subject to oxidation and corrosion above the bath level; as a result some mechanical carbon contamination occurs in the metal, but is removed readily with several water washes. Some of the deposited tungsten crystals are 570 microns (28-mesh) in size. To avoid any possible contamination from residual electrolyte, particularly phosphate, the metal is crushed to pass a 100-mesh screen and treated with dilute hydrochloric acid to dissolve any phosphates that may be present.
Figures 1, 2, 3, and 4 show the equipment and some of the techniques involved in the experiments.
Character of Metal Deposited
The deposition of tungsten on a graphite cathode is shown in figure 5. The metal was deposited from a tungsten trioxide-alkali phosphate electrolyte at a temperature of 900° C. and a current density of 40 a./dm.². The crystal structure of the deposited metal shown in figure 7 varies from spherical grains to clusters of dendritic hexagonal needles in the size range of 28- mesh to minus-325-mesh (570 to less than 35 microns).
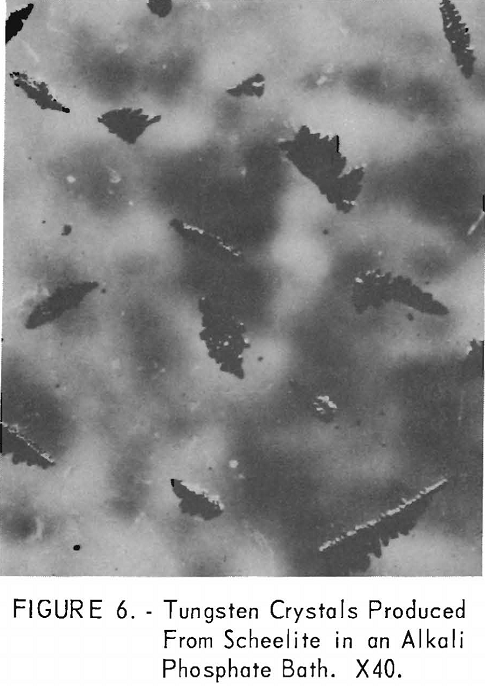
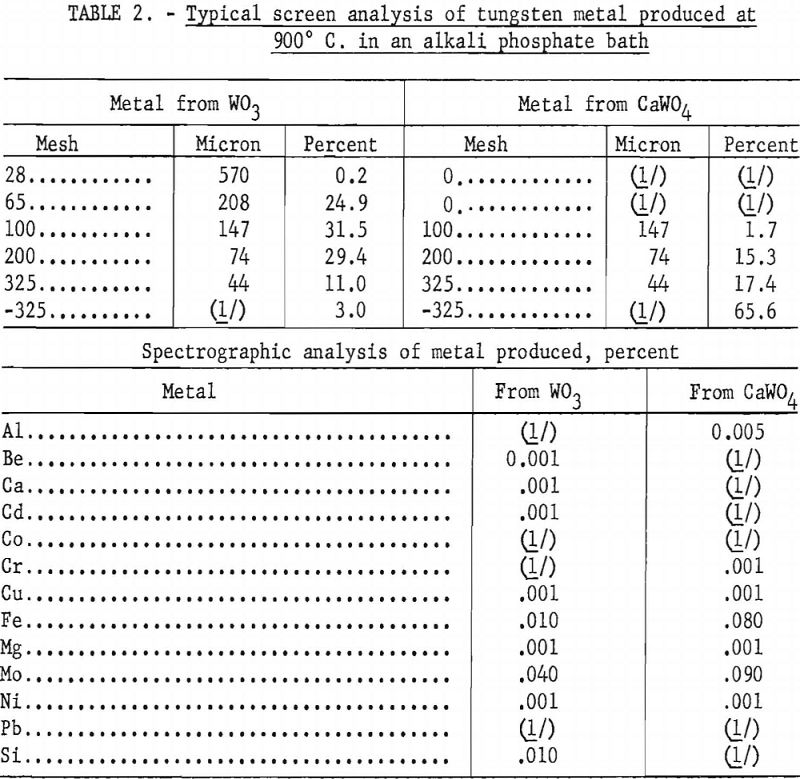
Crystals produced from scheelite under these conditions have similar characteristics in crystal structure but are much smaller. The size range varies from 100-mesh to minus-325-mesh (147 to less than 35 microns). These crystals are shown in figure 6. A typical screen analysis of the two products is given in table 2 with the spectrographs analysis of the metals.
Electrolyte Composition
Preliminary investigations were conducted to determine the most effective electrolyte composition for electrowinning tungsten from impure tungsten com¬pounds and scheelite. Electrolyte compositions investigated were: (1) Alkali phosphate mixtures composed of sodium pyrophosphate, sodium metaphosphate, and sodium chloride, (2) mixtures of sodium tetraborate and sodium pyrophosphate, and (3) straight sodium tetraborate electrolyte.


