Table of Contents
- Research and Technologic Activities
- Explosion, Flame, and. Combustion Research
- Combustion Studies
- Ignition Studies
- Research on Explosion Hazards in Operations Involving Flammable Gases and Liquids
- Determination of Flammability Characteristics of Individual Compounds
- Explosives Research and Testing
- Applied Research
- Fundamental Explosives Research
- Evaluation of Explosives and Blasting Devices
- Experimental Coal Mine and Dust Explosions Research
- Experimental Coal Mine
- Dust Explosion Galleries and Chambers
- Dust Explosion Laboratories
- Meetings and Conferences
- Explosion, Flame and Combustion Research
This circular summarizes the research and technologic activities and publications of the Federal Bureau of Mines Division of Explosives Technology during fiscal year 1959 (July 1, 1958-June 30, 1959).
Part I briefly describes the programs that were active during the report period and presents a summary of some of the information not published elsewhere. In many instances the results are only preliminary and are subject to revision on the basis of continuing investigations. Detailed reports of all programs not security classified will be made later.
Part II lists and describes briefly the publications of the Division during fiscal year 1959. If the published material has some bearing on activities still current during the report period, this is indicated by references in Part I.
Research and Technologic Activities
Explosion, Flame, and. Combustion Research
This program pursues two main lines of inquiry, the one leading to a better understanding of the mechanisms associated with the combustion and explosion of flammable gases and volatile liquids; the other designed to provide information on the flammability and explosibility of individual gases and vapors, especially those of industrial and military interest. The results can be applied in preventing fires and explosions in operations involving these materials and in the safer and more efficient utilization of liquid and gaseous fuels.
The activities of the program include:
- Studies of combustion reactions in gases and volatile liquids, ranging from slow oxidation to detonation.
- Experimental and theoretical investigations of various forms of ignition in the gas and vapor phases.
- Research on hazards associated with production, storage, and utilization of potentially flammable gases and liquids.
- Methods for determining the flammability characteristics of gases and vapors (spontaneous ignition temperature, upper and lower limits of flammability). These methods are developed and applied to combustible gases and liquids under conditions of temperature, pressure and concentration corresponding to those encountered in modern industrial and military operations.
Combustion Studies
A long-term program was concluded on the fundamentals of turbulent flames under the sponsorship of the Office of Scientific Research (Department of the Air Force). Earlier studies were continued on turbulent flame structure and burning. A significant relationship was found to exist between the scale of turbulence and the turbulent burning velocity; this information will find application in the design of combustors with improved space-heating rates.
The Bureau completed a study on the performance of gas burners in terms of appliance design and environment. The study extended earlier findings for flames on monoport burners in free air to flames on multiport burners operating within an appliance. This completes another phase of the extensive investigations, conducted over the past 10 years in cooperation with the American Gas Association (A.G.A.), into factors affecting the stability of burner flames under a wide variety of conditions of gas composition and flow.
Another investigation of interest to the gas industry on the mechanisms of smoking flames was continued in cooperation with the A.G.A. A study of carbon formation in very rich hydrocarbon flames in cooperation with the same organization, yielded new information on the thermal and chemical configuration of the carbon-forming region of such flames. Assistance was also given the A.G.A. in setting up a research project on gas appliances using liquefied petroleum gases. This project was to be carried out at the Cleveland laboratories of the Association.
Scale studies of diffusional flames applicable to fires involving large shallow pools of combustible liquids (such as occur in spillage of liquid fuels) were continued with the sponsorship of the Wright Air Development Center (Department of the Air Force). Trays containing various typical fuels were used to extend scaling factors to heat-transfer processes and to define the role of environmental conditions such as air currents and wind.
To complete information obtained in previous studies on the properties of laminar flames, the effect of pressure on the burning velocity of such flames was measured in a newly designed 8-inch-diameter spherical bomb with a safe pressure rating of 3,500 p.s.i.
An exploratory study of flames of a typical automobile exhaust mixture formed during idling showed that a diffusion flame has wider stability limits than a premixed flame. This information will be applied in a current air-pollution research program sponsored by the Department of Health, Education and Welfare to investigate the feasibility of a noncatalytic afterburner based on diffusion combustion.
Ignition Studies
Considerable progress was made in research sponsored partly by the Office of Naval Research (Department of the Navy) to obtain information on the ignition of flammable atmospheres by hot gases traveling through narrow channels. This problem is closely related to ignition in various combustion chambers, to firedamp ignition by borehole shots, and to explosions in electric housings. Photographic studies of explosions propagating through narrow channels showed that transmission of flame is not essential for ignition; hot gases from a quenched explosion can ignite cold combustible gases at the exit of the channel, A systematic study of ignition by hot laminar jets was made to observe the effect of jet diameter and contact time on the ignition temperature.
The problem of ignition of combustible gases by static electric sparks continued to receive attention. An investigation into the effects of a series resistance on the ignition energy required gave further evidence to support the thermal nature of this type of ignition. Some consideration was given to the use of a high-speed switch for initiating the timing circuit and the spark itself, to be substituted for the more complex auxiliary setup of the high-speed oscillograph.
A motion picture based on Bureau research into electrostatic spark ignition of flammable anesthetics was produced and released with the support of the Abbott Laboratories and in close collaboration with the School of Medicine of the University of Pittsburgh.
The Bureau conducted a very limited study of decomposition and flammability limits of dilute solutions of monochloroacetylene in mixed vinyl and vinylidine chlorides (1:4 ratio) in cooperation with the Ethyl Corp.; monochloroacetylene is known to be extremely reactive to air in the pure form, but its sensitivity in dilute solutions was unknown. Mixtures containing 50 volume-percent of air are nonflammable up to 21 percent monochloroacetylene; the latter mixture is flammable. Mixtures containing 23 to 78 volume-percent monochloroacetylene did not ignite spontaneously upon addition of air at room temperature, but adding air to pure monochloroacetylene down to pressures of 20 mm. Hg absolute resulted in spontaneous ignition. The limiting concentration was not determined.
Investigation of the flammability and detonability of pinane-oxygen mixtures was completed in cooperation with the American Cyanamid Co. to provide safety information applicable in industrial oxidation processes using fuel-oxygen mixtures. Under the experimental conditions employed, mixtures containing 3 to 12 percent pinane could be detonated at 160° C. and an initial pressure of 1 atmosphere; at the same temperature and pressure, the limits of flammability of pinane in oxygen are 0.7 and 43.0 volume-percent.
Flammability limits of mixtures of ethylene and ethylene oxide were determined at room temperature and pressure in cooperation with Vulcan-Cincinnati, Inc. Under these conditions the lower limit of flammability in air ranges from 3.0 to 3.6 volume-percent for all mixtures; the upper limit is between 36.3 percent (pure C2H4) and 100 percent (pure C2H4O).
An investigation was initiated under the sponsorship of the Atomic Energy Commission (AEC) to study the effect of irradiation on the ignition temperature of organic reactor moderators and coolants.
As in other years, flammability characteristics were determined for a number of other potentially hazardous gases and volatile liquids; table 1 gives some particular values.
Investigation of gas explosion disasters and potentially hazardous situations was continued as requested by industries and civil authorities. In addition to a number of small-scale local incidents, two major investigations were made one for a steel producer after an explosion in a waste-gas stack; the other for an oil company on the origin of an explosion in an oil-refinery dephlegmator.
Research on Explosion Hazards in Operations Involving Flammable Gases and Liquids
An investigation is in progress under the sponsorship of the Wright Air Development Center (Department of the Air Force) to obtain information on the potential explosion hazards in large-scale operations with liquid hydrogen, which is of interest as a high-energy fuel. The Bureau developed new techniques to meet the problems associated with explosibility studies at the unusually low temperatures of liquid hydrogen and applied the techniques in model studies on hydrogen fireballs. The resulting information is applicable in establishing the magnitude of the fire and explosion effects anticipated should accidental spillage of liquid hydrogen occur. It will be used to calculate quantity-distance tables for the safe storage of this material.
Investigation was completed into the problem of removing residual fuel vapors from fuel tanks by forced drafts of hot air. The investigation was sponsored by the Bureau of Ships (Department of the Navy). Gas-freeing tests were conducted in a 1,000-cu.-ft. test tank with several combinations of inlet and outlet air ports; research workers determined the time required to reach the upper and lower flammability limits of 20 volume-percent gasoline-air mixtures.
Special consideration was given to the explosion hazards created by fuel mists and foams while loading tanks, to investigate the safety characteristics of kerosene, jet fuel, and motor gasoline, performed in cooperation with the Cities Service Research and Development Corporation. Various concentrations of inert gas were used effectively to reduce the flammability of the different fuel-air mixtures.
