Table of Contents
Increasing usage of aluminum and competition for foreign reserves of bauxite have created a renewed interest in the development of domestic aluminum resources. Investigations were conducted into producing aluminum from domestic clays, anorthosite, and alunite in order to assure a continuing supply in the face of an uncertain world situation. Processes were developed that were technically sound but more expensive than using bauxite in the Bayer-Hall process. Our interest in increasing the productivity of domestic mineral resources has prompted a reinvestigation of these processes using nonbauxitic material to determine whether new techniques and recent innovations might make these processes economically feasible.
An undesirable feature common to all the acid-clay leach processes is coleaching of iron. Cell-grade alumina should not contain more than 0.01 pct iron, but it has been difficult to remove iron from aluminum leach solutions to a correspondingly low level.
The development of solvent-extraction technology has provided a new avenue for iron removal from acid-clay leach solutions at a reasonable cost.
Current investigating processes for recovering aluminum from clay by leaching with nitric acid or hydrochloric acid. These two processes use solvent extraction to purify the aluminum leach solutions of coleached iron. This investigation was initiated to test sulfuric acid as a leachant and to improve the iron-removal step using a continuous solvent- extraction system.
Materials
Leach solutions were prepared from a sample of kaolinitic clay that had been calcined at 750° to 800° C and ground to pass a 20-mesh screen. Table 1 shows the average analysis of three samples.
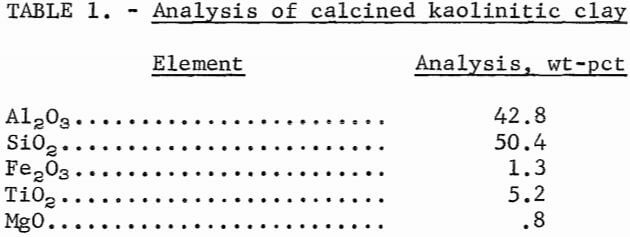
The extractants used in the initial phase of this study are commercially available and include representatives of common extractant classes. Table 2 lists the classes and suppliers of the various extractants used.
The majority of the work was done with Primene JMT, a primary amine having the general formula t-Cl8H37NH2 to t-C22H45NH2. The organic diluents used were Pearl kerosine and Socal 335L, which is a petroleum fraction containing 55 pet aromatics, 18 pct paraffins, and 27 pct naphthenes; both are available from Standard Oil Co. A commercial mixture of decanol isomers was used as a phase modifier. All other chemicals were reagent grade.
Equipment and Procedure
Aluminum sulfate solutions were prepared on a bench scale by leaching clay with sulfuric acid at 105° C in a resin kettle equipped with a mechanical stirrer and a reflux condenser. A larger scale leach was also conducted in a 400-gal fiberglass stirred tank equipped with a steam-heated titanium heat exchanger for startup.
Solvent-extraction experiments were conducted using separatory funnels to determine data points for equilibrium diagrams and measure the effect of individual variables. Elevated temperature studies were conducted either in a constant-temperature water bath or in a controlled-temperature oven. Multi-stage studies were conducted in a 10-cell, mixer-settler unit designed by Bell Engineering Co. This unit has a maximum capacity of 250 ml/min total flow of both phases. Mixing- and settling-chamber volumes were 180 and 400 ml, respectively. The settler area was 61.5 cm². This unit was modified by adding covers and immersion heaters to the settlers and insulating the cells to obtain elevated temperatures when required.
Results and Discussion
Determining Leaching Conditions
A series of bench-scale leaching experiments was performed to select a set of conditions to be used in a large-scale leach test for preparing a stock- leach solution. Batches of calcined clay were added to varying dilutions of 96-pct sulfuric acid in a resin kettle. The clay was reacted under reflux conditions for 2 hours. The percent dissolution of aluminum was determined, and the physical characteristics of the leach slurry were observed (table 3).
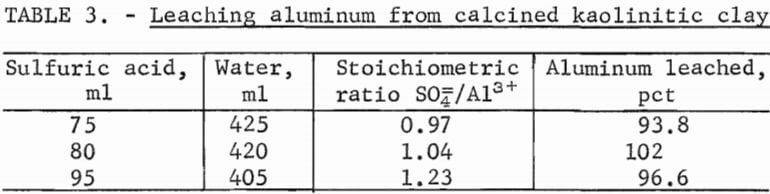
Note.—Conditions: 125 grams calcined clay, 105° C, 2 hours leach time.
The dissolution of aluminum was greater than 90 pct in all three tests; however, there was a noticeable difference in physical characteristics of the leach slurries that affected the settling and filtration rates. In the tests made with 75 and 80 ml of acid, the leach slurry settled to yield a relatively clear supernatant solution that could be decanted. The test made with 95 ml of acid solidified into a solid mass of crystals upon cooling.