Alkali Phosphate Electrolyte
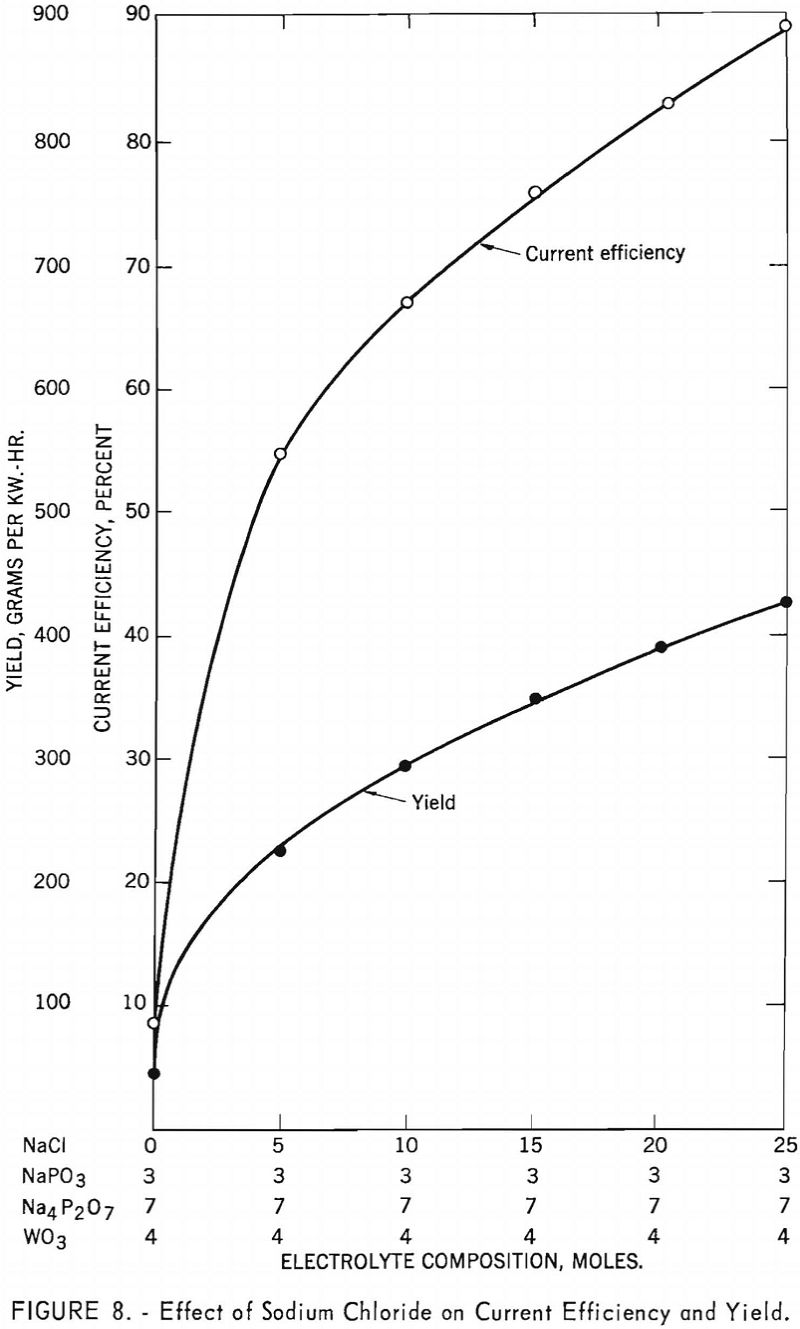
A 7-mole sodium pyrophosphate, 3-mole sodium metaphosphate, and 15-mole sodium chloride electrolyte composition proved satisfactory for tungsten trioxide, yielding an average of 363 grams of tungsten per kilowatt hour at an average current efficiency of 77.0 percent. The yield from scheelite, however, was affected by the amount of sodium chloride in the electrolyte mixture up to 25 mole-percent. A 7-mole sodium pyrophosphate, 3-mole sodium metaphosphate, and 25-mole sodium chloride electrolyte mixture resulted in a yield of 427 grams of tungsten per kilowatt hour at a current efficiency of 89 percent. Figure 8 shows the effect of sodium chloride on current efficiency and yield from scheelite in an alkali phosphate electrolyte.
Alkali Phosphate Borate Electrolyte
Studies were made to determine the effect of additions of sodium tetraborate to the standard alkali phosphate electrolyte, which is composed of 7 moles of sodium pyrophosphate, 3 moles of sodium metaphosphate, and 15 moles of sodium chloride. Several experiments were made replacing 1 mole of sodium
pyrophosphate with 1 mole of sodium tetraborate. The composition was varied from 6 moles of sodium pyrophosphate and 1 mole of sodium tetraborate to 3 moles of sodium pyrophosphate and 4 moles of sodium tetraborate. Current efficiencies for the entire series were more than 80 percent. The best electrolyte mixture was 6 moles of sodium pyrophosphate and 1 mole of sodium tetraborate. The current efficiency was 88 percent, and the yield, 457 grams of tungsten per kilowatt hour.
Sodium Tetraborate Electrolyte
A straight sodium tetraborate electrolyte was effective for tungsten trioxide but ineffective for scheelite because of the formation of the insoluble calcium borate, which greatly increased the viscosity of the electrolyte and interefered with electrolysis. The addition of sodium chloride to the sodium tetraborate reacted with the calcium to form a homogeneous electrolyte with increased fluidity and lowered resistivity. A 46-mole-percent sodium tetraborate and 54-mole-percent sodium chloride electrolyte proved satisfactory for scheelite, yielding 327 grams of tungsten per kilowatt hour at a current efficiency of 76 percent.
Effect of Temperature on Current Efficiency and Yield
Borate Electrolyte and Scheelite
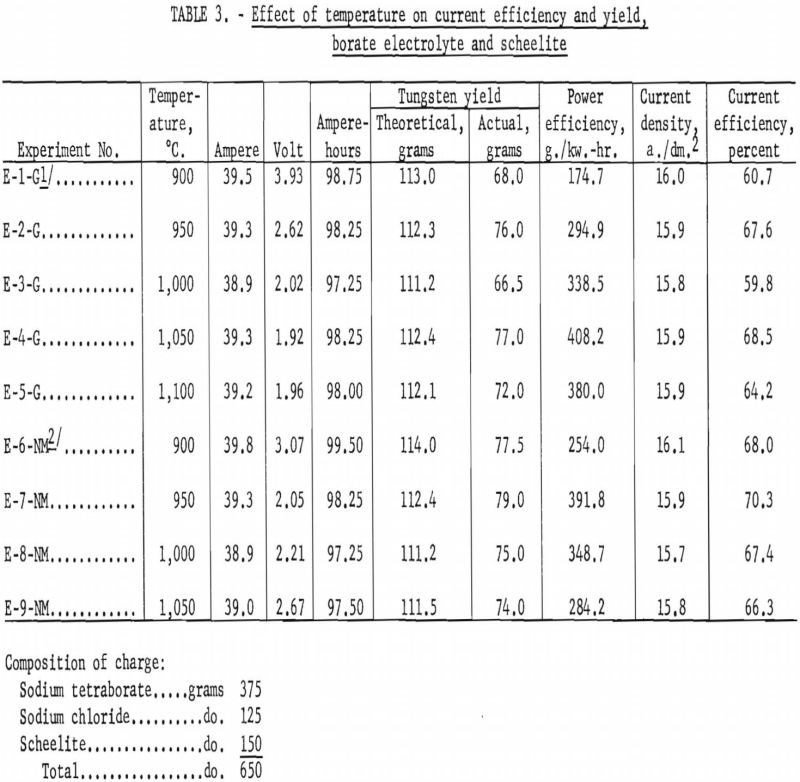
Experiments were conducted to investigate the effect of temperature on current efficiency and yield, using a borate electrolyte and scheelite. Both high- and low-molybdenum-content scheelite were investigated in a sodium tetraborate electrolyte composed of 4.6 moles (46 mole-percent) of sodium tetraborate and 5.4 moles (54 mole-percent) of sodium chloride. For each experiment, 650-gram charges of scheelite electrolyte were used. The current density was held constant at approximately 16 a./dm.², which was the limit of the direct-current power source in using the electrolytic cell as the cathode. Electrolysis was conducted for 2.5 hours. The starting temperature was 900° C. and varied in increments of 50° C. for each experiment. Table 3 shows the results of the experiments. The power efficiency shown in tables is the direct-current power at the cell. It does not include losses in conductors, rectifiers, or power in maintaining temperature of cell.