Determination of Flammability Characteristics of Individual Compounds
In continuing work on explosion hazards of aircraft fuels and hydraulic fluids, begun under the sponsorship of the Wright Air Development Center (Department of the Air Force) in 1950, the Bureau measured spontaneous ignition temperatures, ignition delays, and limits of flammability for 12 thermally stable fuels. Hazards of aerodynamic heating of emptied fuel tanks containing small amounts of fuel also were investigated.
In a new program for the Office of Naval Research (Department of the Navy) to study fire and explosion hazards of new liquid propellants, spontaneous ignition temperatures and minimum electrostatic spark ignition energies were determined for aniline and unsymmetrical dimethylhydrazine in various oxidizing atmospheres.
Explosives Research and Testing
The program on explosives provides information for the explosives industry in developing safer and more efficient explosives, and related equipment for blasting in coal mines and in other industrial and military operations. This is accomplished through:
- Applied research on the properties of explosives compositions and potentially explosive chemicals coupled with the development of new procedures and techniques for evaluating these properties.
- Fundamental research on initiation and detonation processes.
- Testing under provisions of established schedules of new and modified permissible explosives and blasting devices; and of field samples to evaluate the safety of production lots of permissible explosives.
Applied Research
Reflecting the rapid advances in “do-it-yourself” (field-mixed) blasting agents, based on fertilizer-grade ammonium nitrate sensitized with fuel oil, research on the safety characteristics of these compositions continued actively. The sensitivity of these compositions was evaluated, with emphasis on the role of the physical characteristics of the nitrate (prilled, flaked, powdered, coated, uncoated).
Recognizing growing interest in the use of fuel-sensitized ammonium nitrate for underground blasting, the Bureau began a study of the toxicity of the fumes from these compositions and the propagation of detonation in small-diameter charges.
An earlier study of the role of the borehole freespace in the ignition of firedamp by permissible explosives was extended to include a number of other factors. Statistical analysis of data from more than 3,200 shots at the Bureau’s large explosives test gallery (gallery 1, Bruceton, Pa.) showed that the gas content of the gallery, the weight of the explosive in the cannon, and the freespace in front of the explosive charge are all significant in determining the probability of an ignition of the gallery atmosphere by a cannon shot.
The Bureau, in cooperation with the Institute of Makers of Explosives, made significant advances in establishing the effect of inclusion of sodium chloride in American permissible explosives by applying statistically designed experiments. As a result of this investigation, the safety of a number of permissible brands was improved by adding 10 percent sodium chloride.
In cooperation with the Commercial Solvents Corp., the Bureau continued studies to evaluate the sensitivity to shock of systems formed by nitromethane with various process materials that are soluble in it. The principal procedure used in this work was a modification of the card-gap sensitivity test developed earlier for this purpose. The thermal stability of these systems was also investigated.
The card-gap method was also applied to measure the sensitivity of a 50:50 mixture of nitroglycerin and ethylene glycol dinitrate. This mixture will be used as a reference material in other sensitivity measurements for the Department of the Navy. More generally, it can serve as a convenient reference for all commercial explosives investigated by the Bureau. Card-gap sensitivity measurements were also made on monochloroacetylene in cooperation with the Ethyl Corp.; under the specific test conditions used (1-in. steel line); a solution of 10-percent monochloroacetylene in a mixture of vinyl and vinylidine chlorides released considerable energy but did not propagate detonation. The Bureau began a study of the safety characteristics of several potentially hazardous compounds in cooperation with Merck and Co.
The Bureau performed security-classified research on rocket fuels and propellants for the Departments of the Army and of the Navy, for General Electric and for the Thiokol Chemical Corp. In connection with one of these projects, a miniaturized card-gap sensitivity-test configuration was developed.
Fundamental Explosives Research
A program of wide interest and applicability concerns mechanisms involved in the transition from deflagration to detonation. Of great importance in the safety of permissible explosives, this transition may also occur in a solid propellant rocket motor. Initially sponsored by the Department of the Navy, the investigation was continued under the sponsorship of the Advanced Research Project Agency (Department of Defense). Considerable progress was made in developing a resistance-wire technique for continuously observing the progress of the reaction front at the core of the detonating solid charge. This technique, in which the advancing reaction shorts-out elements of a resistance, is also being applied, with high-speed photography, in a study of the mechanism of initiating detonation in the card-gap test used extensively in evaluating the sensitivity of explosive substances.
Preliminary studies of detonations in gaseous mixtures (sponsored by the Department of the Army) showed that detonations of the hydrogen-oxygen system are slowed by passage through a magnetic field of high intensity (about 100,000 gauss); similar measurements are in progress for the carbon monoxide-oxygen system. The effect of electric and magnetic fields on gaseous detonations is being investigated as part of an overall study of the detonation process.
A study was completed on infrared radiation from the gaseous detonation products of cannon shots of permissible explosives undertaken in conjunction with other research on the ignition of firedamp by explosives. Radiation was appreciably higher for shots in methane-air atmospheres than for shots in air alone, even when ignition of the gallery did not occur, indicating that some reaction or combustion of the methane occurs with each shot but is too limited to propagate throughout the gallery and produce a well-defined ignition.
Evaluation of Explosives and Blasting Devices
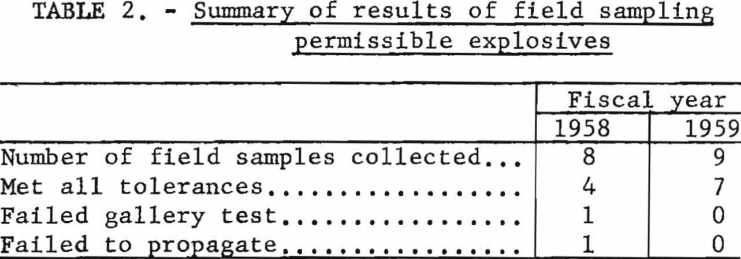
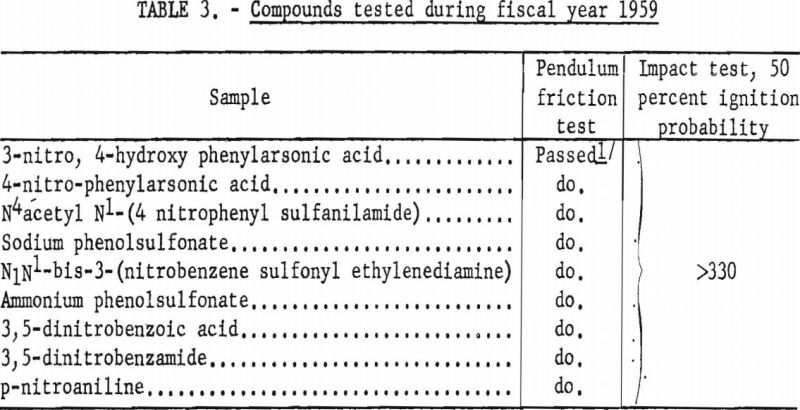
Eleven new or modified compositions tested under the provisions of Schedule 1G met all requirements for permissibility. Nine field samples of permissible explosives, examined for conformance with basic specifications, met the minimum safety requirements (table 2). Impact and friction tests were applied to evaluating the potential sensitivity of several industrial chemicals (table 3).
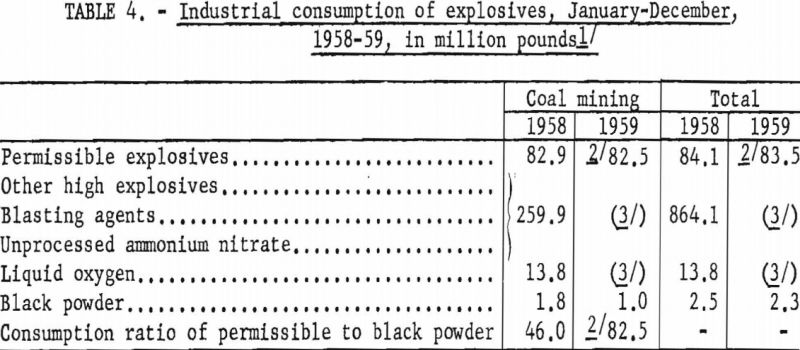
The estimated consumption of permissible explosives by the coal-mining industry in 1959 showed relatively little change from 1958; however, the estimated ratio of permissibles consumed to black powder was markedly higher (table 4), indicating a very encouraging reduction in use of black powder.
Experimental Coal Mine and Dust Explosions Research
The program was originally directed towards the prevention of coal-dust fires and explosions in underground mines but now includes a wide variety of problems arising in industrial operations involving other flammable dusts.
Research under the program is carried out through:
- Large-scale underground experiments in the Experimental coal mine under simulated operating conditions.
