Table of Contents
For the past few years there has been increasing interest among respirator users in determining quantitatively the degree of protection afforded by protective respiratory devices. Overall protection depends on both the efficiency of filters and/or sorbents used and the tightness of seal around the edges of the facepiece. The Bureau of Mines first introduced quantitative leakage tests of complete respirators in its approval schedules in 1965, with the publication of Schedule 21B; other methods were added to the schedule amended in 1969.
A means to study the effect of face fit on leakage is particularly important for nonemergency respirators (designed to be worn in atmospheres not immediately hazardous to life or health) such as chemical-cartridge or particulate-matter respirators. These respirators often are worn in a loose, comfortable manner Some workers realize the atmospheres of exposure are not immediately hazardous and, if there is no objectionable odor or irritation, do not adjust the facepiece very tightly. Wearers of emergency respirators designed for higher, more noxious concentrations of contaminants will endure more discomfort to make sure leakage does not occur.
The ability of filters, cartridges, and canisters to remove contaminants from air is determined readily by machine tests, but face fit can be evaluated only during tests in which the device is actually worn by people. Various systems have been used to evaluate face fit, to train prospective users, and to aid workers m selecting respirators suited to them. All are similar in that the concentration of contaminant within the facepiece is measured while the respirator is being worn in a controlled, known concentration of contaminant. Results are expressed as percent leakage (penetration, into the facepiece, or as “protection factor,” which is defined as the ratio of the test or “challenge” concentration to that within the facepiece.
Test media that have been used are dioctyl phthalate (DOP) aerosols, uranine dye aerosols, sodium chloride aerosols, and Freon vapor-air mixtures thus variously including liquid, solid , and vapor dispersions in air.
This publication describes a quantitative facepiece leakage test for nonemergency respirators. Based mainly on work of Adley and others, a procedure with dichlorodifluoromethane (CCl2F2) has been designed for studying the face fit of chemical cartridge respirators. Wearing ease has been considered of major importance; hence the procedure described is conducted while the respirator is worn in a comfortable manner. Other quantitative face fit tests for emergency respirators are being developed for use in the Bureau of Mines approval program.
Description of Method
The test is conducted while the subject wearing the respirator performs a prescribed series of exercises in a metal-glass chamber containing approximately 1,000 ppm of CCl2F2. Throughout the test, a sample is drawn from within the facepiece (1.72 liters/minute), split into two streams, and conducted to an analyzer and to a plastic storage bag outside the chamber. The analyzer provides a continuous record of CCl2F2 concentration in the facepiece during the test. The CCl2F2 concentration in the plastic bag gives a measure of total leakage. An apparatus arrangement is shown schematically in figure 1.
The respirator is prepared for test by inserting a bulkhead fitting or a large-bore needle as a probe, through the facepiece wall. Cartridges—single or pair, as required are filled with high-activity charcoal to remove CCl2F2 from the inspired air. Following each test the cartridges are retested to make sure the efficiency of removal of CCl2F2 was not impaired during the test and that leakage through the sorbent into the facepiece had not occurred,
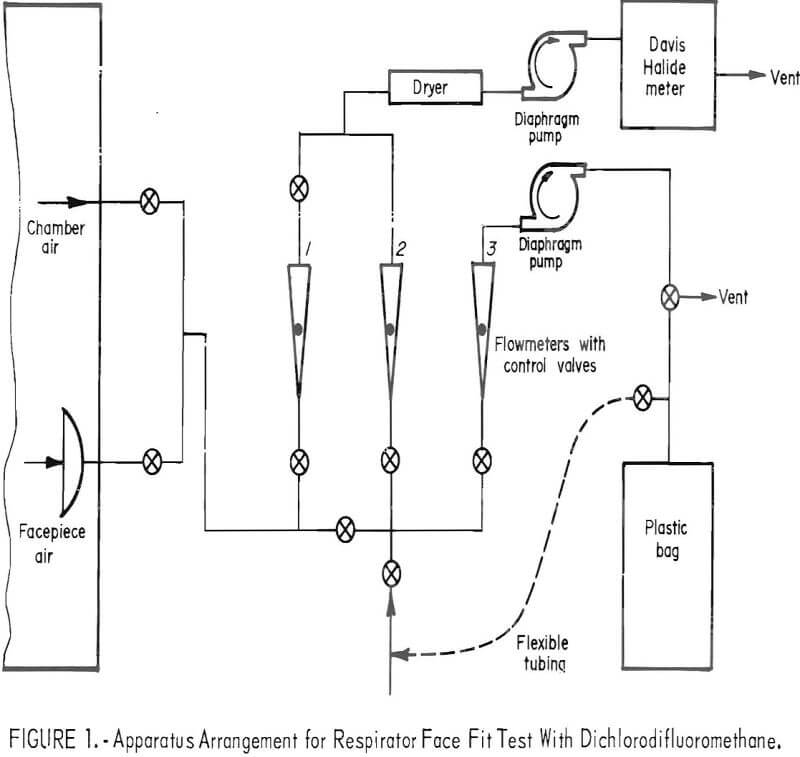
A concentration-response curve is established daily for the analyzer. Dichlorodifluoromethane is added in increments to form 200, 400, and finally, the 1,000 ppm challenge mixture. Response to the first two mixtures is measured directly. Flowmeters 1 and 2 (fig. 1) are utilized to dilute the 1,000-ppm concentration to 23, 50, 87, and 137 ppm. The resulting six-point curve is used to evaluate the test data. Figure 2 shows the apparatus for adding CCl2F2 to the test chamber.