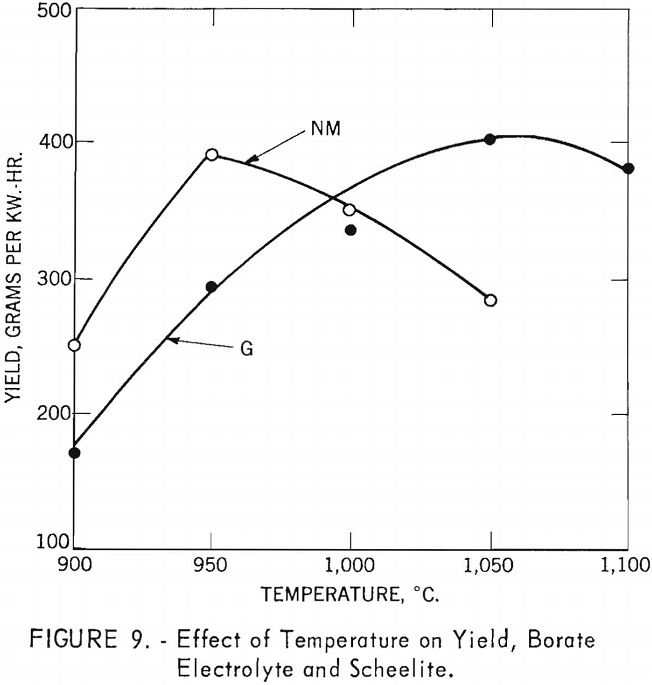
For the high-molybdenum-content scheelite, the effect of temperature on current efficiency is inconclusive, although the general trend appears to be a small increase as the temperature increases, reaching a maximum of 68.5 percent at 1,050° C. However, the effect of temperature on current efficiency for the low-molybdenum-content scheelite shows a small initial increase as the temperature rises, reaching a maximum of 80.3 percent at 950° C., and thereafter decreasing slowly as the temperature increases.
Figure 9 shows the effect of temperature on yield. For the high-molybdenum-content scheelite, the yield increases as the temperature increased, reaching a maximum
of 408.2 g./kw.-hr. at 1,050° C., and then decreasing slowly. The yield from low-molybdenum-content scheelite increased rapidly, reaching a maximum of 391.8 g./kw.-hr. at 950° C., and then decreased rapidly as the temperatures increased.
Table 4 shows the chemical analysis of the high-molybdenum-content metal and electrolyte and the spectrographic analysis of the impurities in the metal The electrolyte is water-soluble, and all the metal contained in the electrolyte can be recovered readily. The spectrographic analysis of the impurities in the metal is given in table 4.
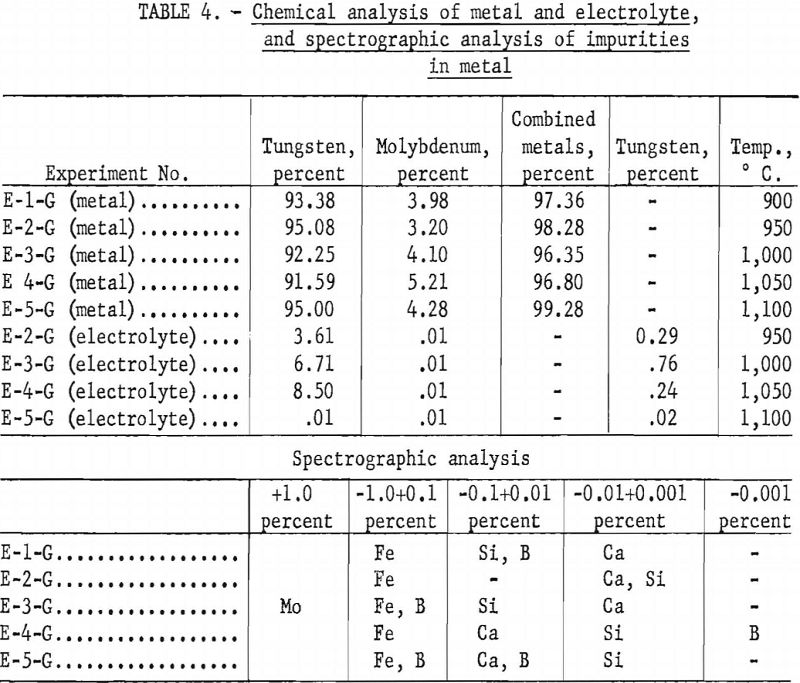
Alkali Phosphate Electrolyte and Scheelite
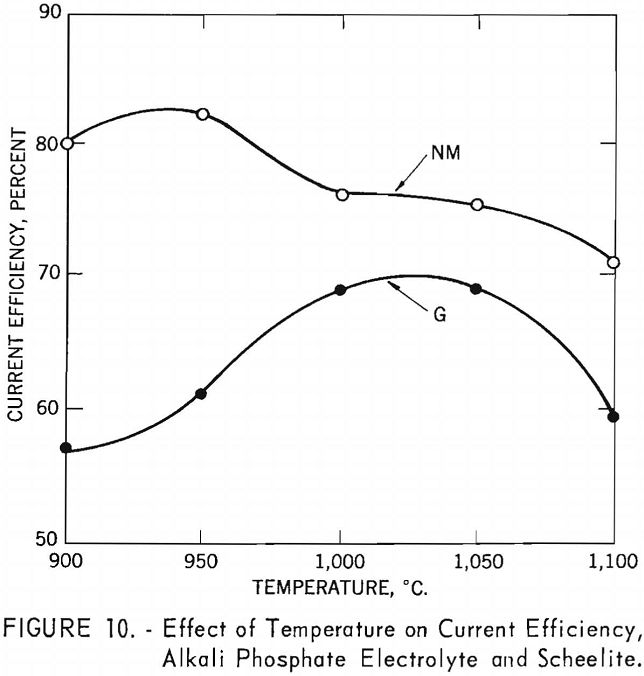
Experiments were conducted to determine the effect of temperature on current efficiency and yield in using an alkali phosphate electrolyte. Both high and low-molybdenum-content scheelites were used in the investigation. The alkali phosphate electrolyte was composed of 255 grams of sodium pyrophosphate, 42 grams of sodium metaphosphate, and 120 grams of sodium chloride, to which was added 176 grams of scheelite-a total charge of 593 grams for each experiment. One-inch-diameter graphite rods were used as cathodes, and graphite cells as anodes. Current density was held at approximately 36 a./dm.² and electrolysis at 2.5 hours. Temperatures were varied in increments of 50° C. between 900° and 1,100° C.
Table 5 and figure 10 show the effect of temperature on current efficiency, and figure 11 shows the effect on yield in grams per kilowatt-hour.