- Studies in galleries and chambers above ground where scaled experiments on initiation and propagation of dust fires and explosions are carried out; these studies serve as a basis for alleviating and preventing such fires and explosions in mines and other industrial installations.
- Laboratory studies, which include small-scale orientation experiments in preparing large-scale work in mines and galleries; fundamental research on properties and reactions of dust flames to provide basic information on combustion in dust clouds and layers; and determination of explosibility and flammability of individual dusts and powders by standard methods.
Experimental Coal Mine
The Bureau continued its research on the control of mine fires through high-expansion foams, a process first developed at the British Safety in Mines Research Establishment. Essentially, the procedure consists of spraying a water solution of a foaming agent (detergent) onto a net stretched across a mine entry through which ventilation air is flowing. The resulting bubbles form a plug of foam, which is transported by the ventilation current to the fire; this seems to provide a rapid and economical means of attacking mine fires at a distance. The main problems were to generate and maintain a foam plug that could be transported rapidly without excessive drainage of the contained water and to provide adequate airflows to move the plug. These problems were solved, and both coal and oil fires were attacked successfully (table 5). A portable foam-generating unit was developed, which consisted of a fan, a net, sprays, and metering equipment. Tests in multiple entries of a commercial mine showed that this unit can be used effectively by ordinary mine personnel.
The Bureau also concluded an experimental investigation of the hazards of firedamp ignition by a cutoff charge. The investigation completes a long-term program on the safety of multiple short-delay blasting in coal mines, which is now being promoted by the Bureau.
An earlier study was resumed transporting coal and limestone float dusts by mine ventilating currents. In addition to general information on the movement of dusts in mine entries, research workers are seeking correlation between dust collected on trays and dust deposited on mine rib and floor surfaces. Analysis of the deposits on the trays could then provide information on the local dust-explosion hazards and show when rock dusting is required again.
In another study associated with the problem of rock dusting in coal mines, wet- and dry-applied asbestos dust and limestone dust were compared. Less coal float dust adhered to the dry-applied asbestos, which also was more easily dislodged by a low-velocity air jet. Wet-applied asbestos caked harder then wet-applied limestone.
Continuing the long-standing program to inform the public of the importance of safe mining practices, the Bureau held a large-scale demonstration witnessed by some 400 people at the Experimental coal mine on June 6, 1959.
Dust Explosion Galleries and Chambers
Although much information is now available on the explosibility of coal dust in clouds and in layers and on that of firedamp atmospheres, very little is known of the explosibility of mixtures of coal dust and firedamp. Because such mixtures are frequently encountered in mines, the Bureau has begun an investigation of the effect of small amounts of methane on the explosibility of coal dust-air mixtures and of small amounts of coal dust on the explosibility of methane-air mixtures.
An apparatus was designed and tested for determining the minimum dust-cloud concentration for ignition in atmospheres containing small amounts of methane.
Researchers studied the effect of dust concentration on explosion pressures and rates of pressure rise using various vent openings; such information is of great importance in designing industrial installations subject to dust-explosion hazards.
The Bureau investigated the spontaneous heating tendencies of dusts during storage, using the increase in temperature of air or oxygen passed through a coal-dust sample to evaluate the tendency of the sample to heat spontaneously; the surface area of the sample appears to be one of the factors promoting the tendency to self-heating. An investigation was begun to observe if and how aging affects the explosibility of coal dust stored for experimental purposes.
The good reproducibility of results obtained in the galleries and those obtained in the laboratory was checked through a statistical analysis based on data from 10 repetitive trials.
Dust Explosion Laboratories
Work was concluded on the experimental ignition of firedamp by sparks such as are produced by friction of roof-bolt steel against mine rock; gas was ignited by friction of steel on rock and by rock on rock but could not be ignited by steel on steel under the conditions employed.
Minimum electrical energies required to ignite clouds of coal dust were measured with standard electrostatic spark equipment and the Wimshurst electrostatic machine. The effect of the oxygen concentration of the atmosphere on the pressures and rates of pressure rise in coal-dust explosions was studied to establish a value applicable to most industrial installations.
Explosion hazards of 50 mineral, agricultural, and industrial dusts were evaluated by standard procedures in the laboratory. Table 6 gives characteristics of some of the more typical samples.
In fundamental studies of dust flames supported partly by the Office of Ordnance Research (Department of the Army), the burning times for single particles of graphite, aluminum, and magnesium were measured as a function of the particle size. For particles of more than 20 microns, diffusion of oxygen to the solid surface was the controlling factor; for smaller particles, the chemical reaction rate was controlling showing that the rate-determining process undergoes a change.
Meetings and Conferences
Besides conferences organized by sponsors and cooperative agencies in the interest of current activities, the Division participated in a number of international and national meetings. International meetings included the Seventh International Symposium on Combustion, Oxford, Great Britain, at which four papers were presented ; the 31st International Congress of Industrial Chemistry, Liege, Belgium, where one paper was presented, and a meeting of Directors of Safety in Mines Research, Verneuil, France, (October 1958) where results of six projects were reported.
In the United States, work of the Division, particularly in the field of fuel-sensitized ammonium nitrate blasting agents, was presented at the Fourth Annual Symposium on Mining Research, Missouri School of Mines; the 1959 Annual Convention of the National Crushed Stone Association; the Third Symposium of Rock Mechanics; the 1959 Annual Convention of the National Fire Protection Association; the May 1959 meeting of the Executive Committee of the Cement, Quarry and Mineral Aggregates Section, National Safety Council.
Explosion, Flame and Combustion Research
Most combustible-oxidant systems have two limits of flammability at any specified temperature and pressure. These limits are ordinarily defined so that all included mixture compositions are flammable.
Researchers calculated flame temperatures by trial and error to obtain further information on the effect of temperature, pressure, and inert diluents on adiabatic-limit flame temperatures. The heat of combustion of each limit mixture was equated to the enthalpy change required to increase the temperature of the combustion products to that of the flame. Because of the relatively low temperatures encountered, dissociation of the products was neglected. Experiments demonstrated that the adiabatic-limit flame temperature (Tf) is independent of the initial temperature over a rather wide temperature range. Since Tf is essentially constant for a given combustible, the law of Burgess and Wheeler may be written as follows:
L · C + H = k………………………………………………………….(1)
where L is the lower limit composition, C is the heat of combustion of the combustible involved, and H is the heat energy added to the limit mixture to raise its temperature from the reference temperature (for example 27° C.) to the test temperature; k depends on the combustible and the direction of flame propagation.
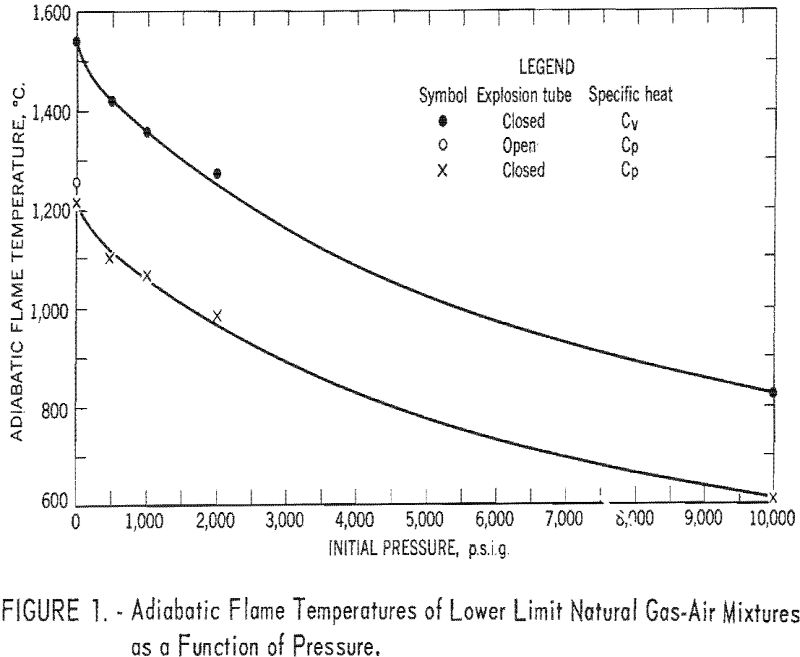
As the lower limit of flammability is decreased by an increase in pressure at a fixed Initial temperature, the heat liberated by the limit mixtures and the corresponding adiabatic flame temperatures also decrease. Flame temperatures of upper limit mixtures are approximately the same as those of the corresponding lower limit mixtures at atmospheric pressure; they appear to decrease quite rapidly with pressure, and yield extremely low values at moderate pressures.
