Table of Contents
The management of solid waste is an ever increasing concern in the United States. Every year our nation generates between 140 to 160 million tons (Lichstein, 1990 and Thayer, 1989) of municipal solid waste. Only about 10 pct of the waste is recycled, mostly aluminum and paper. About 135 million tons of this waste end up in landfills. Although plastics make up only 7 pct of the solid waste generated, they are a fast growing segment of such waste. Today, less than 1 pct of the plastics in municipal solid waste is recovered. In 1987, the plastics industry produced 28 million tons of plastics, and the public discarded about 11 million tons. By the year 2000, plastics production is expected to reach 38 million tons, and the public is expected to throw away about 19 million tons, accounting for 10 pct of municipal wastes. Although they are not a major proportion by weight, plastics have been targeted as a waste problem (Lichstein, 1990, Thayer, 1989, and Rotman, 1989).
Rising costs of landfilling and of alternative waste treatment methods such as incineration are making it prohibitively expensive to handle large amounts of plastics waste. Recycling can be less costly than other disposal methods because there is value in the reused materials that can offset some of the landfill costs (Lichstein, 1990, Thayer, 1989, and Rotman, 1989).
Five thermoplastic types are commonly used in consumer packaging applications (Thayer, 1989). Polyethylene (PE) is the most widely used thermoplastic. High-density PE is used for rigid containers such as jugs, household product containers, and motor oil bottles.
Low-density PE is used in films and bags. Polyethylene terephthalate (PET) is used extensively in rigid containers, particularly carbonated beverage bottles. Polystyrene (PS) is best known as a foam in the form of cups, trays, and food containers. In its rigid form it is used in cutlery. Polyvinylchloride (PVC) is a tough plastic often used in construction and plumbing. It is also used in some food, shampoo, oil, and household product containers. Polypropylene (PP), frequently interchanged for PE and PS, is used in a variety of areas including snack food packaging, battery cases, and disposable diaper linings. Research on recycling municipal solid waste has developed several techniques and processes for separating the waste into recyclable components (Lichstein, 1990, Thayer, 1989, Rotman, 1989, and Sullivan, 1973). The waste is usually shredded to about 1-cm pieces and then separated into its major components. These processes are effective for recovering aluminum, paper, and light weight plastics, but a heavy weight plastics fraction is obtained that has a very limited reuse market. Over 90 pct of this heavy weight plastics fraction, called the “mixed plastics” fraction, is made up of PS, PET, and PVC. Recovery of these plastics into high purity products will greatly improve the markets for these recycled waste plastics.
For the mixed plastics fraction, PS can be separated in a brine solution around sp gr 1.1, leaving a mixture of PET and PVC. Froth flotation has been explored as a possible separation method with limited success (Saitoh, 1976a, Saitoh, 1976b, and Holman, 1974). Recent flotation hydrodynamics research by the Bureau of Mines (Jordan, 1986, Jordan, 1989, and Hood, 1990) has shown improved flotation kinetics of minerals flotation by optimizing the hydrodynamics for both bubble-particle attachment and bubble-pulp separation. This expertise was used to develop a flotation technique to separate mixed plastics into its components. A cooperative agreement was negotiated with wTe Corporation, a municipal waste treatment corporation, to devise and evaluate a flotation method for separating the mixed plastics from municipal wastes. A combination of selective flotation chemistry and improved flotation hydrodynamics was utilized in this separation technique.
The composition of the mixed plastics product from a municipal waste would depend upon the previous recycling efforts on the waste stream. For instance, if PET drink bottles were hand-picked from the waste stream, 85 pct of the PET would not be present in the mixed plastics product. This low PET, mixed plastics may respond differently than the high PET, mixed plastics that still contain the PET plastic drink bottles. To develop an effective flotation method, both of these mixed plastics compositions were tested. As shown in previous research (Holman, 1974), PS can be recovered prior to the flotation step with a Na2CO3 brine separation. Subsequent flotation would only be needed to separate the PET and PVC. This research was designed to investigate the brine PS separation followed by PET flotation using both low and high PET feed compositions. Table 1 summarizes the various mixed plastic feeds and process sequences that were considered for this flotation test work. The scope of this research was confined to the flotation process. Consequently, as shown in table 1, for the brine separation-flotation sequence, complete removal of the PS was predicted leaving only a mixture of PET and PVC in the same proportions as the feed to the brine separation circuit.