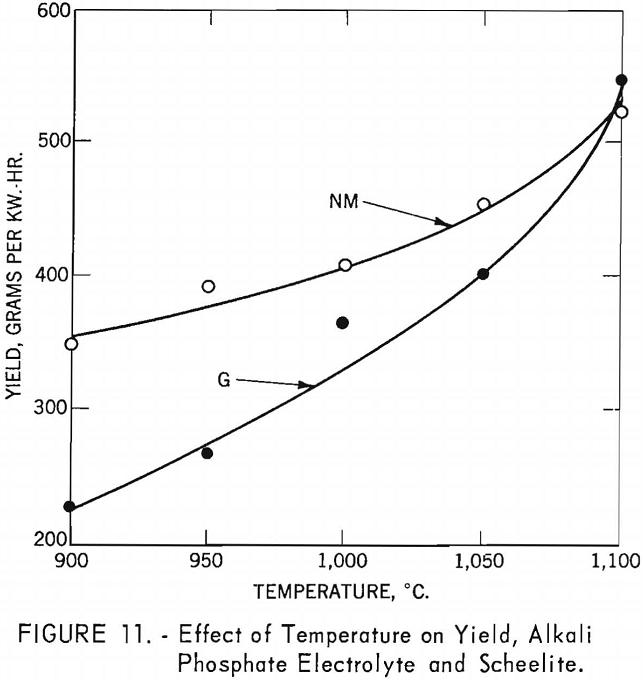
In the latter figures, as in subsequent ones, the variation of individual results has been noted, but the main trends appear valid, at this stage of investigation.
For the high-molybdenum-content scheelite, an increase in temperature resulted in an increase in current efficiency, reaching a maximum of 69.1 percent between 1,000° and 1,050° C. and then decreasing at higher temperatures. For the low-molybdenum- content scheelite, a maximum current efficiency of 81.9 percent was reached at a temperature of 950° C.; thereafter current efficiency decreases as the temperatures increased.
The effect of temperatures on yield for the high-molybdenum-content scheelite shows a steady increase from 900° to 1,100° C., reaching a maximum yield of 546.7 g./kw.-hr. For the low-molybdenum-content scheelite, the initial yield of 349.9 g./kw.-hr. is much higher than for the high- molybdenum-content scheelite at the same temperature; however, the increase in yield at elevated temperatures was not as rapid, reaching a maximum of 528.2 g./kw.-hr. at 1,100° C. Significant in these experiments is the drop in voltage as the temperatures increased, indicating that elevated temperatures increase fluidity and lower the resistivity of the electrolyte, which aids electrolysis and may account for the increased yield.
In comparing the effect of temperature on current efficiency and yield for the borate and alkali phosphate electrolytes over the same temperature range of 900° to 1,050° C., the average current efficiency for the high-molybdenum-content scheelite and borate electrolyte was 64.1 percent and the average yield 304.1 g./kw.-hr. For the low-molybdenum-content scheelite, the average current efficiency was 68.0 percent and the average yield 319.7 g./kw.-hr. For the high-molybdenum-content scheelite and alkali phosphate electrolyte, the average current efficiency was 64.3 percent and the yield 319.9 g./kw.-hr. For the low-molybdenum-content scheelite, the average current efficiency was 78.2 percent and the yield 398.5 g./kw.-hr. These results show that higher current efficiency and yield are obtained with the alkali phosphate electrolyte than with the borate electrolyte.
Effect of Current Density on Current Efficiency and Yield
Experiments were conducted to determine the effect of current density on current efficiency and yield. Materials used were: (1) Impure tungsten tri-oxide, (2) synthetic scheelite, and (3) high- and low-molybdenum-content scheelite. A standard alkali phosphate electrolyte, composed of 255 grams of sodium pyrophosphate, 42 grams of sodium metaphosphate, and 120 grams of sodium chloride, was used throughout the experiments. The weight of tungsten material added to the standard electrolyte depended on the materials used for each experiment 126 grams for tungsten trioxide, 126 grams for synthetic scheelite, and 176 grams for either high- or low-molybdenum-content scheelite. The temperature was held constant at 900° C., and electrolysis time at 2.5 hours. The current density was varied for each experiment. The graphite cell was used as the anode, and 1-inch-diameter graphite rods were used as the cathodes.
Current densities reported in these experiments are based on the initial current density computed on the area of the 1-inch-diameter cathode occupied by the electrolyte. As electrolysis continues, the metal deposited on the cathode increases the surface area (fig. 5), decreases the space between the anode and the cathode, and reduces the available ions. All these factors influence the true current density.
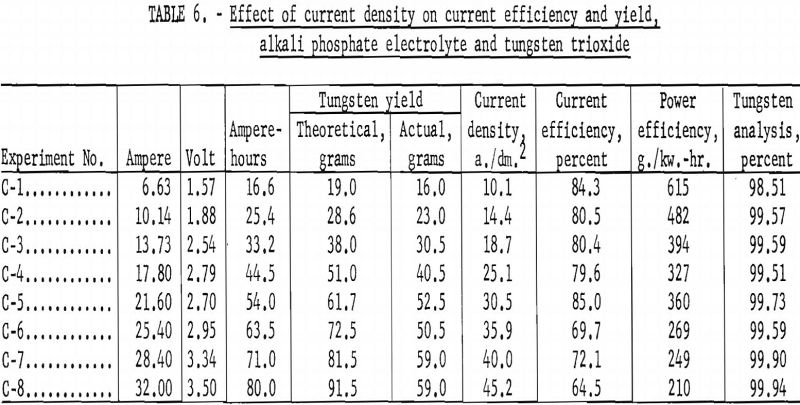
Alkali Phosphate Electrolyte and Tungsten Trioxide
Table 6 shows the tabulation of results on the effect of current density on current efficiency and yield for tungsten trioxide. Figure 12 and 13 are curves of these results. Figure 12 shows that increased current density decreases current efficiency. The decrease is modest up to a current density of 30 a./dm.²; thereafter, current efficiency decreases rapidly. Experiments were not conducted beyond a current density of 45.2 a./dm.²; however, from the rapid decrease in current efficiency at the higher current densities, the curve can be expected to continue in that direction. The curve for yield, while somewhat irregular, shows a pronounced decrease in yield as current density increased up to a current density of 25.1 a./dm.²; thereafter the decrease is more gradual. Analysis of the tungsten metal shown in table 6 indicates that the higher current densities favor the purity of the metal.