A stock-leach solution used in the solvent-extraction experiments was prepared by conducting a large-scale leach using the following conditions (equivalent to leaching 125 grams calcined clay with 80 ml sulfuric acid): (1) A 400-pound batch of calcined clay was added in 20-pound increments to 160 gal of water plus 32.5 gal of sulfuric acid; (2) clay was added over a 3-hour period to control the rise in temperature caused by the exothermic reaction; (3) the slurry was stirred for an additional 2 hours at 105° C and allowed to settle overnight; (4) the supernatant solution was decanted and clarified by filtration; and (5) the tailings were reslurried with wash water, settled, decanted, filtered, and added to the leach solution. The combined leach-and-wash solution was analyzed as follows, in grams per liter: Al 46.7, Fe 1.53, Mg 0.095, and Ti <0.001. The measured acid concentration was 0.4N sulfuric acid.
Determining Extraction Conditions
A series of experiments was conducted to select the most efficient extractant for removing iron from the aluminum sulfate leach solution. Extractants were contacted with the leach solution in separatory funnels at ambient temperature at a 1:1 phase ratio. Kerosine was used as the diluent in all cases. Table 4 shows the iron extraction in percent.
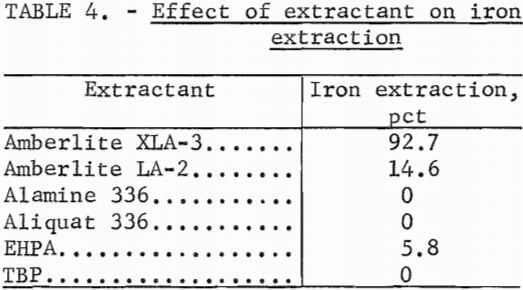
A 10 vol-pct concentration of extractant was used in each case, with the exception of the test made with TBP where 50 vol-pct was used. In the tests made with amines, 5 vol-pct decanol was added to the diluent to improve phase disengagement.
The primary amine was the only extractant that demonstrated an appreciable affinity for iron. Since Amberlite XLA-3 is a purified fraction of less expensive Primene JMT, the remainder of the experiments were conducted using the latter extractant.
During an earlier investigation into the extraction of iron using a tertiary amine from an aluminum chloride solution, ferrous iron was shown to be a nonextractable species (JL). The possibility that a similar effect might occur in a sulfate system was investigated by contacting two portions of aluminum sulfate leach solution with 10 vol-pct Primene JMT in kerosine. In the first portion, the iron was oxidized with hydrogen peroxide; the iron concentration after extraction was measured for comparison with the result obtained from the second portion, which had not been oxidized. The iron concentration in the raffinate varied between 0.14 g/l without oxidant and 0.004 g/l after oxidation indicating that the presence of ferrous iron has a marked detrimental effect on overall iron extraction. Hydrogen peroxide was added to all subsequent portions of leach solution prior to extraction studies.
The effect of using a diluent having a high-aromatic content was investigated by contacting portions of the stock-feed solution at an organic-to-aqueous phase ratio of 1:3 at 50° C using 10 vol-pct Primene JMT as the extractant. The diluents were kerosine plus 10 vol-pct decanol and Socal 355L. The percent extraction of iron was determined to be 67.8 for the Socal 355L and 56.6 for the kerosine-decanol; however, the phase separation was significantly better when the kerosine mixture was used. Iron loading in the organic phase was calculated to be 2.4 g/l iron using kerosine-decanol and 2.9 g/l using Socal 355L. Since bulk prices of Socal 355L are nearly 50 pct higher than for kerosine and phase separation is not as effective using Socal 355L, kerosine was used as the diluent for all the remaining work.
To determine the effect of acid strength on the extraction of iron, a series of tests was conducted on a synthetic solution prepared by dissolving aluminum sulfate and ferric sulfate in varying concentrations of sulfuric acid. These solutions were contacted with 10 vol-pct Primene JMT in kerosine at an organic-to-aqueous phase ratio of 1:1. The iron concentration in the aqueous phase was determined before and after contact (table 5).
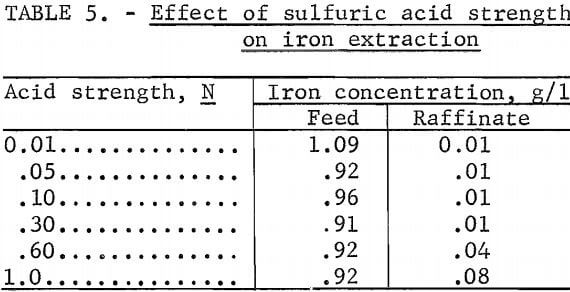
The extraction of iron was constant up to an acid strength of at least 0.3N but began to decrease at 0.6N. Since the stock-leach solution had a measured acid strength of 0.4N, extraction should be near maximum efficiency.
Since amines are organic bases, an experiment was conducted to determine whether the primary amine would extract appreciable quantities of acid from the leach solution. A portion of the stock-leach solution was contacted with a solution of 20 vol-pct Primene JMT in the free amine form and 10 vol-pct decanol in kerosine at a 1:1 phase ratio. The pH of the aqueous phase, as measured before and after contact, increased from 0.4 (0.4 N H2SO4) to 0.5 (0.3 N H2SO4). Since the final pH is within the range that yields satisfactory extraction of iron, it should not be necessary to pretreat the amine solution with acid prior to startup of the system. The only condition that might cause a loss in extraction efficiency would be the carryover of entrained acid from the stripping circuit.
