Table of Contents
The behavior of collectors at the mineral-solution interfaces is usually explained in terms of an ionic adsorption process. Through the distribution of collector ions between the solid surface and the co-existing solution phase the mineral is believed to acquire a water-repellent surface coating.
Quartz
A —100 mesh ground crystalline quartz was infrasized; the products of the third and fourth cones were mixed together and reserved for experimental purposes. This stock material was cleaned by leaching in boiling concentrated HCl.
Collector
The distribution of dodecylammonium acetate between the quartz surface and the solution phase was determined by the radiotracer method of analysis with carbon 14 as the tracer element. The radioactive amine salt with C synthesized into the hydrocarbon chain was supplied by Armour and Co.
Adsorption Tests
Two different experimental methods were used. In the first, to be designated as the agitation method, a weighed amount of quartz and a measured volume of amine salt solution were agitated in a 100-ml or 50-ml glass-stoppered pyrex graduated cylinder.
Vacuum Flotation Tests
The vacuum flotation technique used by Schuhmann and Prakash in the study of the quartz-barium chloride-oleic acid system was adopted for the determination of the critical pH curves for quartz using different hydrocarbon chain-length amines.
Solubility of Dodecylamine
When alkaline solutions are dealt with it is essential that the solubility of dodecylamine be known. Adsorption densities cannot be determined when dodecylamine is precipitating because in experimentation no distinction can be made between adsorbed amine and precipitated amine.
The light scattering method involved the observation of Tyndall cone formation in aqueous solutions of known amine salt concentration and alkaline pH. In the adsorption measurements the clear equilibrium solution was radioanalyzed for amine content when a precipitate was observed. In the agitation test, 67 ml of a solution which initially contained 1.95×10 -4 mols per liter of radioactive dodecylammonium acetate were contacted with 8.55 g of quartz.
The values obtained by potentiometric titrations were determined by Brown. This method consisted of a potentiometric titration of an aqueous dodecyl-ammonium chloride solution with NaOH and with a
platinum black hydrogen electrode. Table I shows that the solubility values obtained by the various methods are in good agreement. A value of 2×10 -5 mols per liter shall be assumed in this paper.
Adsorption Isotherm
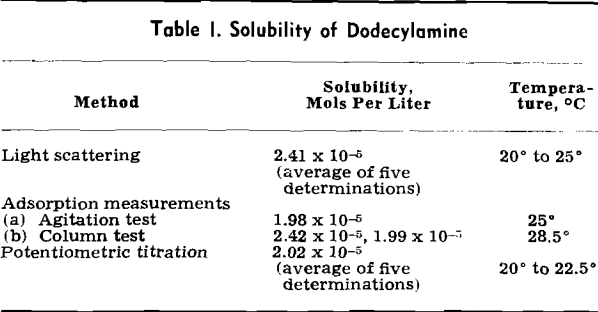
Results of the investigation of the adsorption density of dodecylamine on quartz for a ten thousandfold range of solution concentration and neutral pH are shown in Fig. 1. In this figure the solid circles represent the data determined by the agitation method and the open circles those obtained by the column method. The graph clearly illustrates that the relation between Γ (adsorption density) and c (equilibrium concentration of amine salt) is independent of the particular experimental procedure that was used.
Under these conditions of solution concentration, the experimental adsorption isotherm can be described by the empirical relation
Γ = 8.1 x 10 -9 √c………………………………………………………..[1]
where Γ and c have the dimensions of mols per cm² and mols per liter, respectively.
Beyond a concentration of 2×10 -4 mols per liter, the surface concentration increases more rapidly with collector concentration. The following empirical equation describes this branch of the isotherm:
Γ = 2.2×10 -6 c 1.16………………………………………………………[2]
The highest concentration of amine salt used in this investigation (4.08×10-³ mols per liter) is still below the critical concentration of micelle formation (1.3×10-² mols per liter).
The percentage surface coverage may be obtained from the adsorption densities by assuming a value of 23.4 A² for the area per available surface site.
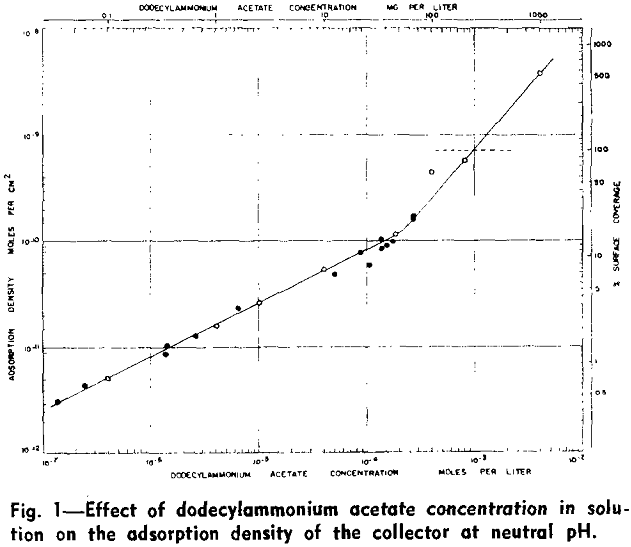
Effect of pH on the Adsorption Density of the Collector
The variation of collector adsorption density with pH for five different solution concentrations (8.16×10 -4, 1.85×10 -4, 4.08×10 -5, 4.08×10 -6, and 4.08×10 -7 mols per liter) is represented graphically. The effect of alkaline pH on the adsorption density has been investigated only at two collector salt concentrations, 4.08×10 -5 and 4.08×10 -6 mols per liter, since experimentation in the alkaline pH range is complicated by precipitation of undissociated amine.