Substituting an inert diluent for part or all of the nitrogen in the ‘air’ comprising a limit mixture does not appear to affect the limit flame temperature appreciably, unless the thermal conductivity, specific heat, and molecular weight of the substituted diluent vary appreciably from those of nitrogen. Thus, adiabatic flame temperatures of limit mixtures appear to be relatively independent of initial mixture temperature for many combustibles; they are dependent on initial pressure, direction of flame propagation, and combustible and inert gas compositions. For any particular combustible-inert gas-air system, the limit flame temperatures decrease with increase in pressure; upper limit flame temperatures decrease more rapidly with increase in pressure than lower limit flame temperatures.
The effect of temperature on the lower limit can he determined by assuming a constant flame temperature or by using a modified Burgess-Wheeler law. The effect of pressure cannot be assessed in the same manner, although some trends in the lower limits (fig. 1), minimum oxygen requirements, and limit flame temperatures have been noted.
Liberation of methane and formation of hydrogen and carbon monoxide in sealed areas of coal mines can create explosive atmospheres if enough oxygen is present to support combustion. However, the oxygen concentration may be reduced through combustion processes, and the concentration of inert gases may be increased enough to render the atmosphere nonexplosive. Before such a sealed area is opened, it is essential to know whether the flammable gases are present in concentrations that can form explosive mixtures when air is introduced. Various experimental methods are used to determine the explosibility of a particular mine atmosphere, but they are not generally well suited to field use.
To meet this need, the Bureau has developed a simplified graphic method for determining the explosibility of a mine atmosphere under mine-fire conditions, if the composition of the atmosphere is known. An explosibility diagram (fig. 2) is used to determine whether a given mixture of gases (for example, methane, nitrogen, and air) is explosive (A), will become explosive when mixed with air (B), or is nonexplosive (C). As the concentrations of the gases in a sealed area may vary with time, the composition of the gas for a given situation can be represented by the composition point calculated according to a procedure outlined.
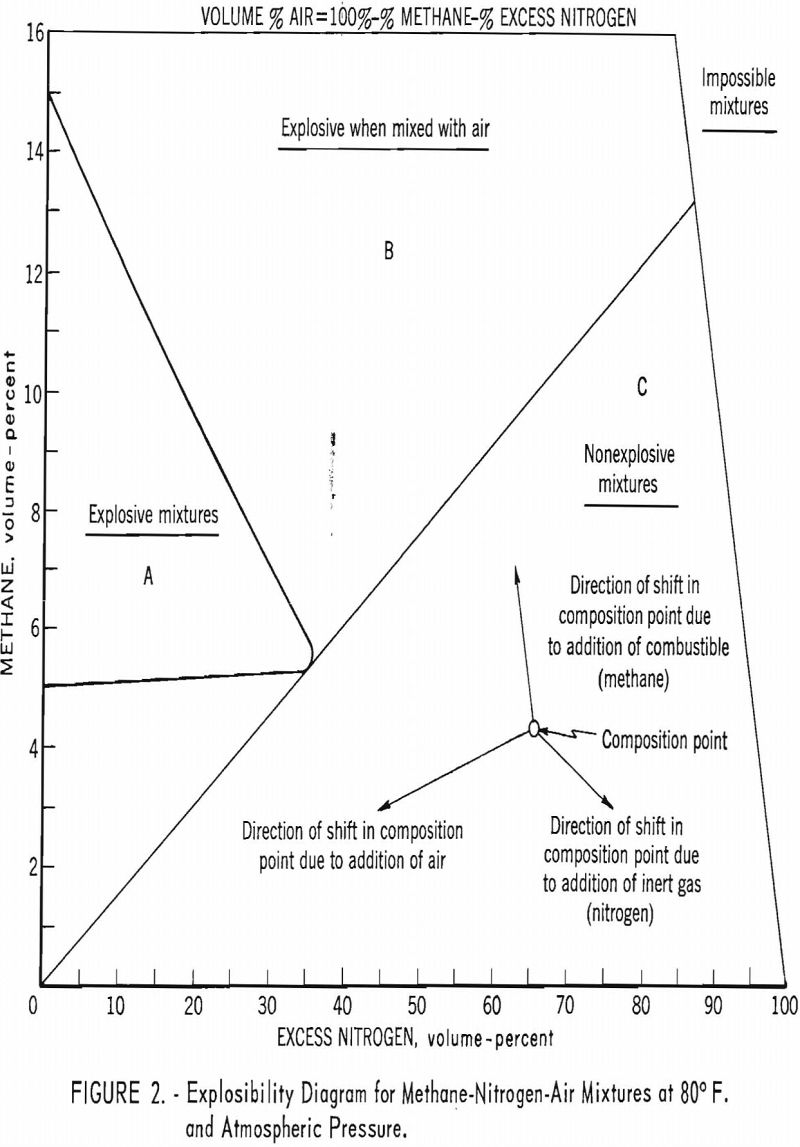
When the atmosphere also contains hydrogen and carbon monoxide, the diagram must be expanded, because methane, hydrogen, and carbon monoxide do not have the same lower limits of flammability; appropriate conversion factors are used to give the same effective lower limits for each gas. Similarly, appropriate conversion factors must be used to calculate the effective inert-gas concentrations for nitrogen and carbon dioxide.
A ratio scale is given for the rapid determination of the maximum allow-able oxygen concentration as a function of a methane-to-total combustible ratio.
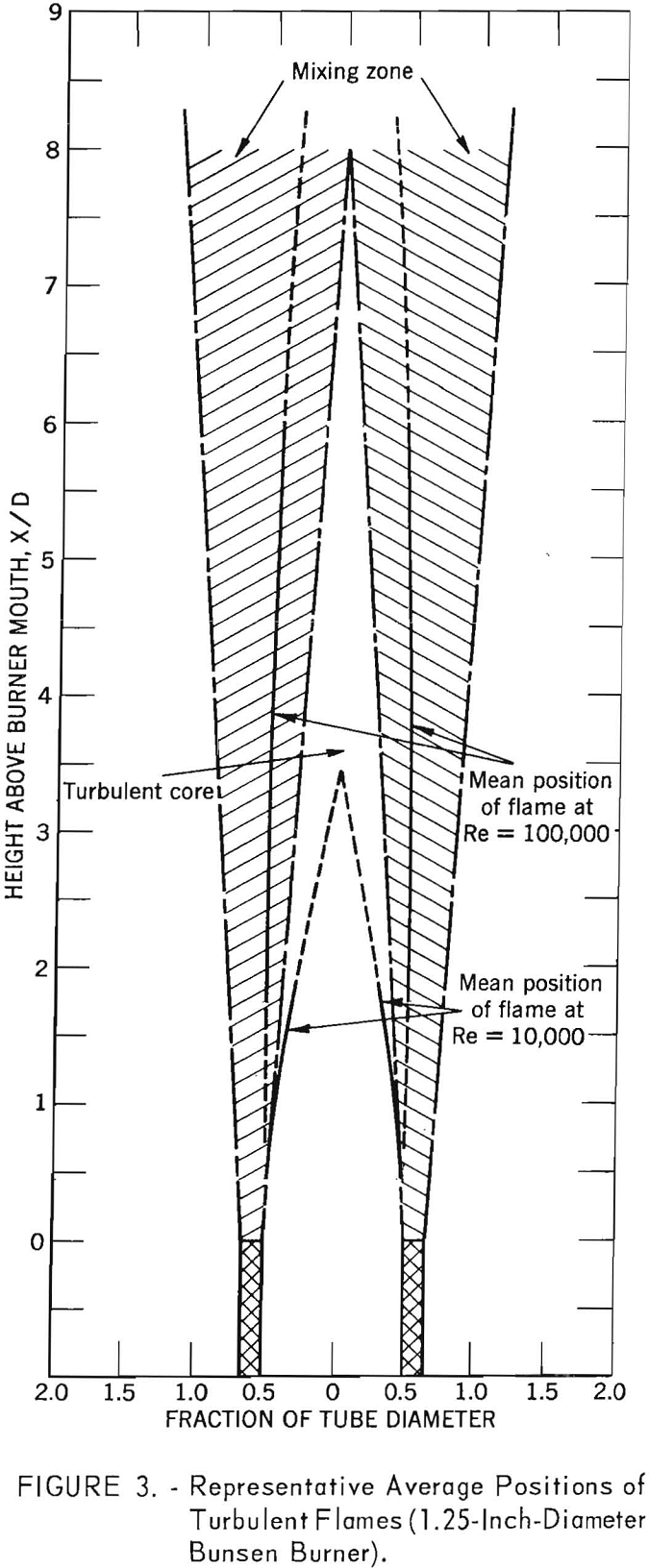
Work with bunsen-type flames affected by pipe-flow turbulence suggests that a useful distinction may be drawn between flames that burn (1) in the turbulent core of the approach flow and (2) in the wake of the burner rim, in what is commonly known as the “mixing zone” (see fig. 3). In the turbulent core, the average gas flow velocities, the turbulent fluctuation velocities and, presumably, the scales of turbulence, are close to their values at the mouth of the burner; a flame at low Reynolds number lies almost entirely within this core. In the wake of the burner rim there are steep velocity gradients; the scale of turbulence in this wake zone is markedly larger than in the core.