To investigate the effect of temperature, portions of the stock-leach solution were contacted with 10 vol-pct Primene JMT in kerosine at a 1:1 phase ratio at temperatures from 0° to 60° C. Iron remaining in the raffinate is shown in table 6.
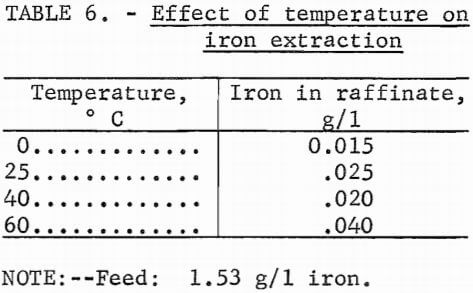
Phase separation improved with increasing temperature, but extraction efficiency decreased. At 60° C, however, 97 pct of the iron was extracted, indicating that even at these higher temperatures, the system could be used effectively for extracting iron.
An important consideration in developing a solvent-extraction system is the extent to which metals other than the metal of interest are coextracted. Since the goal of this investigation is to remove iron from aluminum sulfate leach solutions, experiments were conducted to determine the extent of aluminum extraction by the primary amine. A portion of the stock-leach solution was contacted with 10 vol-pct Primene JMT in kerosine at an aqueous-to-organic phase ratio of 2:1. Analysis of the organic phase indicated that 0.027 g/l aluminum was present, which corresponds to 0.03 pct extraction. In another test, a solution of aluminum sulfate free of iron was contacted at a phase ratio of 1:1 with a 10-pct amine solution; the organic phase contained 3.3 g/l aluminum, which corresponds to 7.5 pct extraction. In multistage experiments using stock-leach solution, the concentration of aluminum in the organic phase decreased from 2.2 g/l in the first extraction cell to 0.009 g/l in the fourth extraction cell. Thus, aluminum appears to be extracted when insufficient iron is present, but it is displaced by iron as the organic phase becomes loaded.
An experiment was conducted to determine the effect of adding various concentrations of decanol on loading of iron into the primary amine. Organic solutions containing increasing amounts of amine and decanol were contacted with the stock-leach solution at a constant ratio of amine to iron at 50° C.
The concentration of iron in the amine solution is shown in table 7.
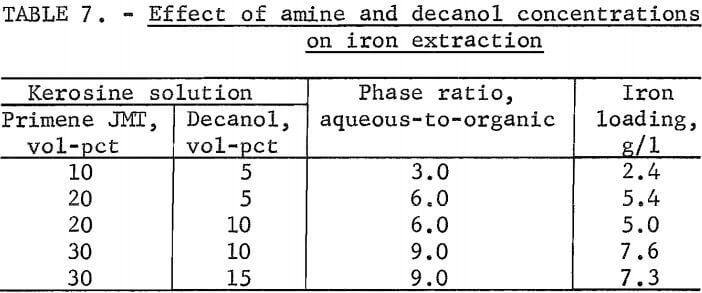
Although somewhat higher iron loading may be achieved with lower decanol concentrations, phase separation was much better with the higher decanol concentrations; therefore, in general, a ratio of 2 amine to 1 decanol was used in further investigations.
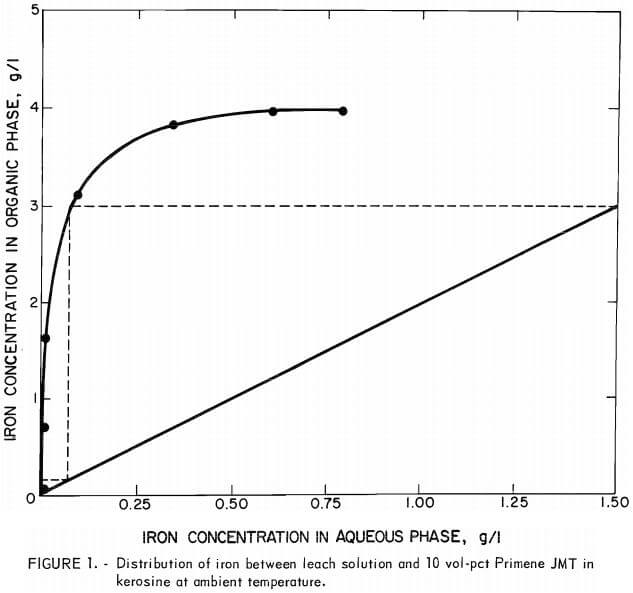
To evaluate the efficiency of the extraction system devised in the preceeding experiments, a series of equilibrium diagrams was developed for the distribution of iron between the stock-leach solution and primary amine solutions in kerosine. The first diagram was developed using 10 vol-pct amine at ambient temperature without adding decanol. Figure 1 shows that iron may be extracted under these conditions to a loading of 4 g/l. A countercurrent extraction system operating at an aqueous-to-organic phase flow ratio of 2:1 should be capable of lowering the iron concentration from 1.5 g/l to near zero in two stages using this extractant mixture as illustrated (fig. 1) by the McCabe-Thiele plot (2).