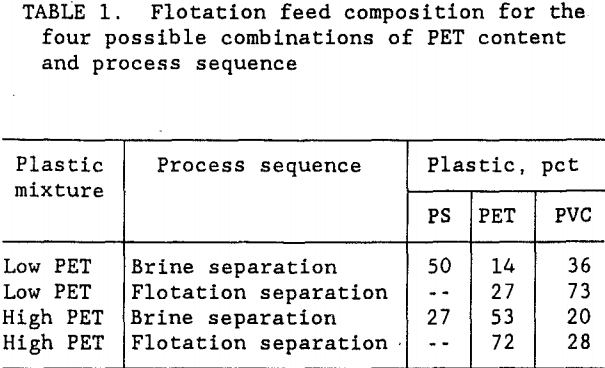
The purity of each product from the plastic separation processes would be an important consideration. However, purity requirements largely depend upon the end-user for recycled plastics. A relatively high product purity standard of 99.5-pct was targeted for this research. Test work was conducted to attain that goal for each of the three plastic types.
Description of Sample
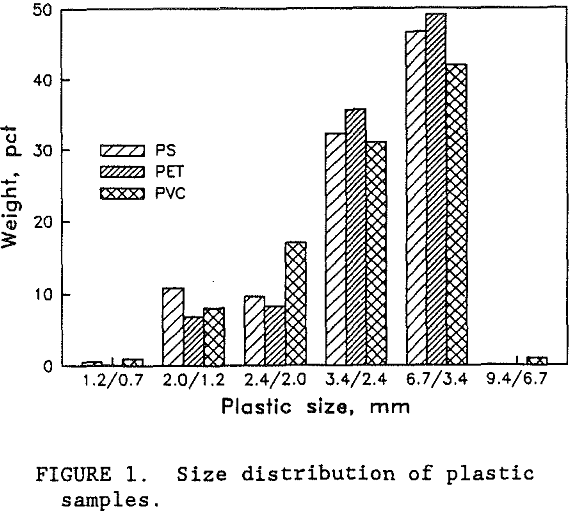
Samples of PS, PET, and PVC were provided by wTe. Each plastic type was a different color so that visual analysis could be used to evaluate the test results. Normally, waste bottles would have small residual deposits of their former contents. However, the wTe waste recovery process includes a detergent wash step for the mixed plastics product. This washing step effectively removes the residual chemicals. For this research, unused colored bottles were obtained from suppliers. The colored plastic bottles were shredded by wTe to minus 9.4 mm which is typical for the municipal waste recovery process. Figure 1 shows the sample size distribution of each plastic type. Virtually all of the plastic was between 0.8 and 6.7 mm. Almost all of the particles were shaped like flakes with thicknesses ranging from 0.05 to 0.3 cm. The thickness of the shredded plastic is expected to be a function of the original plastic container’s thickness. As shown in figure 2, the thickness of the plastic particles were distributed over a wide range and the mean plastic thickness was around 1.5 mm. Figure 3 is a photograph of a typical group of these plastic pieces. To help characterize the particle size and shape, a circular disk shape particle model was used. Representative portions of each size fraction were weighed and counted. The mean particle weight and circular disk shape model were employed to calculate the mean particle thickness. According to the model, the mean
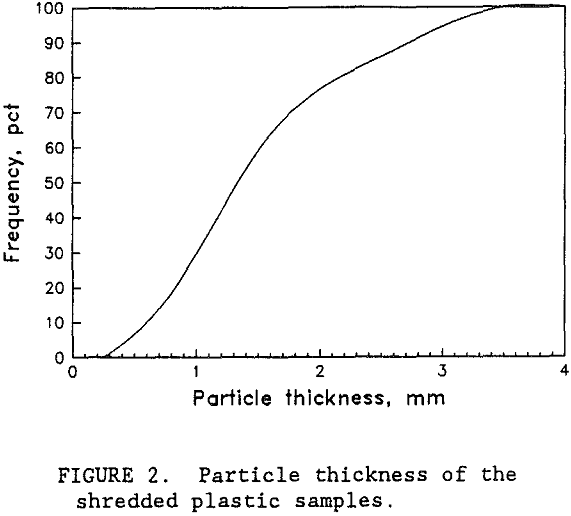
particle thickness was 1.3 mm and the measured mean particle thickness was 1.5 mm. This indicated that the circular disk shape model was a fairly good representation of the actual shapes of the plastic particles.