Flames within the central core are probably the flames of practical importance. However, there are unresolved controversies over their structures, which may depart from the traditional model of a wrinkled laminar flame front.
For this reason, a study was made of flames in the wake zone where the “classical” model of a wrinkled laminar flame explains the salient features of structure and propagation rate. For flames burning in the turbulent core, this simple model may still give useful predictions, even ‘though the flame brush structure is much more complex.
Flashback and blowoff limits of laminar flames have been explained previously by a concept based on boundary velocity gradients, and it has been shown that the critical-boundary velocity gradient for blowoff can be the same for laminar and for unpiloted pipe-flow turbulent flames. On the other hand, flashback and blowoff gradients have been reported to be greater in turbulent than in laminar flow. An explanation of these differences could contribute to the understanding of turbulent combustion and to the design of industrial equipment and engines.
In a preliminary study, the need for further information on stability limits of turbulent flames has been indicated, and new data have been
presented on laminar tilted-flame limits, as well as turbulent flashback and blowoff limits; only unpiloted flames on unobstructed ports have been considered.
A correlation was sought between tilted flame gradients for flashback in laminar flow and turbulent flashback gradients, based on the assumption that turbulent flashback is produced by events in the laminar sublayer. This sublayer is more likely to be significant on ports of small diameter. Blowoff gradients of unpiloted pipe-flow turbulent flames have shown that stabilization of turbulent flames is not always in the laminar boundary sublayer. In unpublished experiments at the Bureau of Mines, the volumetric flow at blowoff of pipe-flow turbulent unpiloted flames was raised considerably by adding a cold, short, slightly but sharply expanded cylindrical section of tubing (a skirt stabilizer) atop a long pipe burner These skirt stabilizers were operated hot, water-cooled, and with inserts of varying geometry. In figure 4, on a 15-cm. long water- cooled skirt, the flame base shows an interesting abrupt expansion at the exit of the skirt, which suggests that the cross-sectional area of the flame within the skirt is reduced by a boundary layer of burned gas along the wall. This inward thrust on the flame due to confinement of burned gas by the wall of the skirt may be important in achieving the great gain in flame stabilization that this geometry provides.
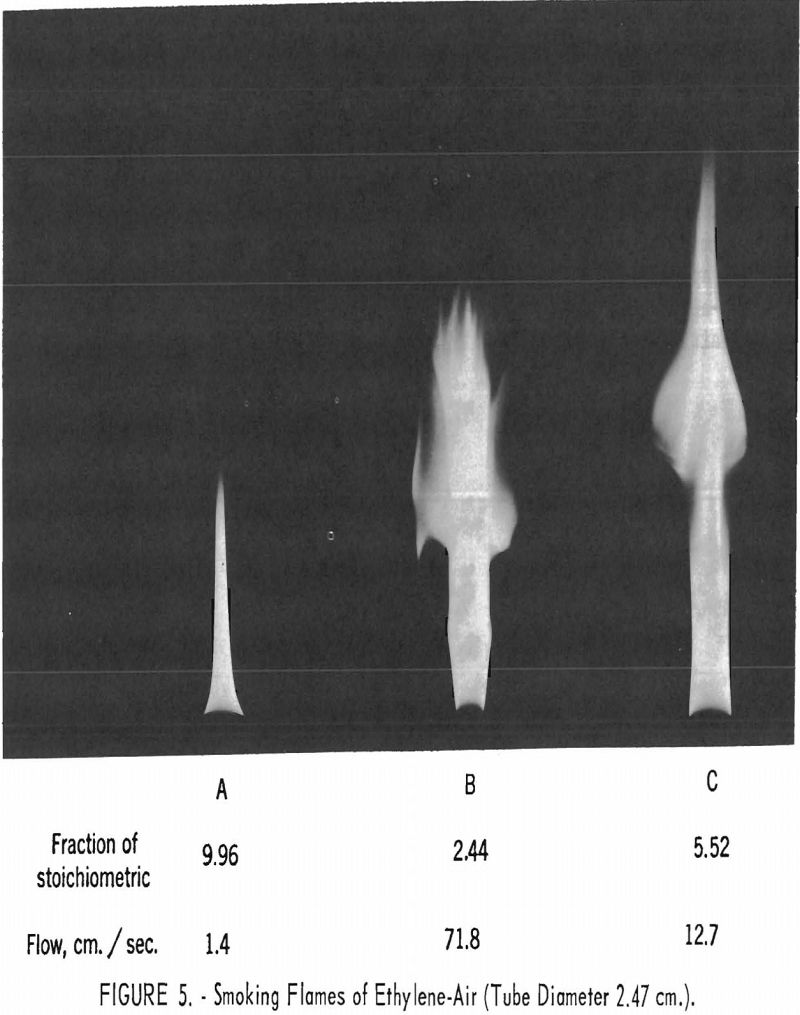
Carbon deposits from smoke in gas applicances and engines are a serious problem, and the smoke limit of the burner flame must be considered in designing gas-burning appliances. To obtain information on the mechanism of smoke production in flames and on the fuels and fuel-air ratios that can be used on gas appliances without evolving smoke, a study was made in which streams of ethylene and air were permitted to burn in free air. Hot gases leaving the flame were viewed under even illumination against a black background. Particulate matter in the nonluminous hot gas from the flame was made visible by the reflection of a strong beam of light. The smoke limit determined in this way is defined as the point where smoke is first observed leaving the flame, and is expressed in units of flow against fuel-air composition of the premixed stream.

Researchers found that there are no smoke limits characteristic of the fuel gas and independent of the burner diameter and fuel rate. No smoke limits leaner than the constant yellow-tip limit for the particular fuel (characteristic of the fuel-oxidant system alone) were observed.
Two types of smoke-limit flames were observed: Flames that are richer and primarily a function of flame rate (fig. 5A); and flames that are leaner and depend primarily on the fuel-air ratio (fig. 5B) . The availability of unlimited ambient secondary air does not prevent escape of smoke from flames. Gases within smoking ethylene-air flames do not contain significant quantities of oxygenated and/or high molecular hydrocarbons.
Flat and conical flames of very rich propane-air and ethylene-air mixtures were examined in the region from the initial blue zone up to late in the yellow zone. Analysis of the flame gases showed that the main reaction products were carbon monoxide, carbon dioxide, hydrogen, and water. No compound heavier than toluene was observed, even in concentrated samples. The small quantities of low-molecular-weight aromatics and unsaturated hydrocarbons and their absence in the smokier region of the flame indicate that carbon is not formed mainly by polymerization. Carbon may form directly by splitting of C-C and C-H bonds. The CO2/CO ratios were approximately five times larger in diffusion flames than in premixed flames. Rich propane-air and ethylene-air flames were not in thermochemical equilibrium.
Temperature profiles based on thermocouple measurements through a rich flat flame were higher than theoretical in the vicinity of the initial blue zone and lower in most of the yellow zone.