Single-stage studies demonstrated that decanol additions and elevated temperatures were required to obtain adequate phase separations. Therefore, equilibrium diagrams were developed at 50° C using 10 vol-pct amine-5 vol-pct decanol (10:5 extractant mixture) and 20 vol-pct amine-10 vol-pct decanol (20:10 extractant mixture) in kerosine. These modifications lower the iron loading to 2.5 g/l when the 10:5 extractant mixture is used (fig. 2); however, loadings of 5.2 g/l were obtained (with the 20:10 mixture (fig. 3). Iron
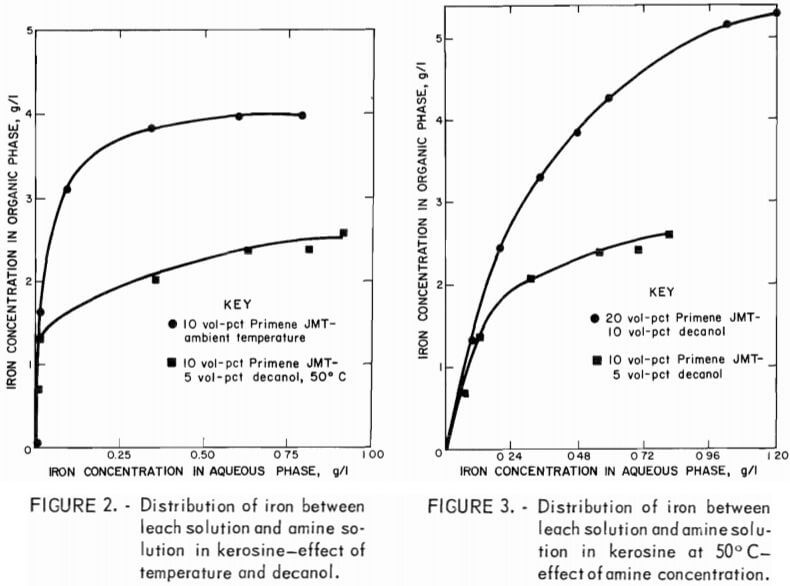
extraction was not as efficient as shown in figure 1; however, adequate iron separation can be accomplished in a reasonable number of stages when employing a multistage countercurrent system.
Determining Stripping Conditions
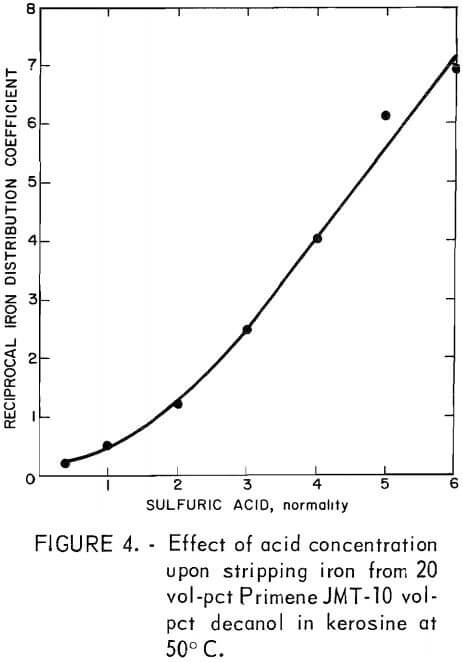 Methods for stripping iron from the amine solution were studied to devise techniques for regenerating the extractant for recycle. Using sulfuric acid in the system would prevent the introduction of new ionic species in the system. Therefore, the equilibrium concentration of iron in the organic and aqueous phases was determined at various sulfuric acid strengths. The ratio of the concentration of iron in the aqueous phase to the concentration in the organic phase (1/Ko) was plotted versus acid strength. Figure 4 shows that this dependence is nearly linear above 2N acid.
Methods for stripping iron from the amine solution were studied to devise techniques for regenerating the extractant for recycle. Using sulfuric acid in the system would prevent the introduction of new ionic species in the system. Therefore, the equilibrium concentration of iron in the organic and aqueous phases was determined at various sulfuric acid strengths. The ratio of the concentration of iron in the aqueous phase to the concentration in the organic phase (1/Ko) was plotted versus acid strength. Figure 4 shows that this dependence is nearly linear above 2N acid.