Flotation Separations
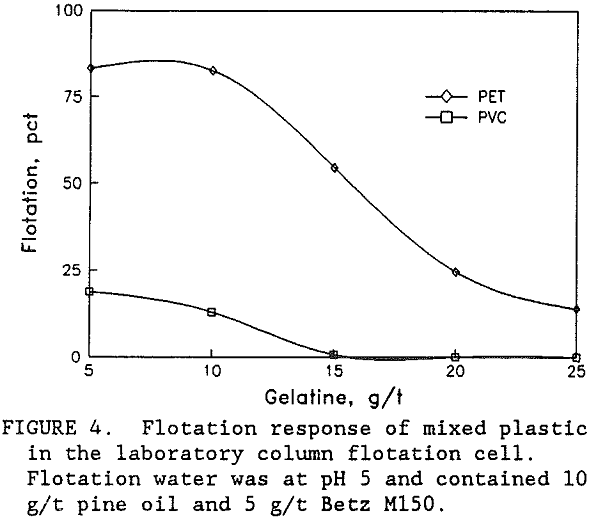
All three of the plastic types were naturally hydrophobic and would float in the presence of only frother. Depressants such as tannic acid, goulac, starch, glue, amine acetate, and gelatine were all expected to depress flotation for all three plastic types (Lichstein, 1990, and Sullivan, 1973). Preliminary laboratory flotation tests were conducted with all these depressants. The best results were obtained using gelatine in pH 5 water to float a high purity PET product from the mixed plastics. As shown in figure 4 selective flotation was obtained between PET and PVC with only a small amount of gelatine in the solution. Complete flotation of the PET was not obtained. A portion of the PET never did float even without the gelatine depressant. This unfloatable PET was typically the coarser (plus 2.4 mm size) and was also around 3 mm thick. The PET particle size and shape would have a significant effect upon flotation.
After completing the preliminary flotation tests, a laboratory flotation cell, shown in figure 5, was constructed to simulate a commercial flotation unit. The flotation cell consisted of a 7.5-cm-diam tube equipped with a bubble slurry sparger located near the bottom of the cell, through which the fine bubble slurry was pumped. Two hundred grams of plastic was conditioned for 5 min with reagentized water in the feed chamber prior to being released into the flotation cell. The 2,000-mL feed chamber was attached to the flotation column, as shown in figure 6. During conditioning a moveable gate inside the feed chamber closed the feedport. The conditioned

feed slurry entered the flotation cell through a 3-cm-diam feedport, located 75 cm above the bubble slurry sparger. Bubbles were generated in a Denver DR flotation cell at 1,400 r/min using 10 g/t pine oil and 5 g/t Betz M150. Pine oil was used to stabilize the large size bubbles and Betz M150 was used to help stabilize the small bubbles. The bubble slurry was pumped to the flotation unit at 1,000 mL/min. The bubble slurry flow into the flotation cell produced an upward flow velocity of 22 cm/min between the bubble sparger and the top of the flotation column, as shown in figure 7. Virtually all of the water in the bubble slurry exited the column cell with the flotation concentrate. This net upward flow of the water through the column flotation cell is completely opposite in conventional column flotation. The upward flow tended to elutriate the bubble coated PET particles which assisted their transport to the top of the cell. In addition, the upward flow of water also helped the floated particles overflow at the top of the cell. However, the upward flow had to be low enough to prevent elutriation of the gelatine-covered PVC particles and allow them to be recovered in the flotation tailings.