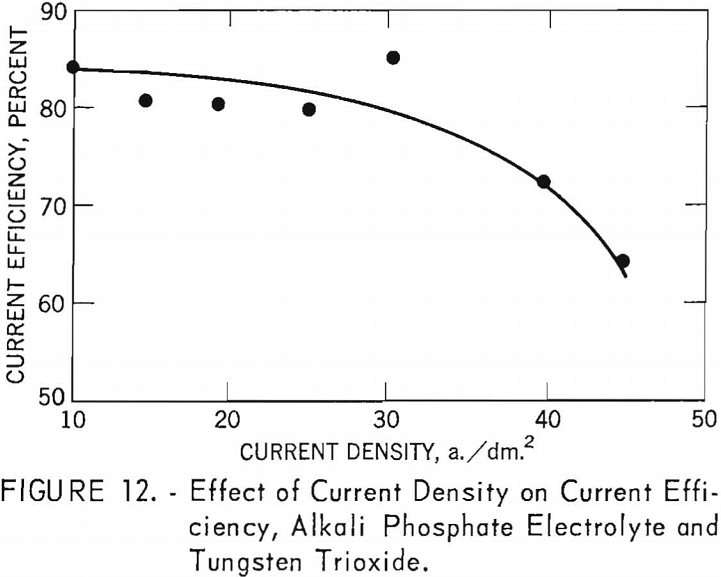
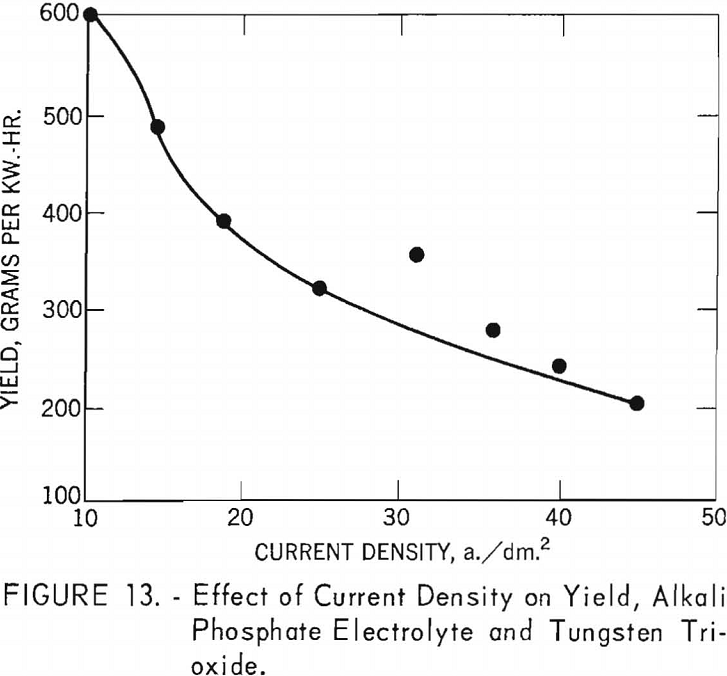
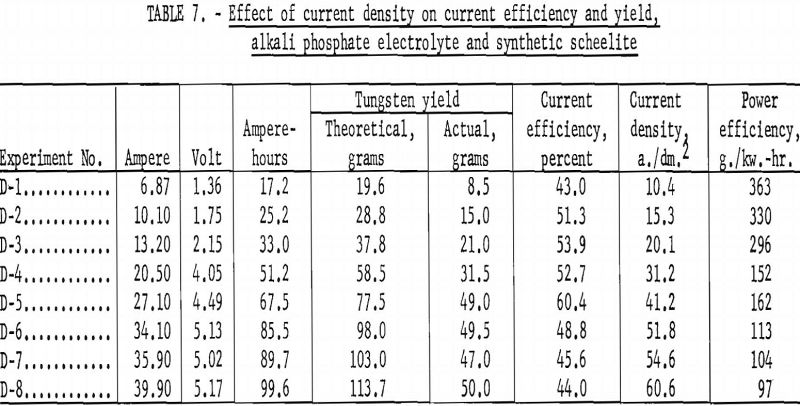
Alkali Phosphate Electrolyte and Synthetic Scheelite
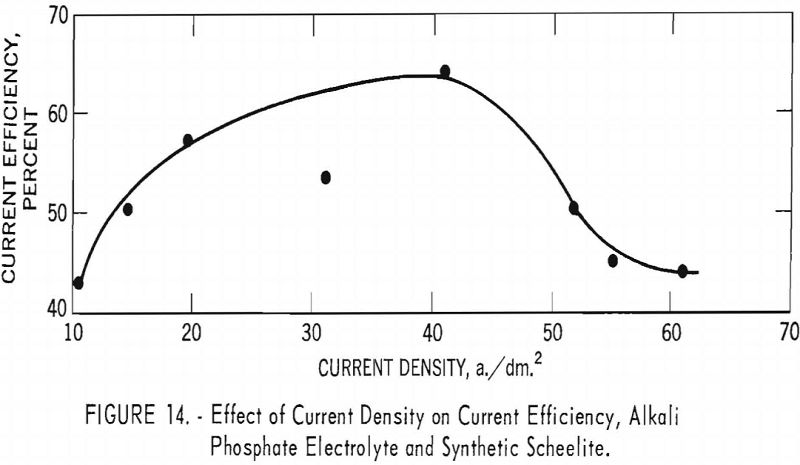
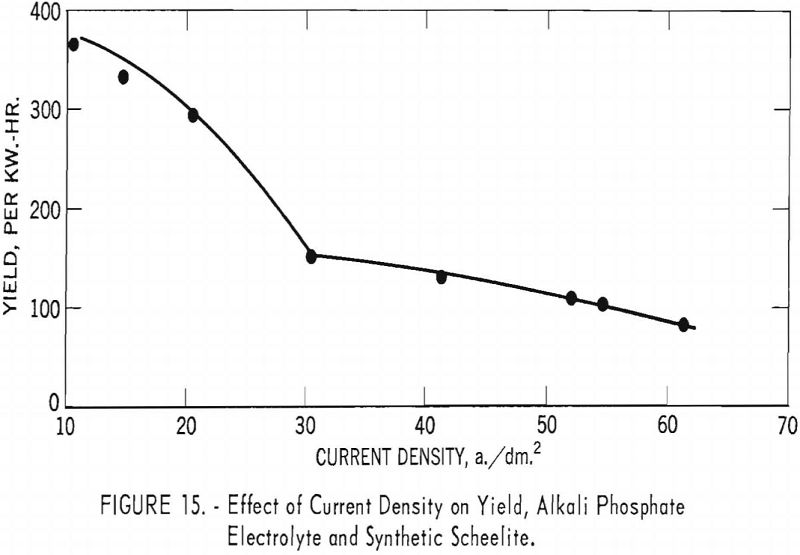
Table 7 and figures 14 and 15 show the effect of current density on current efficiency and yield for synthetic scheelite. Unlike the results obtained for the tungsten trioxide, the current efficiency for synthetic scheelite increases as current density increased, up to a maximum of 60.4 percent at a current density of 41.2 a./dm.², and thereafter decreased as current density increased. The effect on yield was a pronounced decrease between 10 and 31.2 a./dm.²; thereafter the decrease was slower as current density increased.