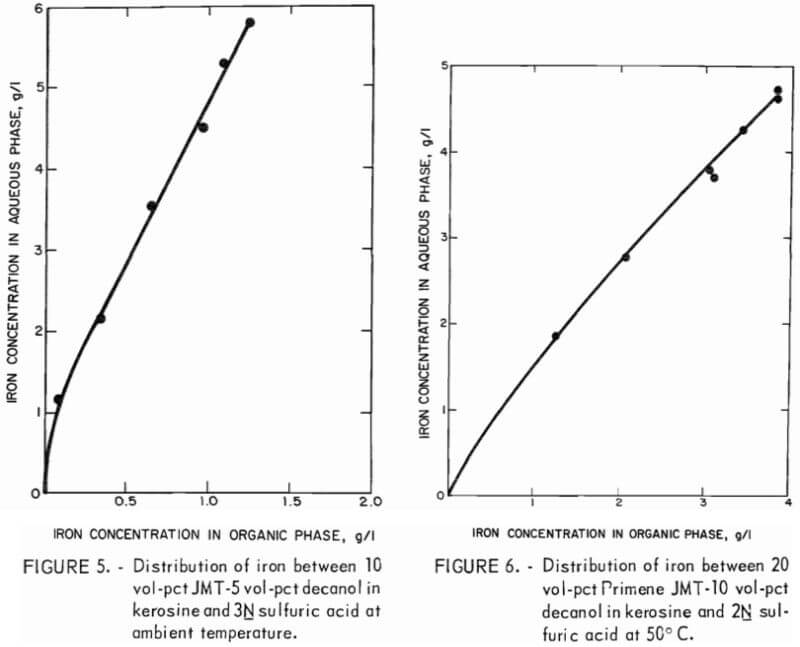
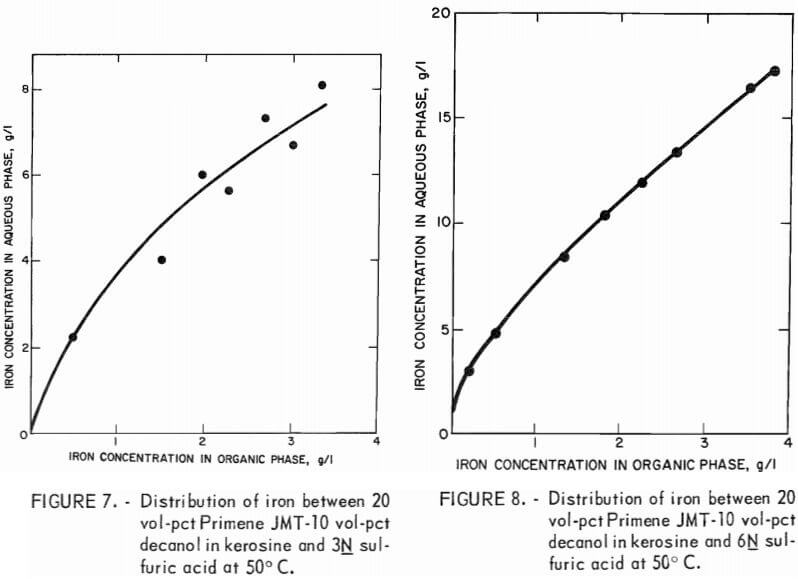
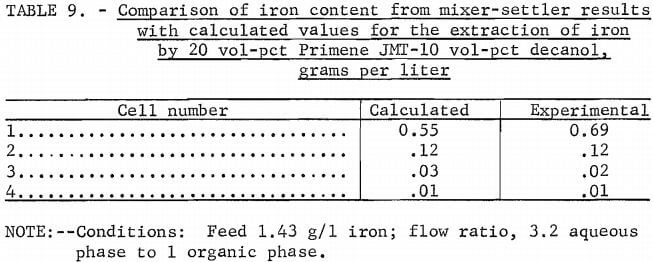
A series of equilibrium diagrams was developed for this system of stripping iron from a primary amine solution at various sulfuric acid concentrations. Figure 5 is an equilibrium curve for stripping iron from a 10 vol-pct amine-5 vol-pct decanol in kerosine solution using 3N sulfuric acid at ambient temperature. The equilibrium curve shows that iron favors the aqueous-acid phase and that the amine solution could be regenerated at an aqueous-to-organic phase ratio as low as 1:3. This would yield a waste stream containing less than 10 g/l iron. Since it is generally desirable to obtain high metal concentrations in waste streams, a series of equilibrium diagrams was developed for a 20:10 extractant mixture at 50° C versus varying strengths of sulfuric acid.
The equilibrium curve for 2N acid stripping is nearly linear with a slope of approximately 1.0 (fig. 6). This acid concentration would limit the choice of phase ratio in the stripping circuit so that an iron-waste stream having a concentration only slightly greater than the iron loading of the amine solution could be obtained. A 3N or 6N acid concentration would allow the use of higher ratios of organic-to-aqueous phases in the stripping circuit and, therefore, yield higher concentrations of iron in the waste stream (fig. 7-8). Iron can be effectively removed to a low level in the recycled organic solution by any of these acid solutions.