The top of the flotation cell was 50 cm above the feedport. The hydrophilic plastic pieces settled 75 cm through the swarm of bubbles and were collected beneath the bubble sparger. The bubbles became attached to the hydrophobic plastic pieces and these pieces floated 50 cm above the feedport before overflowing into the concentrate. The flotation kinetics for the PET plastic were very fast. The large particles settled quickly down the 75 cm column. Based upon the particle sizes and shapes, the expected residence times ranged from 7 to 24 s. The fluid flow past the particles was characteristic of transitional to turbulent flow. Bubbles quickly collided with the falling particles and attached to the hydrophobic surfaces.
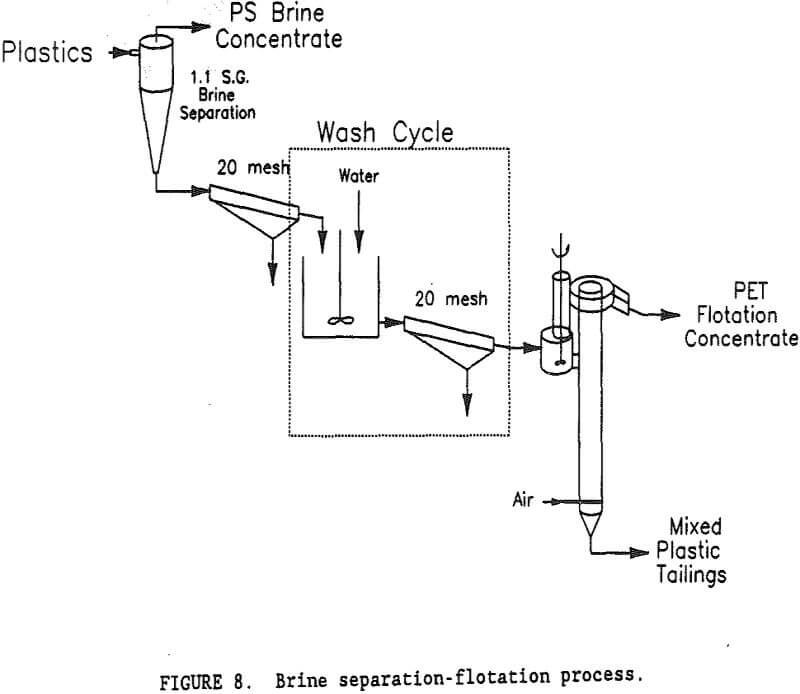
Brine Separation-Flotation Process
A flowsheet of the brine separation-flotation process is shown in figure 8. While the separation of PS in a Na2CO3 brine was beyond the scope of this research, one test was conducted to determine its effectiveness and the impact that separation would have on subsequent PET flotation. A sample of mixed plastics was slurried in a sp gr 1.1, Na2CO3 brine. The PS (sp gr 1.03) rose to the surface and the PET (sp gr 1.35) and PVC (sp gr 1.35) sank. Ninety-eight percent of the PS was recovered in a 99.7-pct PS concentrate. Only a few, small pieces of PET were found in the PS concentrate. The sink product contained virtually all of the PET and PVC. Preliminary flotation tests showed that Na2CO3 interfered with the depressant properties of gelatine, so that the sink product would need to be washed to remove the Na2CO3. The degree of washing required was not known, so a simple multi-stage wash process was devised for test purposes. A
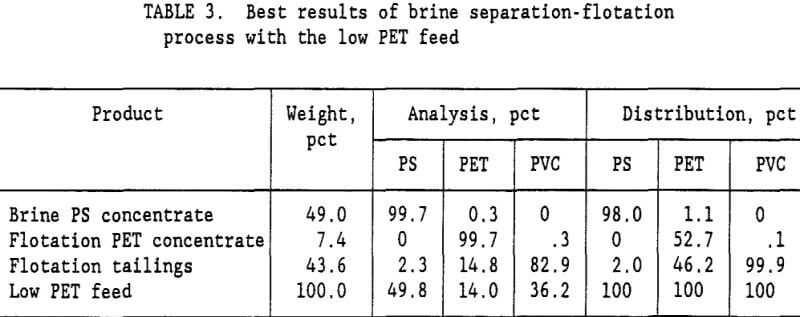