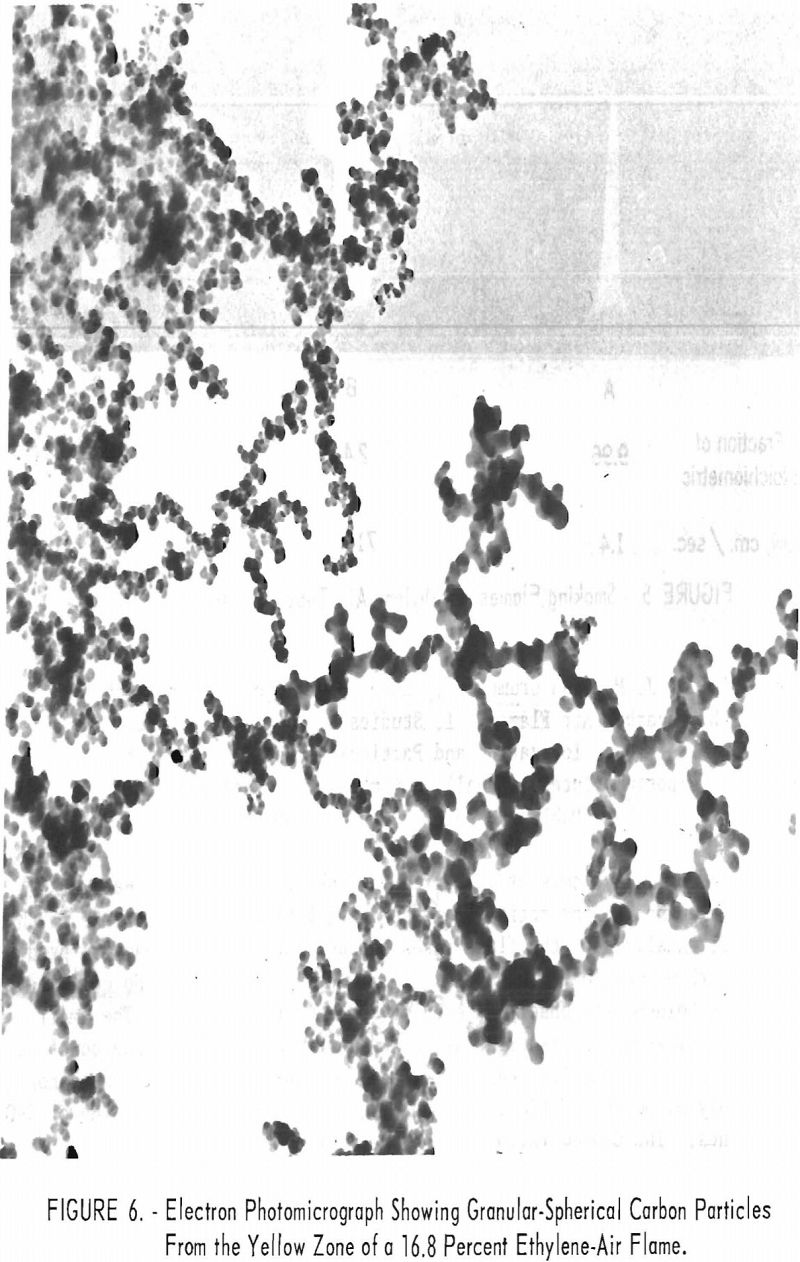
The ionization maximum in flat flames occurred a short distance beyond the start of the yellow flame zone at a position where temperatures were falling, suggesting that carbon nuclei or possible carbon precursors were ionizing. Electron micrographs showed that particulates within these flames differ in physical appearance. The carbon particles were granular-spherical for the ethylene-air flames (fig. 6) and filamentary to granular-spherical for the propane-air flames (fig. 7).

Igniting combustible mixtures by hot burned gases from flames is important in many applications such as ramjets, burners, and rocket-combustion chambers. Following an investigation, which showed that igniting methane-nitric oxide mixtures by pilot flames depends on the size and temperature of the flame, researchers attempted to extend this experimental procedure to hydrocarbon-air mixtures. However, minimum ignition conditions could not be established in this way for these mixtures. Accordingly, the investigator developed a procedure in which a gas such as nitrogen or air was heated in a furnace and injected continuously as a jet (4 mm. diameter) into a cold combustible mixture at a flow of 35 cc./sec. Luminous reactions were observed within the jet and, under favorable conditions, ignition of the cold outer mixture occurred at the end of the luminous jet at distances up to 30 cm. from the furnace. Ignition temperatures were measured for two conditions: (a) Ignition by a diffusion flame formed when hot air flows into pure cold fuel, and (b) ignition of an explosive fuel-air mixture by hot nitrogen. Ignition temperatures are lower for (a), where only the fuel must diffuse into the jet, than for (b), where both fuel and air must diffuse into a hot region to start a reaction.
The author notes that the “hot-gas ignition temperatures” do not correlate with other flame characteristics, especially with the “spontaneous ignition temperatures” measured in furnaces.
Detonation of an explosive in a shothole produces a shock wave which issues from the hole and possibly contributes to the ignition of the firedamp atmosphere into which it emerges. This is followed by a jet of hot gases, which is the most probable ignition source.
To study the mechanism of this ignition the researchers extended the investigation of the action of hot-gas jets under conditions designed to reproduce ignition of firedamp by hot spent detonation gas on a smaller scale. Factors that were considered included composition and oxygen index of the firedamp atmosphere, effect of turbulence of the jet, and composition of the detonation gases to simulate conditions with under- or over-oxidized explosives.
Turbulence of the jet reduced the ignition temperature. The presence of 9-percent ethane in methane (the approximate composition of firedamp) reduced the ignition temperature about 30° C. The role of the oxygen index was somewhat ambiguous. The most significant observation was that the concept of a “most ignitible mixture” is useful only if the hot jet is inert. If oxygen is present in the jet, the most ignitible mixture may be close to the rich limit. Small amounts of hydrogen and/or carbon monoxide in the detonation products can reduce the ignition temperature as much as 500° C. Thus, explosives that liberate small amounts of hydrogen and carbon monoxide may offer an increased hazard. Further experiments with short bursts of hot gas into explosive mixtures are in progress.
Previous investigation had shown that the ignition temperatures of a variety of fuels ignited by continuous jets of hot inert gas follow an order that is very different from that observed for spontaneous ignition or spark ignition. On the other hand, hot-gas ignition temperatures are close to flame temperatures for limit mixtures, suggesting that the reactions governing limit flames are similar to those leading to hot-gas ignition. If this is true, the temperatures should be of the same order in both instances.
To verify this, researchers compared hot-gas ignition temperatures with limiting flame temperatures, determined by producing a diffusion flame in an initially stoichiometric fuel-air mixture, which was progressively diluted with nitrogen. The nitrogen concentration required to quench the flame was noted, and the limiting flame temperature was calculated, assuming stoichmetric combustion. With a few exceptions, a correlation was found between hot-gas ignition temperatures and limit, flame temperatures.
The effect of characteristic flame inhibitors (such as bromine, chlorine, and methyl bromide) was investigated, and this corroborated the conclusion.
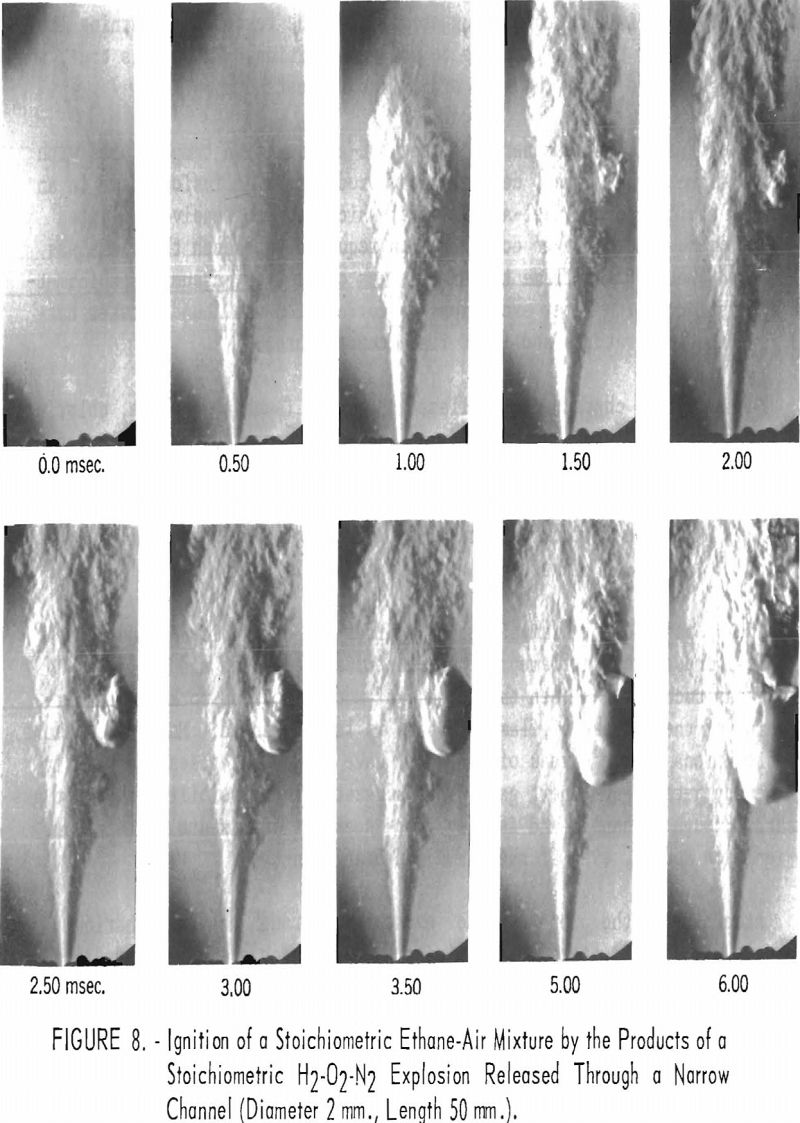
The propagation of flames through cylindrical or annular channels has practical importance in flameproofing electrical equipment and for flame traps. As such, it has been the subject of much practical work, but understanding of the process of flame stoppage is less advanced. Moreover, recent investigations at the Bureau of Mines have shown that gases are readily ignited by jets of hot inert gas. This suggested the possibility that hot gas pushed through a channel and issuing into an explosive mixture could become an ignition source, even if the flame had been quenched.