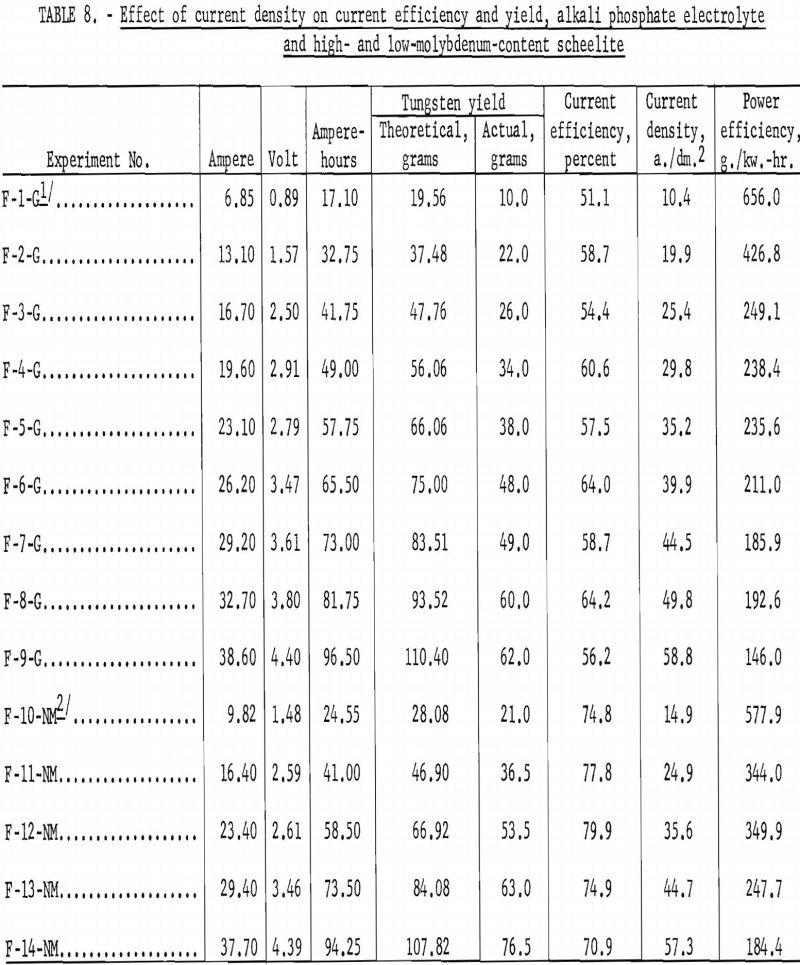
Alkali Phosphate Electrolyte High- and Low-Molybdenum-Content Scheelite
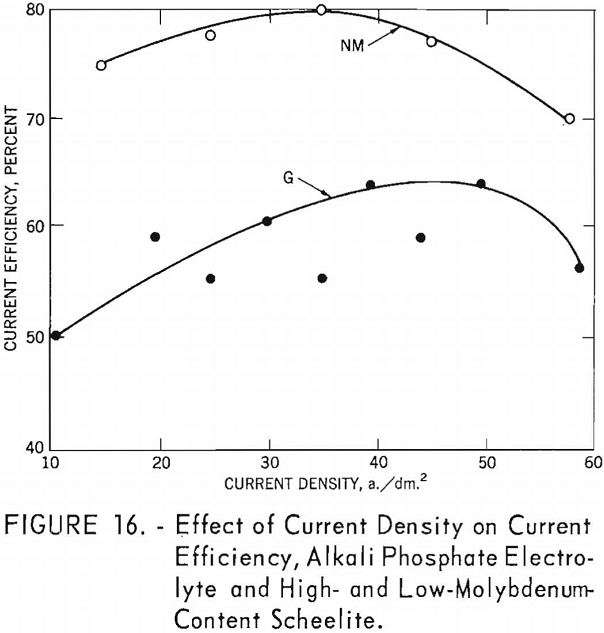
Table 8 and figures 16 and 17 shows the effect of current density on current efficiency and the yields for high- and low-molybdenum-content natural scheelite. Curve G shows the effect of current density on current efficiency for the high-molybdenum-content scheelite and is somewhat irregular; but the general trend indicates an increase in current efficiency as current density increased, reaching a maximum of 64.16 percent at a current density of 49.8 (experiment F-8-G). The maximum current density and current efficiency for this experiment are comparable with the maximum current density and current efficiency of experiment D-5 for synthetic scheelite. Curve NM for the low-molybdenum content scheelite is more consistent, reaching a maximum current efficiency of 79.94 percent at a current density of 49.8 a,/dm.² (experiment F-12-NM).
The lower current efficiency for the high-molybdenum-content scheelite can be partially attributed to the molybdenum content, which is approximately six times more than for the low-molybdenum-content scheelite. Current efficiencies were calculated for tungsten only; if calculations were adjusted for molybdenum, the current efficiencies for the high-molybdenum-content scheelite would be 8 to 11 percent higher than shown in table 8.
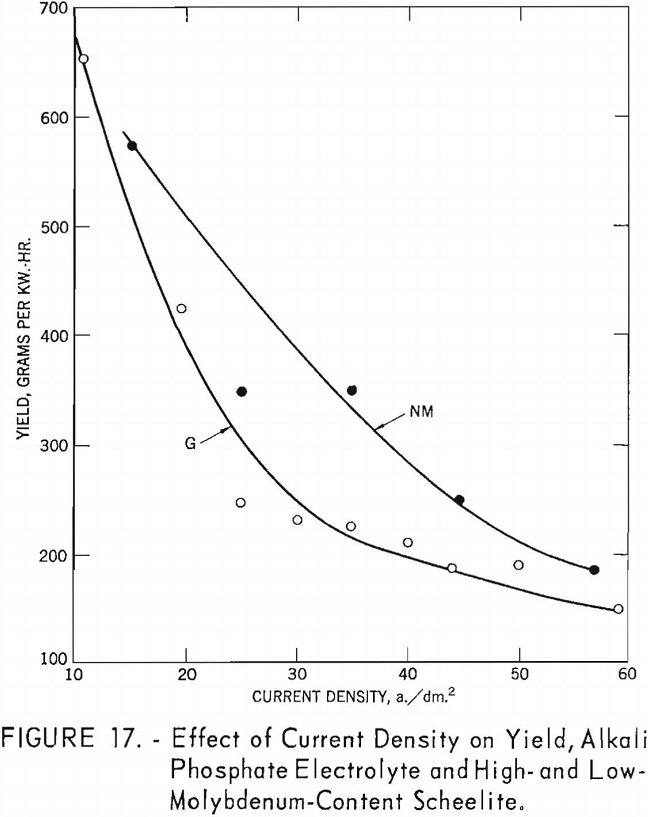
Curve G shows that the effect of current density on yield for the high- molybdenum-content scheelite is somewhat irregular, but the trend shows a pronounced decrease in yield up to a current density of 29.8 a./dm.² thereafter decreasing more slowly as current density increased. Curve NM for the low-molybdenum-content scheelite shows almost a consistent decrease in yield as current density increased.