brine slurry of PVC and PET plastics was drained on a screen with 0.85-mm openings. The wet plastic removed from the screen contained 35-pct moisture. Fresh water was added to the wet plastic to form a 15-pct solids slurry, which was again drained on the 0.85-mm opening screen. This single wash step reduced the Na2CO3 content of the water from 10.5 pct to 0.99 pct Na2CO3. The ratio of Na2CO3 to plastic was reduced to 5,400 g/t. A second wash cycle lowered the Na2CO3 to plastic ratio to 500 g/t, and a third wash cycle lowered it to 46 g/t. Flotation tests were conducted at all three Na2CO3 levels representing a 1, 2, or 3 cycle wash. Table 2 summarizes the results of these tests. For the low PET mixed plastic feed (27 pct PET and 73 pct PVC), a 99.7-pct PET product was obtained with the 3 cycle wash. It required 45 g/t gelatine in the flotation water to depress the PVC and recover 53 pct of the PET in the flotation concentrate. With a 2 cycle wash, the best concentrate grade was only 97.8 pct PET. Increasing the gelatine level to 150 g/t resulted in no flotation at all. For the low PET feed, at least a 3 cycle wash was required to obtain a suitable PET purity in the flotation concentrate. The best results for the brine-flotation process with the low PET feed are shown in table 3.
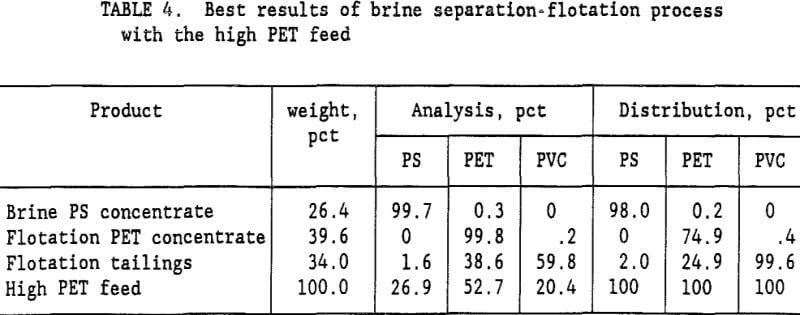
The Na2CO3 brine separation produced a 99.7- pct PS concentrate that recovered 98 pct of the PS. After 3 wash cycles, 53 pct of the PET was recovered in a 99.7-pct PET flotation concentrate. The flotation tailings contained 82.9 pct PVC with only a 2.3 pct PS and 14.8 pct PET. Two clean plastic products were obtained and the third product was high in PVC. For the high PET feed (72 pct PET and 28 pct PVC), using a single wash and 60 g/t gelatine, a 99.8-pct PET concentrate was obtained that recovered 75 pct of the PET. Adding a second wash reduced the required gelatine to 45 g/t and produced a 99.5 pct PET concentrate at about the same PET recovery. The optimum process would depend upon the relative cost of additional wash stages versus the savings obtained in the required amount of gelatine. A more complete presentation of the single wash, high PET feed results is shown in table 4. Again, the Na2CO3 brine separation produced a
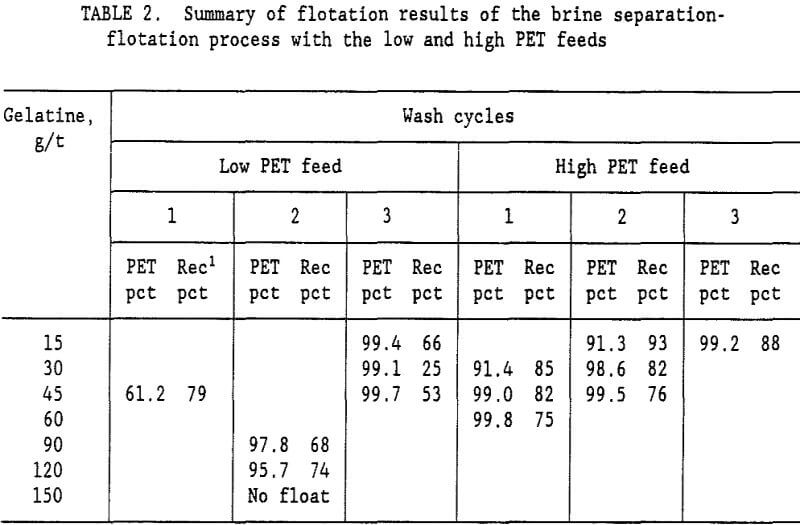