Multistage Studies
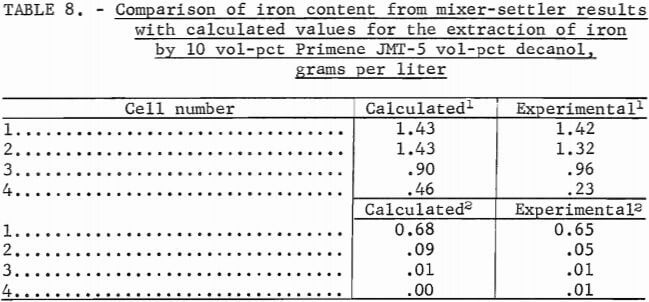
Results of multistage experiments were compared with the data plotted on the equilibrium diagrams (figs. 1-3) as follows: An operating line was incorporated with a slope equal to the flow ratio employed and steps were drawn from the operating line to the equilibrium curve as illustrated in figure 1. Values calculated for the extraction of iron by a 10:5 extractant mixture at two different flow ratios were compared with the analysis of the aqueous phase of each cell after an actual run in the mixer-settler (table 8).
A similar comparison was conducted using a 20:10 extractant mixture. The results of this experiment were compared in the same manner as the preceding results (table 9).
The close agreement of the calculated values with experimental results shows that equilibrium diagrams may be used to predict the number of stages required to remove iron from an aluminum sulfate leach solution starting with a known concentration of iron and a chosen flow ratio.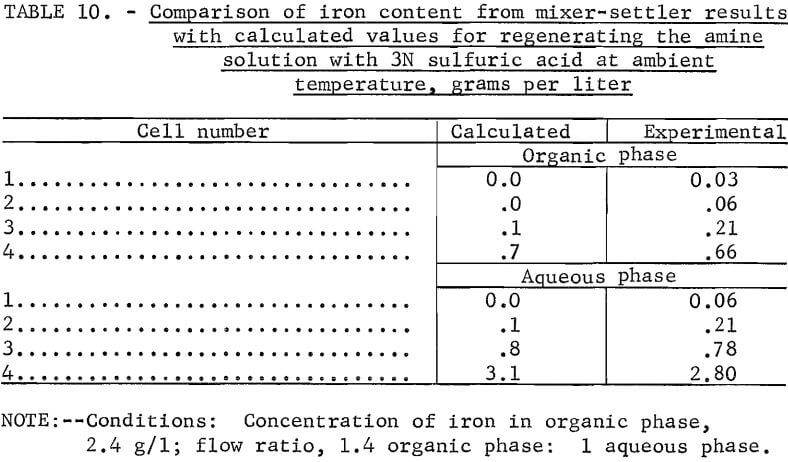
A subsequent set of experiments was conducted to study stripping of iron from amine solutions under multistage conditions. An iron-loaded solution of 10 vol-pct amine-5 vol-pct decanol in kerosine was contacted with 3N sulfuric acid at ambient temperature. The iron concentration in each phase of each settler is shown in table 10.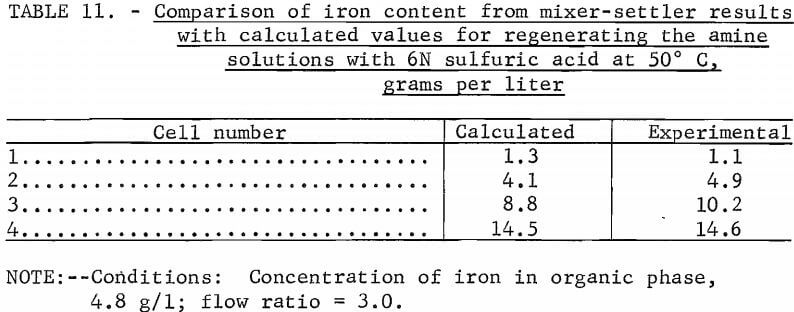
As in the case for extraction, there was close agreement between values calculated from equilibrium diagrams and experimental results. The calculated values predict that the iron concentration in the organic phase will be 0.0 after three stages. The experimental data show that although the iron concentration is very low (0.06 g/l) after three cells, a small amount of iron would be recycled. This recycle load could be diminished by adding a fourth regeneration cell if desired.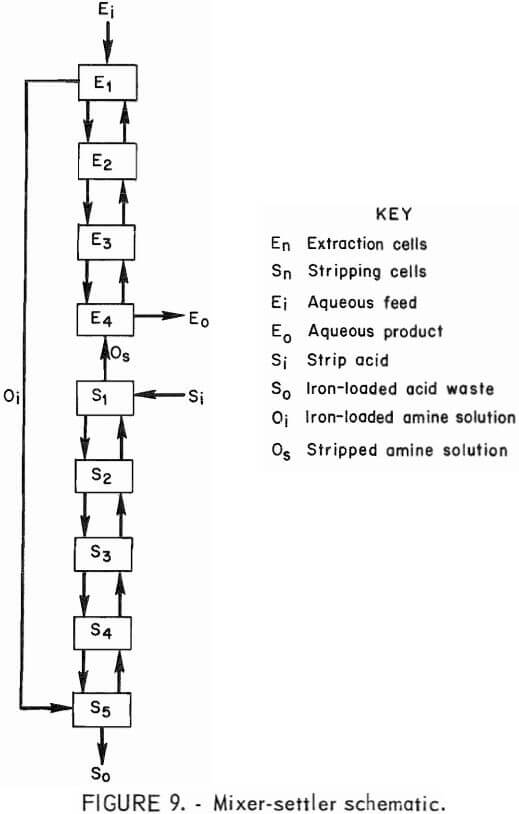
Another stripping experiment was conducted using an iron-loaded solution of 20:10 extractant mixture versus 6N sulfuric acid at 50° C. The aqueous phase of each settler was analyzed for iron after reaching equilibrium. These values were then compared with values calculated from the equilibrium diagram (table 11).