Effect of Current Density on Codeposition and Separation of Molybdenum and Tungsten
Codeposition of Molybdenum and Tungsten
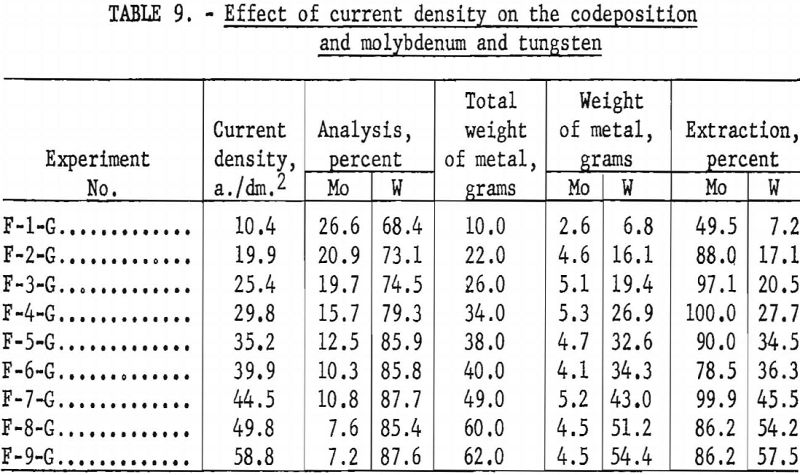
To determine the effect of current density on the codeposition of molybdenum and tungsten from a high- molybdenum-content scheelite, the products obtained from series F experiments, shown in table 8, were analyzed and the results tabulated. Table 9 and figure 18 show the effect of current density on the codeposition of molybdenum and tungsten. The curve for molybdenum shows that at the lower current densities molybdenum deposits vary rapidly, reaching 100- percent extraction at a current density of 29.8 a./dm.² in figure 18 the anomaly in the molybdenum curve at higher current densities is believed to be due to sampling and analytical techniques. The increased voltage used to maintain the higher current densities also may be a contributing factor. At higher voltages the deposition of molybdenum in preference to tungsten may not be as pronounced as at the lower voltages. The curve for tungsten shows a steady increase and forms a nearly straight line as the current density increased. This observation led to further investigations as a possible technique in separating molybdenum from tungsten.
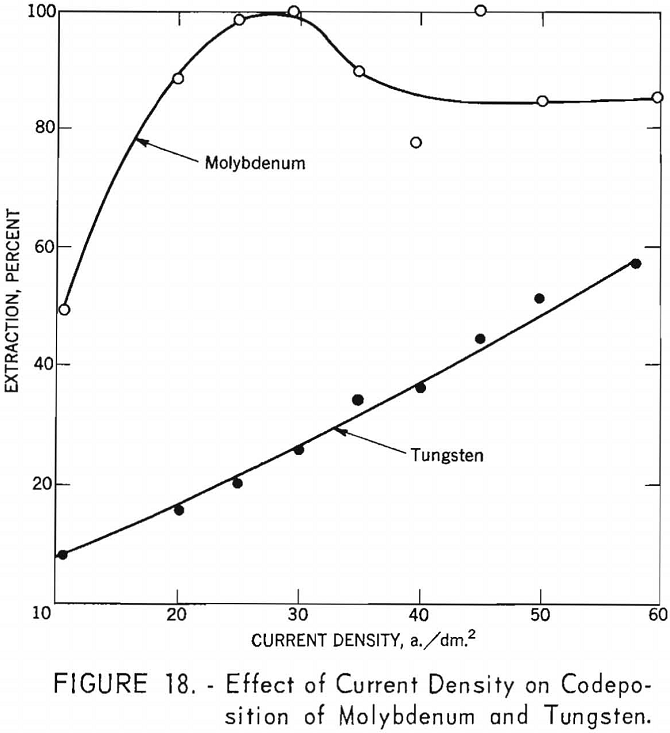
Separation of Molybdenum From Tungsten
Stationary Cathode
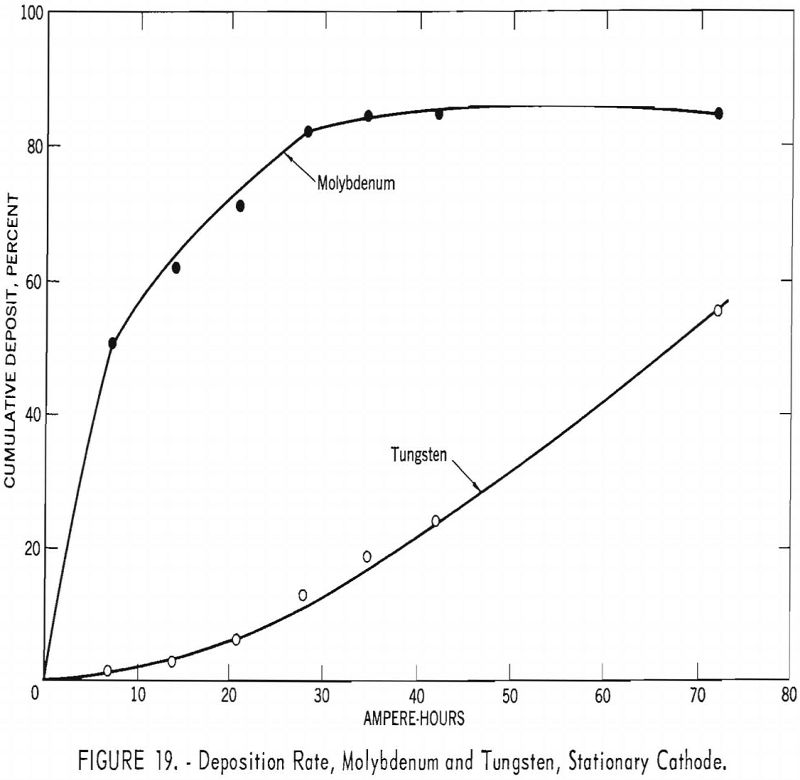
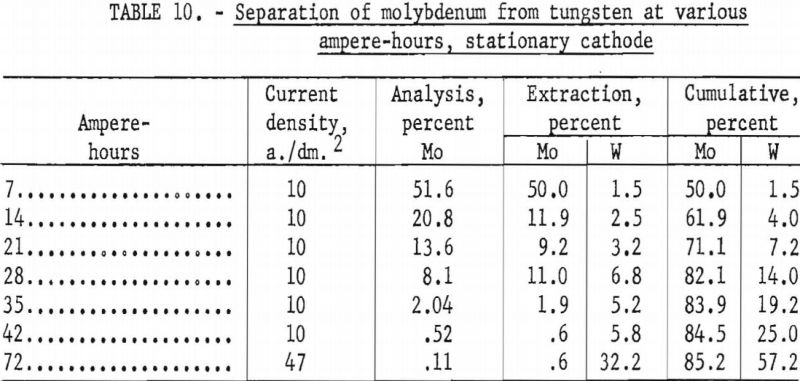
For investigating the separation of molybdenum from tungsten, the same electrolyte mixture and high-molybdenum-content scheelite used in series F experiments were used. A graphite cell was used as the anode, and 1-inch diameter graphite rods were used as stationary cathodes. The temperature was held constant at 950° C. The current density was held at 10 a./dm.² for the removal of molybdenum during the first six intervals of 7 ampere-hours and then increased to 47 a./dm.² for the deposition of tungsten. At the end of each 7-ampere-hour interval, the cathode was removed, and a new one inserted. The metal deposited on each cathode was collected and analyzed. Table 10 shows the percent extraction of molybdenum and tungsten as the ampere-hours of operation increased. Figure 19 shows the cumulative percent of molybdenum and tungsten deposited for the given ampere-hours. The curve for molybdenum rises sharply, achieving 