Note: Flotation water at pH 5.0, 10 g/t pine oil and 5 g/t Dowfroth 1012. Recovery.
Note: Flotation water at pH 5.0, 10 g/t pine oil, 5 g/t Dowfroth 1012, 45 g/t gelatine, and 3 wash cycles.
Note: Flotation water at pH 5.0, 10 g/t pine oil, 5 g/t Dowfroth 1012, 60 g/t gelatine and 1 wash cycle.
99.7- pct PS concentrate that recovered 98 pct of the PS. After 1 wash of the sink product, flotation recovered 74.9 pct of the PET in a 99.8- pct PET concentrate. The flotation tailings were mixed plastics.
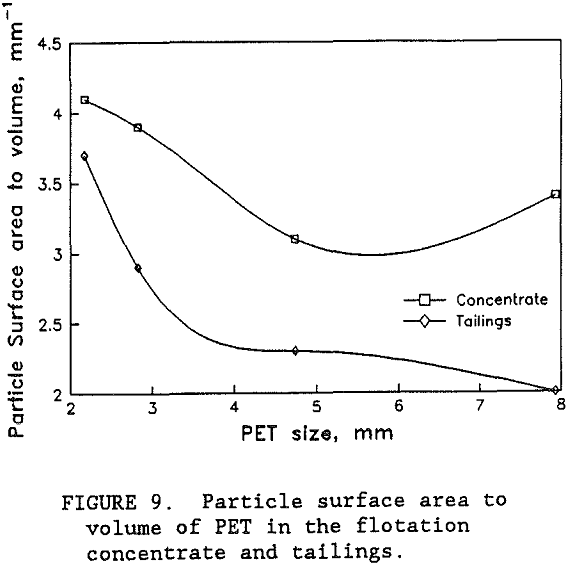
While the flotation grade of the PET product was good, the PET recovery appeared to be limited to around 75 pct. A size analysis of the products from the best separation with the high PET feed revealed that the PET recovery hovered around 75 pct until the particle size was below 2 mm screen size. Over 90 pct of the PET smaller than 2 mm screen size was recovered in the flotation concentrate. Unfortunately, most of the PET was coarser than 2 mm. Using the circular disk shape particle model and representative sample weights and counts, the mean particle thickness of the PET in the concentrate was 1 mm. However, for the PET in the tailings, the calculated mean thickness was 2 mm. It was the coarse and thick PET particles that were not being recovered. As shown in figure 9, the coarse PET particles have a relatively small surface area to volume ratio which limited the number of bubbles that can attach to the available particle surface. However, the thinner PET particles have a larger ratio of surface area to volume giving them more available surface area for enough bubbles to attach and to float the particle. Upon close examinati on, the wet PET particles in the tailings have numerous bubbles attached to their particle surfaces, but not enough to lift the particles to the top of the flotation cell. So that while the flotation chemistry appears to produce selective attachment of the bubbles on the PET surfaces, the available area for bubble attachment on the coarser and thicker particles was not enough to float these particles.