The analyzer is a modified Davis Halide meter. Halogenated vapors (for example, CCl2F2) are pumped through a gas cell containing two electrodes between which an electric spark is provided to enhance the normal nitrogen band spectra by a mechanism that is not fully understood. The enhanced intensity can be related to the concentration of CCl2F2. Light energy from the spark source passes through an ultraviolet-transmitting glass filter to a blue-sensitive phototube. The electrical signal from the phototube is amplified and recorded graphically on a strip-chart recorder. The recorder tracings may be integrated, if desired, for comparison with total leakage values obtained from analysis of the composite bag samples. Two ppm of CCl2F2 is readily detectable; for a 1,000-ppm challenge mixture this represents a leakage of 0.2 percent. Smaller leakages can be determined by increasing the challenge concentration.

Several changes were made to improve the sensitivity and stability of the Halide meter. As recommended by Nelson, an improved ultraviolet-transmitting filter (Corning No. 7-54) was installed. The copper electrode was replaced with 0.32-inch-diameter platinum wire to improve stability and minimize maintenance. The flowrate was reduced to 0.86 liter per minute. A switch was installed to permit the use of either panel meter or strip-chart recorder. Particular improvement in electrical stability resulted with electrode holders redesigned to improve electrical contact and facilitate adjustment or removal for cleaning.
Before entering the chamber the subject dons the respirator, adjusts it in a fashion that is comfortable to him, and makes the tightness test positive pressure or suction recommended by the manufacturer. A pressure tightness test is made by blocking the exhalation valve, exhaling to develop pressure in the facepiece, and noting whether outward leakage around edges can be detected. A suction test is made by blocking the inlet openings to the cartridges, inhaling, and noting whether the facepiece remains collapsed against the face. In these tests the subject is instructed to adjust the respirator straps just tight enough to satisfy the tightness test and not so tight as to bring undue pressure on the nose and other parts of the face. The subject then enters the chamber and performs the following activities :
- 3 minutes : Nodding and turning head, coughing.
- 1-½ minutes: Smiling.
- 1-½ minutes: Frowning.
- 3 minutes: Reciting alphabet aloud.
- 3 minutes: Talking aloud.
- 3 minutes : Deep and shallow breathing.
- 3 minutes: Pumping air with hand pump into a 1-cubic foot cylinder
Figure 3 shows subject in chamber and wearing respirator fitted for test. Leakage into the mask is followed throughout on the recorder while a sample from the facepiece is pumped into a plastic bag for later analysis. The overall average leakage is calculated,
Leakage (percent) = Concentration in bag/Challenge concentration x 100
Leakage for a particular activity period during test is determined by relating the area under the curve recorded during that period to a standard area determined from the calibration concentration-response curve.
Measurements
Leakages into six respirators were measured; five of the respirators were Bureau of Mines approved and one was nonapproved. Three subjects of medium stature and similar facial characteristics wore each respirator during duplicate tests. The results of each pair of tests are shown in table 1. The reported average leakages were calculated from the composite air samples. Corresponding leakages based on integration of recorder tracings agreed satisfactorily but data reduction was tedious and time consuming.