50-percent extraction of the molybdenum at 7 ampere hours and accompanied only by 1.5 percent of the tungsten. At 28 ampere hours, 82.1 percent of the molybdenum was extracted and 14 percent of the tungsten. The tungsten curve is more steady, rising slowly at the beginning and increasing more rapidly as the ampere-hours increased. It will be noted that molybdenum extraction only reached 85 percent. This is due to the loss of molybdenum-bearing electrolyte adhering to the cathode when exchanged at each 7-ampere-hour interval.
Rotating Cathode
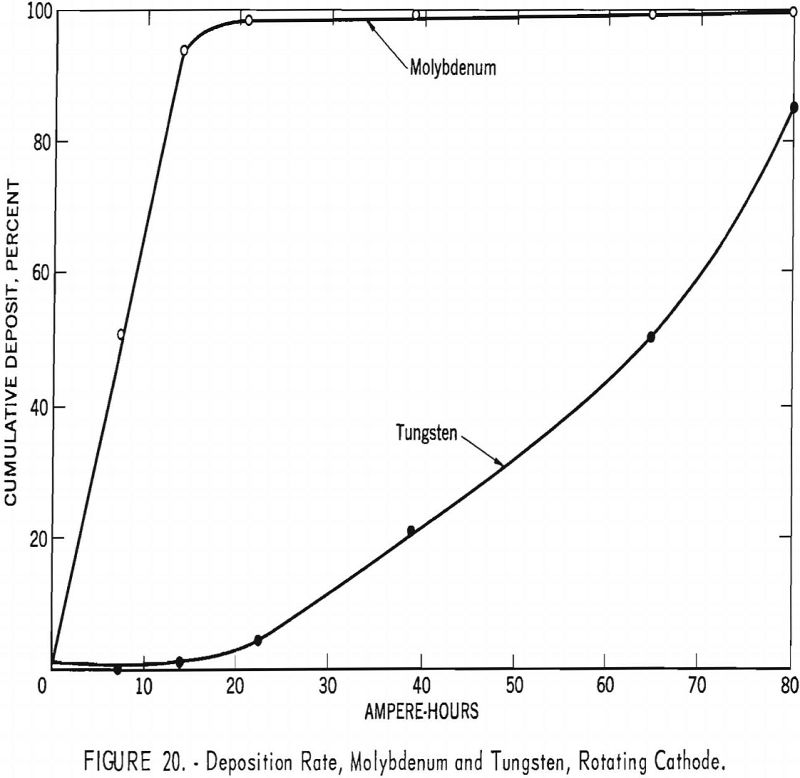
To determine the possible effect of local depletion of metal in the vicinity of the cathode and to maintain a homogeneous electrolyte, the rotating 1-inch-diameter graphite cathodes, which stirred the electrolyte, were substituted for stationary cathodes. The experimental technique was the same as for the stationary cathode, except that the current density was reduced to 7 a./dm.² for the first three intervals for the removal of molybdenum and then increased for the last three intervals for the removal of tungsten. Table 11 shows the results obtained by using a rotating cathode to separate the molybdenum from the tungsten at the given ampere-hours and current density, and figure 20 shows the cumulative percent of molybdenum and tungsten deposited. These results show that stirring the electrolyte with a rotating cathode assists in preventing metal depletion around the vicinity of the cathode and is partially responsible for the extremely high separation of the molybdenum from the tungsten. At 7.5 ampere-hours, 52.1 percent of the molybdenum and only 0.1 percent of the tungsten were extracted; at 21.25 ampere-hours, 98 percent of the molybdenum and 5.1 percent of the tungsten were extracted.
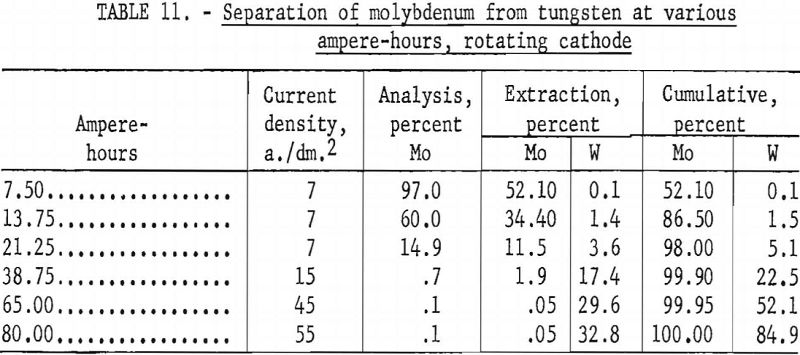
These results show that, under controlled conditions of electrolysis at low current density, it is possible to separate molybdenum from tungsten to a satisfactory degree from the same electrolyte, thus obviating prior chemical purification of the scheelite.
Recovery of Reagents From Spent Electrolyte
Spent electrolyte contains insoluble compounds and impurities, comprising about 33 percent of the total. The remaining 67 percent comprise useful reagents, which are water-soluble and can be regenerated by solution and crystallization.