While improved PET recovery may not be necessary to obtain a useful PET product, the recovery of additional PET would enhance the quality of the PVC-rich flotation tailings. Two techniques could be considered. The PET/PVC mixture could be sized prior to flotation at about 4 mm size. Each size fraction would be floated separately. The fine size fraction would be floated in the manner previously described in this paper. The coarse size fraction would be floated with a larger vertical fluid flow aimed at enhancing the PET flotation.
The second technique to consider would be shredding the PVC-rich flotation tailings to
reduce the overall size of the PET particles should enable these particles to float, but shredding will also lower the size of the PVC particles. The flotation recovery of PVC above 2.0 mm screen size was less than 1 pct. However, below 1.2 mm screen size, the PVC recovery in the flotation concentrate jumped to 60 pct. Shredding the PVC-rich flotation tailings to enhance PET flotation will probably increase the PVC flotation. More gelatine will be needed to depress the large surface area of these small PVC plastic particles. The economics of this process would determine if the additional shredding and gelatine costs would be justified.
Discussion
Compared to the low PET feed, the high PET feed required less washing to remove the residual brine to allow effective gelatine depression of the PVC particles. After 2 wash cycles with the high PET feed, 45 g/t gelatine in the flotation water was enough to mask the harmful effects of the residual brine and depress the PVC. For the low PET feed, 3 wash cycles and 45 g/t gelatine in the flotation water were required to depress the PVC. After the third wash cycle for the high PET feed, only 15 g/t gelatine in the flotation water was required for the high PET feed. The residual Na2CO3 brine after three washes had no measurable effect on the flotation response.
The low PET feed had 2.6 times more PVC than the high PET feed and also required 3 times more gelatine to depress the PVC. After 3 wash cycles, the ratio of gelatine to PVC in the flotation water was roughly equal for both low and high PET feeds. For the low PET feed (73 pct PVC), a clean separation required 45 g/t gelatine. For the high PET’feed (28 pct PVC) which contained 62 pct less PVC than the high PET feed, a clean separation used 66 pct less gelatine in the flotation water at 15 g/t gelatine. Clearly, the amount of gelatine required for selective flotation of PET was proportional to the amount of PVC in the flotation feed.
While Na2C03 brine separation recovered a high purity PS product and flotation recovered a high purity PET product, no flotation method was found to obtain a high purity PVC product. The PVC did not require much gelatine for depression but, even at low levels of gelatine, a portion of the PET was too large and thick for flotation and was recovered in the PVC product. The best PVC concentrates for the low and the high PET feeds were only 92 pct PVC and 74 pct PVC, respectively. In both cases the PET particles in the PVC concentrates were over 2 mm thick and coarser than 2 mm screen size.
Summary and Conclusions
A combination brine separation and flotation process was used to obtain high purity PS and PET products from mixed plastics. The Na2CO3 brine separation produced a 99.7-pct PS concentrate that recovered 98 pct of the PS. Three wash cycles with fresh water were required to remove the residual Na2CO3 brine. After washing, flotation recovered 53 pct of the PET from the low PET feed in a 99.7-pct PET concentrate. For the high PET feed, over 98 pct of the PS was recovered in a 99.7-pct PS concentrate by brine separation. Only two washes were required to remove the residual brine from the high PET feed. After washing, 75 pct of the PET was recovered by flotation in a 99.7-pct PET concentrate. The PVC-rich flotation tailings could not be effectively treated to recover a clean PVC product. The amount of gelatine required to depress the PVC was proportional to the amount of PVC present in the flotation feed.