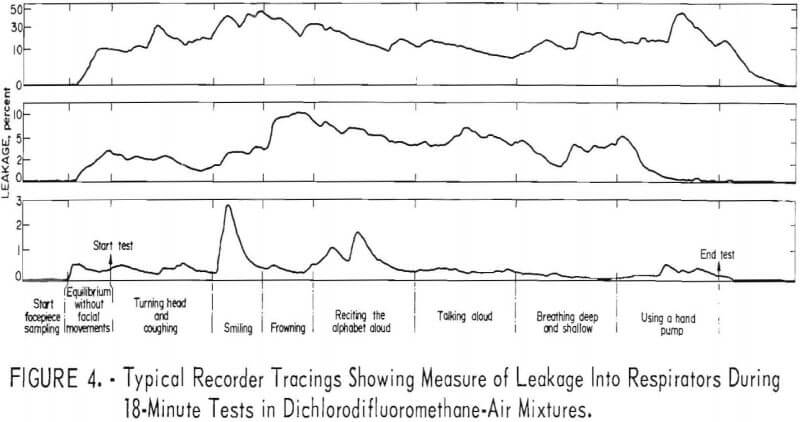
Three recorder tracings (fig. 4) typify the relation between leakage and physical activity

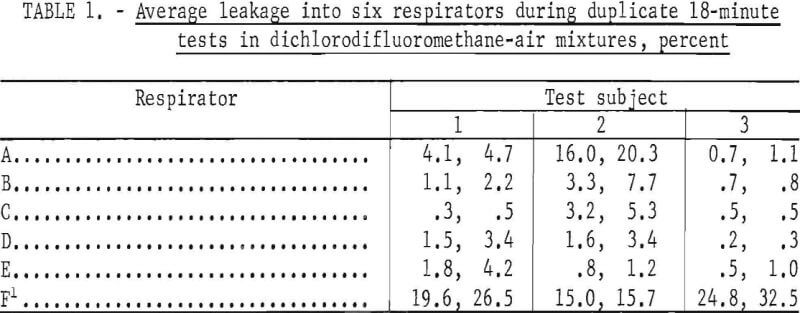
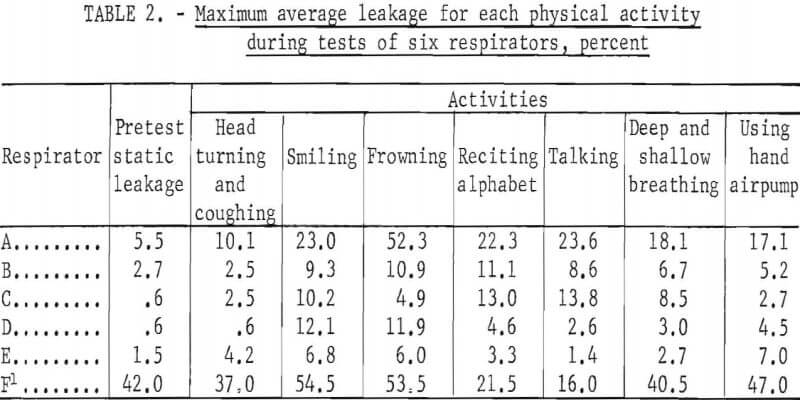
Table 2 shows the maximum average leakage for each activity and respirator during all tests of the series. Shown also is the maximum “static” concentration; that is, when the subject was standing motionless before starting the schedule of timed physical activities.
Possible loss of CCl2F2 by lung absorption was studied briefly. Tests in a 100-ppm challenge concentration and without charcoal in the cartridges indicated a loss of less than 3 percent. This would be equivalent to 0.3-percent leakage in a test with a 1,000-ppm challenge mixture.
Discussion
This procedure is designed for testing nonemergency respirators with half-mask facepieces worn in a manner simulating that observed in industrial usage. Minimal tension on facepiece straps is used to hold the facepiece just tight enough to satisfy the simple, commonly used, pressure or suction check of tightness. The test serves two purposes to determine quantitatively the overall leakage, and to observe the combined effects of facepiece design, face shape, and facial movements on leakage. The data presented are not intended to critically compare the various respirators and subjects but to illustrate the kind of information the test affords. They reveal rather large leakages that many may find surprisingly high, particularly for approved respirators. These respirators already have satisfactorily passed other tightness tests under Bureau of Mines Schedule 23B, in which they were worn by several different subjects but were adjusted more carefully to obtain a tight face seal. Consequently, the leakages measured in the tests described in this paper more nearly represent those to be expected in practical usage than those in usual laboratory tests.
Of 30 tests of approved respirators, 2.6 overall average leakages were less than 5 percent for the 18~minute test periods (table 1). Leakage for one respirator and one subject was about 20 percent. The variations between duplicate test values indicate that each person may adjust the facepiece in a slightly different fashion each time he dons it.
The order of magnitude of leakage caused by facial movements is shown in table 2. Maximum values only are shown for each respirator. Although these are quite variable and of short duration, they illustrate that frequent or extreme facial movements may significantly increase leakage into halfmask respirators.
Similarly, the “pretest static” measurements showed leakage that resulted from the initial adjustment of the facepiece when donned and not from facial movements or physical activity. Careful initial adjustment when donning a respirator is essential to minimize leakage.
Conclusion
The described method is adequate for quantitatively measuring leakage of vapor into chemical-cartridge, halfmask-type facepieces. Complementing other tests of tightness, it is useful for measuring overall leakage and for studying the effect of facial movements on leakage.