The calculated and experimental values were found to be in close agreement. The iron concentration in the regenerated amine solution was determined, by analysis, to be 0.1 g/l. As will be demonstrated in later experiments (fig. 10, cell number S1), this level of recycled iron will cause no difficulty in obtaining an aqueous product containing less than 0.01 g/l iron.
To verify the conclusions drawn from operating the extraction and stripping circuits as independent units, experiments were conducted in which the amine solution was run directly from the extraction circuit to the stripping circuit and recycled back to the extraction circuit. This should approximate the operation of an integrated system and yield information on the recycle characteristics of the amine solution.
In the first experiment, the mixer-settler was operated for 8 hours at 45° to 50° C. Four cells were used for extracting iron from the stock-leach solution by a 20:10 extractant mixture; five cells were used for regenerating the amine solution with 3N sulfuric acid. Samples taken after 5, 6.5, and 8 hours of operation showed little change in the composition of the exit streams or in the iron concentration of the recycled amine solution. The unit was operated at an aqueous-to-organic phase flow ratio of 3.2:1 in the extraction circuit and an organic-to-aqueous phase flow ratio of 1.5:1 in the stripping circuit. Each phase of each settler was analyzed at the conclusion of the run. A flow scheme for the mixer-settler operation is represented in figure 9.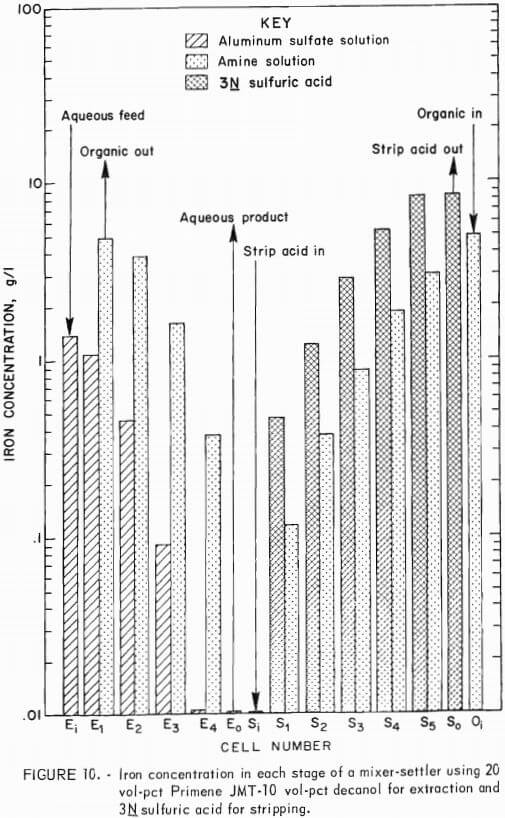
Figure 10 shows that the purified aluminum sulfate solution contained 0.01 g/l iron (cell number) and that the recycled amine solution contained 0.12 g/l iron (cell number S1) . These values are very close to those predicted from earlier results (tables 9-10). Since no change was detected between 5 and 8 hours of operation, the recycle characteristics of the amine solution appeared favorable for continuous operation. Analysis of aluminum in these solutions showed 0.92 g/l present in the organic phase after the first extraction stage decreasing to less than 0.01 g/l after the fourth stage and 0.08 g/l aluminum reporting to the iron-waste stream. Since the iron- waste stream is about one-fifth the volume of the aluminum sulfate stream, this level of aluminum amounts to 0.03 pct of the aluminum fed into the system.
The amount of soluble organic material present in the aqueous exit streams was determined from this test by collecting the organic material by filtration through activated charcoal. Organic material was recovered by leaching the charcoal with acetone and then evaporating the acetone. Of the 1.2 pounds of organic material recovered per ton of contained alumina, 1.1 pounds was in the purified aluminum sulfate solution.
Another experiment was conducted using 6N sulfuric acid in place of the 3N acid for stripping the amine solution. This operation was conducted continuously for 14 hours. The aqueous-to-organic phase ratio was maintained at 3.2:1 for the extraction circuit, but the phase ratio was increased to 3 organic phase to 1 aqueous phase in the stripping circuit. The number of stripping stages was reduced to four. After 7 hours of operation, the purified aluminum sulfate solution contained 0.019 g/l iron, and the acid-waste stream contained 15.5 g/l iron. After 11 hours these solutions contained 0.013 g/l iron and 15.4 g/l, respectively. Each phase of each settler was analyzed at the end of the run (fig. 11).