Accordingly, the propagation of explosions through short cylindrical channels was investigated, using an apparatus in which a single cylindrical channel formed a connection between a small chamber in which an explosion was initiated and a larger chamber filled with suitable gas mixtures. Researchers found that transmission of flame through the channel was not essential to induce an explosion in the larger vessels. Schlieren photographs of flames of hot-burned gases emerging from the channel into the explosion chamber showed that vortexes of hot gas ejected from the jet into the cold explosive mixture are responsible for ignition (fig. 8).
An earlier investigation showed that although flames do not propagate through narrow passages (≤ 3 mm.), hot gases can travel through such passages to ignite the atmosphere into which they emerge. To support and complete these findings, researchers made systematic measurements to determine the effect of channel geometry and of the properties of the gas mixtures. The influence of quenching diameter and flame temperature was also considered.
Ignition was shown to originate in eddies that form in the outer boundary of the jet. This demonstrates that the ignition process is influenced not only by the nature and temperature of the hot gases issuing from the channel but also by the properties of the cold outer gases. The aerodynamic properties of the jet are the important parameters determining ignition. They, in turn, are influenced by the length and width of the channel.
Against the background of extensive information on the spark ignition of combustible gas mixtures resulting from the Bureau’s long term investigation of the problem, a study was made of the gap energy for ignition in a circuit containing dissipative elements.
Factors considered were (1) the energy stored on the condenser, (2) the energy that may be dissipated in any resistance in the external circuit, (3) possible electromagnetic radiation from the circuit elements; and to a lesser degree (4) effects such as voltage, phase angle, and discharge time.
The resulting information indicates that the minimum gap energy is essentially the minimum stored ignition energy as measured in the absence of dissipative elements in the external circuit; in other words, there is no indication that the presence of series resistance reduces ignition energy requirements significantly if electromagnetic radiation is neglected.
To implement the study of the ignition of explosive gas mixtures by low-energy capacitance sparks of very short duration (1 mµ sec. to 1µ sec.), investigators assembled auxiliary equipment around a commercial dual-channel oscillograph. The completed setup is described, and performance characteristics are given. The equipment permits observation of current and voltage transients in sparks capable of igniting gas mixtures. Its resolution time is about 2 mµ sec.; the instantaneous amplitude of the wave forms can be used to within 4 percent of maximum deflection. Oscillographic measurements of the total energy dissipated during a spark discharge correspond within about 10 percent to the known initial stored energy, for energies of the order of 1 mj, or more.
Explosives Research and Testing
Aware of the rapid advances of field-mixed do-it-yourself explosives, based on fuel-sensitized fertilizer-grade ammonium nitrate, the Bureau has undertaken a study of the safety characteristics of these explosives.
In summing up the present status of this investigation, three problem areas are stressed:
- Ammonium nitrate-oil mixtures should be treated as true explosives that can detonate accidentally, even though they may be relatively insensitive in most forms.
- Fire can be a serious problem. Fire in ordinary raw ammonium nitrate can be fought quite successfully with copious amounts of water, whereas other extinguishing agents are not effective. However, there is some question as to the advisability of attacking established ammonium nitrate-oil fires, except by remote means.
- Fumes from blasting may present a serious hazard, especially in underground operations.
The relation between the borehole freespace and the probability on fire-damp being ignited by a borehole shot has been studied by Audibert, Denues, Greenwald, Grant, and others. To obtain more quantitative information of this relationship, the Bureau began an investigation in which 700 shots of a typical permissible explosive were fired from a ¾-inch steel cannon with several freespace conditions (fig. 9). The effect of a second variable (the charge weight) was also considered.
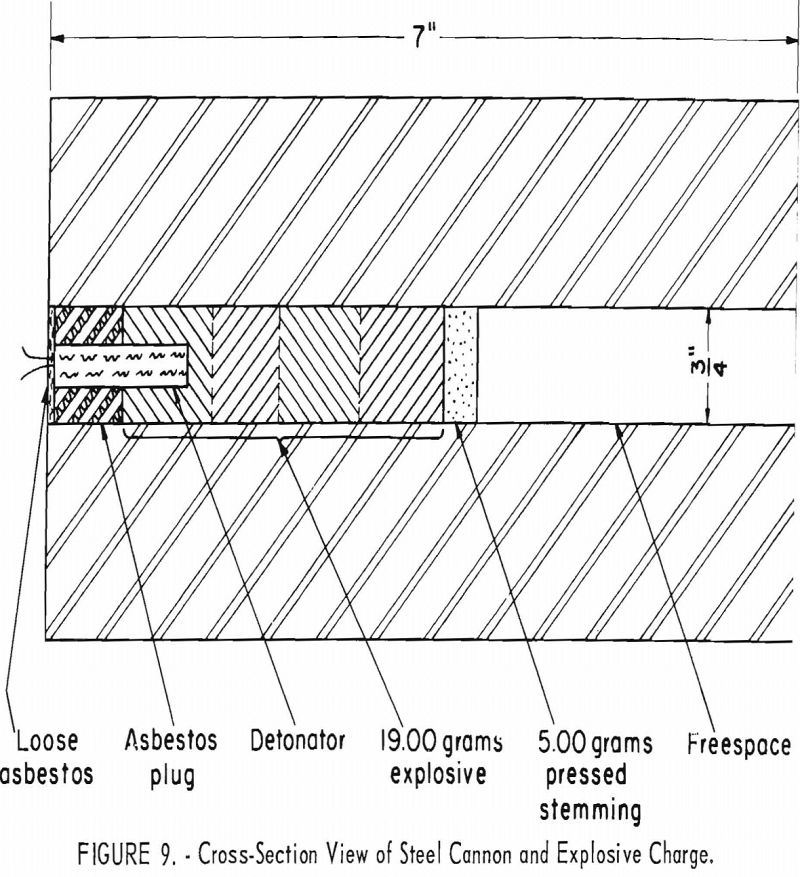
Two basic procedures were used. In the first, the charge weight was varied according to the Bruceton up-and-down method to determine the W50 value (weight of explosive giving a 50-percent ignition probability under the experimental conditions). In the second procedure, the charge weight was maintained at this W50 value and the freespace was varied from 0 to 6 inches (fig. 10).
Statistical treatment of the resulting data by analysis of variance showed that the freespace means were different; this conclusion is not impaired by possible differences in the explosive used. For the given experimental configuration, the incendivity increased with increasing freespace up to a maximum; beyond this maximum, there was indication of decreasing incendivity.
Although the records show that permissible explosives have not caused a single explosion disaster when used in prescribed ways, some ignitions occur annually owing to improper usage. Moreover, recent developments (“stump” blasting, multiple short-delay blasting) in coal-mining methods in the United States have created a need for explosives that are even less incendive than the existing formulations. To meet these new conditions, a program of research was initiated, designed to give a better understanding of the relative incendivity of explosives and of the basic variables that affect this incendivity in firedamp or firedamp-dust atmospheres.

The first step was the choice of a test method capable of giving a more realistic picture of the ignition probability than the classical method, in which testing is performed at the tail of the probability curve. In the belief that more reliable and economical comparisons between explosives are obtained by comparing the 50-percent ignition probability points, the Bureau applied the Bruceton up-and-down method experimentally in gallery testing of permissible explosives modified by the addition of small percentages (0 to 9 percent) of sodium chloride.
A study of the results increased confidence that the up-and-down technique is capable of distinguishing differences in incendivity (under gallery test conditions), not only for different explosive compositions, but for lots of the same explosive. The experimental data also show that addition of small quantities of sodium chloride tends to reduce the incendivity of the explosive to firedamp, although it cannot Insure reduced incendivity, as chemical composition alone does not define the incendive character of an explosive. The investigation must be extended to a number of other factors that may affect the safety of permissible explosives.