The purified aluminum sulfate solution contained 0.01 g/l iron, and the recycled amine solution contained 0.05 g/l iron. There was no detectable decrease in extraction or stripping efficiency over a period of 14 hours of operation, which corresponds to nine total exchanges of the amine solution. The concentration of aluminum in the amine solution showed the same trend as in the previous experiment with 0.29 g/l aluminum reporting to the acid-waste stream. This corresponds to 0.06 pct of the aluminum fed into the system.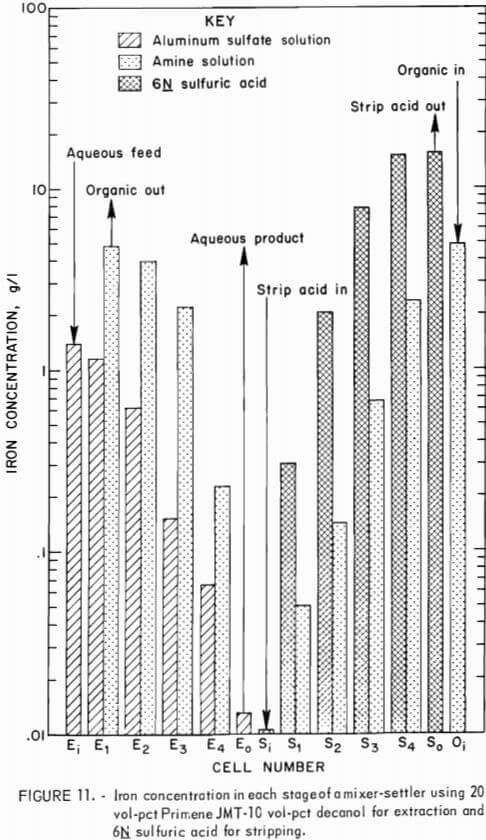
The organic material in the aqueous stream was determined as before and found to total 1.6 lb/ton of alumina of which 1.1 pounds was in the aluminum sulfate solution and 0.5 pound was in the acid-waste stream.
Both of the above integrated solvent-extraction systems yield an aluminum sulfate solution from which alumina containing less than 0.01 pct iron may be prepared. The system containing 3N sulfuric acid requires an extra stripping stage and results in a slightly higher recycle of iron in the amine solution; however, the losses of aluminum to the acid-waste stream are slightly smaller as is the soluble loss of the amine solution. These differences in the systems would have to be evaluated with respect to other unit operations in an overall process to determine which system would be best suited to a particular use.
Determining Alumina Purity
A portion of purified aluminum sulfate solution was evaporated to obtain crystals of aluminum sulfate, which were calcined to produce alumina. The alumina was analyzed for impurities, and these analyses were compared with target values for alumina (table 12).
Table 12 shows that many of the values are close to or less than the target requirements. For example, the values obtained for iron are below those acceptable to private industry; other impurities such as chromium and magnesium do not meet the specifications. The level of these impurities might be lowered by a carefully controlled fractional crystallization or by recovering the aluminum as aluminum chloride by sparging with hydrochloric acid. These possibilities were not investigated in this study.
Conclusions
This investigation has demonstrated that iron may be removed from an aluminum sulfate solution by continuous countercurrent extraction with a primary amine, The amine solution may be regenerated with no loss in extraction efficiency. However, some precautions must be taken to minimize entrainment losses of the amine solution in the aqueous-product streams.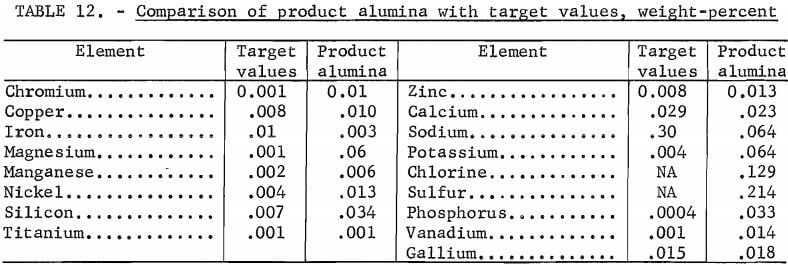
Recovering alumina from domestic resources, methods for extracting iron from aluminum sulfate solutions (obtained by leaching kaolinitic clay with dilute sulfuric acid) were investigated. In countercurrent extraction experiments at a 1-gal/hr flow rate of aluminum sulfate leach liquor, the iron concentration was decreased from 1.4 g/l to 0.01 g/l after four stages of using a primary amine extractant. The iron-loaded amine solution was regenerated in five stages using 3N sulfuric acid or in four stages using 6N sulfuric acid. The aluminum sulfate solution obtained was suitable for the preparation of alumina meeting cell-grade standards of less than 0.01 pct iron.