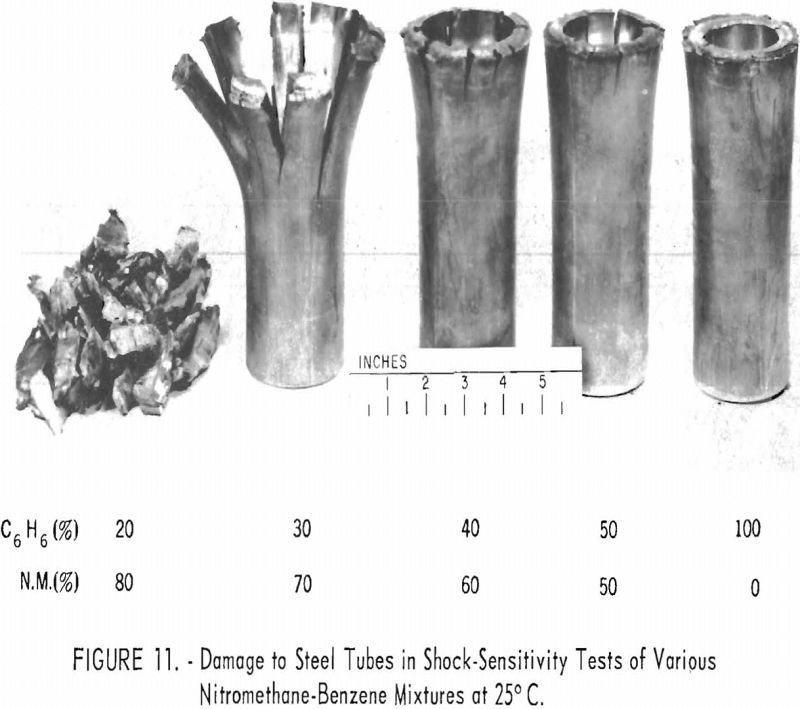
Applying nitromethane as a solvent, in the synthetic-fiber and petroleum-refining industries depends upon the sensitivity characteristics of the systems formed when process materials are dissolved in this compound. In the course of an extensive study of the sensitivity of nitromethane, the Bureau developed a modification of the card-gap test, which,with the experimental Bruceton up-and-down method, gives dependable and very reproducible results. Liquid systems that yield negative results at zero card gap but still show considerable release of energy were subjected to a more rigorous confinement test which gave semiquantitative data (fig. 11).
A systematic study of the action of various sensitizers and desensitizers showed that bases are strong sensitizers; acids and a wide variety of other compounds are mild to weak sensitizers or desensitizers, with evidence of competing effects. Temperature has a marked influence on the sensitivity level; higher temperatures increase sensitivity.
Experimental Coal Mine and Dust Explosions Research
In 1956 the British Safety in Mines Research Establishment issued a report describing the initial phases of research for the remote control of timber fires with high-expansion foam plugs in a rock tunnel. As the method promised to be useful in controlling coal-mine fires that cannot be attacked by standard fire-fighting measures and frequently require extensive sealing, an investigation was begun in 1957 by the Bureau of Mines to study the applicability of the method under American mining conditions (room-and-pillar extraction, multiple entries).
Preliminary laboratory studies with some 35 foaming agents and 20 stabilizers or additives showed that there are wide variations in the rate of liquid loss and in the stability of foams produced by various agents. Large-scale studies were made at the Experimental coal mine, using two basically different types of detergents as active ingredients: Alkyl aryl sulfonates
and lauryl sulfates. More than 70 transport experiments were made in the Experimental coal mine to study the variables governing the formation and movement of foam plugs through mine entries and to find or develop foams that can be transported at a low water gage over long distances and still retain enough moisture to control fires.
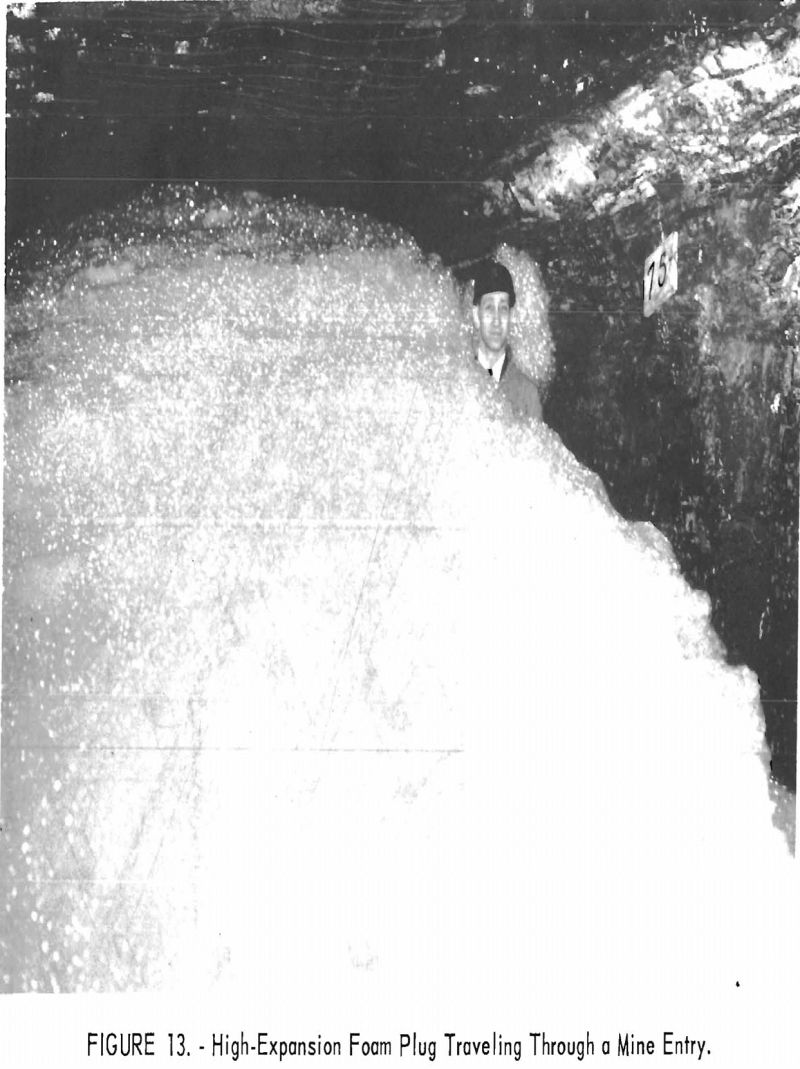
The foam is formed by spraying a water solution of foaming agent on a net stretched across the mine entry (fig. 12). As the solution penetrates the net, air-filled bubbles are produced quickly filling the cross section of the entry and creating a light-weight foam plug, which is moved toward the fire by the ventilating current (fig. 13).
In the transport experiments, the structure and stability of the foam, liquid loss at the net, liquid drainage during transport, and final liquid content varied appreciably, depending upon the foaming agent. Investigation showed that the foam can turn sharp corners and move over obstructions. The expansion ratio of the foam is about 500:1 to 1,000:1 as compared with about

10:1 for ordinary fire-fighting foam. The length of foam plug that can be formed depends primarily on the available air pressure, the physical characteristics of the road surface, and various properties of the foam. As the plug moves forward, its rate of advance decreases.
Nine fire experiments were performed in the Experimental coal mine (fig. 14). For effective control of a floor-rib-roof coal fire, the estimated water content of the foam transported through the fire zone was about 100 to 150 gallons. In an earlier investigation, 450 gallons of water applied as a spray from a 1-Inch nozzle were required to quench a similar fire.
Thus, by selecting a suitable foaming agent and by proper control of the air pressure and the initial air speed at the net, relatively long foam plugs may be generated, with a wide range of moisture contents. Although transporting foam over a rapidly advancing, deep-seated fire is not likely to quench it completely, the procedure may suppress flaming combustion, thus enabling fire-fighters to approach the fire near enough to complete quenching by applying
large volumes of water or other standard measures. When the air pressure available at the foam-generating net is limited and not quite adequate to drive the plug to the fire, it may be possible to augment the pressure by installing an auxiliary blower in the mine entry or by advancing the net toward the fire in several steps. The practicability of the latter method is being investigated, as are many other phases of the problem.
In 1949, research at the Experimental coal mine established that the gas ignition hazard of permissible explosives fired at short-delay (millisecond) intervals, is low and of about the same order as equivalent single-shot blasting. The hazard is less than with simultaneous initiation of the charges. Moreover, other things being equal, short-delay blasting reduces exposure of the shotfirer to dust, fumes, and weakened roof as compared with equivalent single-shot blasting. Compared with equivalent simultaneous blasting, it reduces roof disturbances. Nevertheless, recommendations for short-delay blasting were delayed by the Bureau pending consideration of the potential hazards of misfires and cutoff charges (charges exposed through dislodgment of the burden by a previous shot).
The problem of misfires was met by a testing schedule for a permissible blasting unit, which provides adequate current for initiating 20 short-delay detonators wired in series. The present Information Circular on multiple blasting with millisecond-delay detonators, prepared for the guidance of safety engineers, shotfirers, and mine inspectors lists the precautions to be followed to minimize cutoffs, misfires, and other potential hazards of multiple shooting.