An alkaline pH appears to have a more pronounced effect on the adsorption density of the collector. The amount adsorbed is seen to increase more rapidly as a function of hydroxyl ion concentration in basic than acid solutions, especially for a collector salt concentration of 4.08×10 -5 mols per liter.
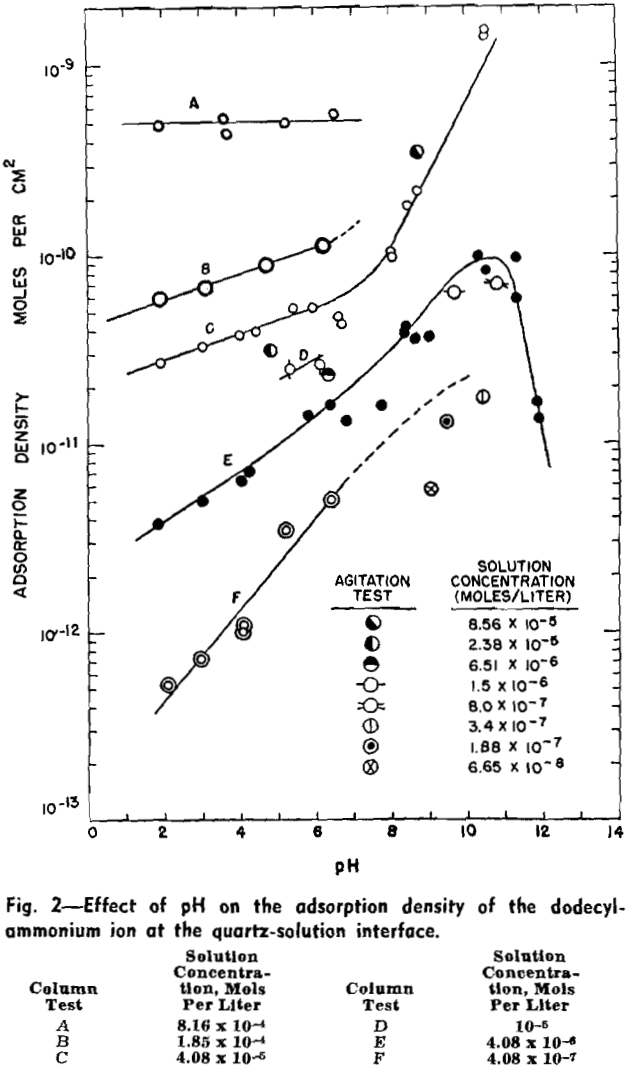
Critical pH Curves
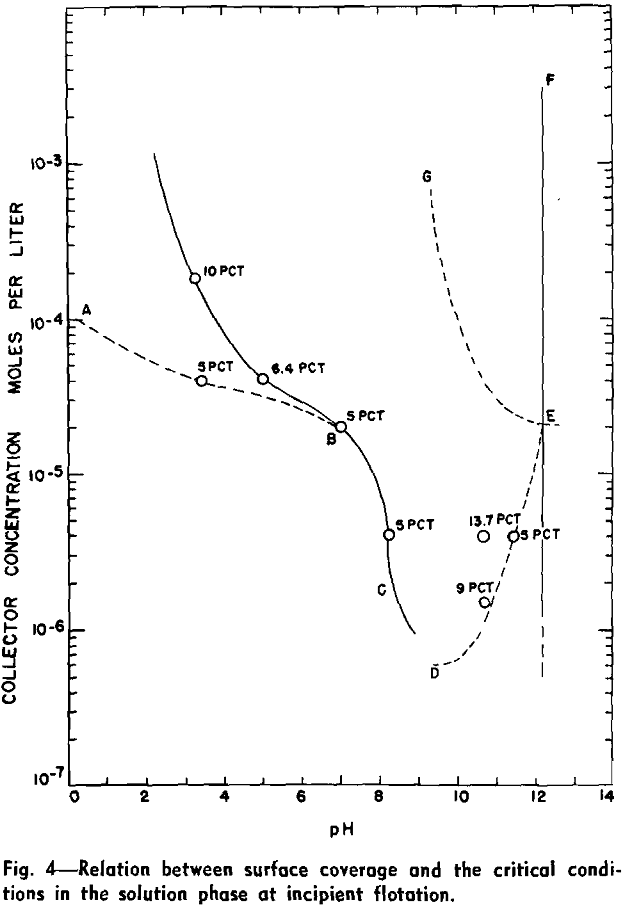
Flotation occurs under conditions above the curve for the specific collector, towards the left of the vertical line at pH 12.2 and towards the right of the dotted line at pH 1.4. Regardless of the specific collector and its concentration, it will be noted that the transition of quartz from a flotative to a nonflotative condition is obtained at substantially the same upper critical pH of 12.2. With decreasing pH of the pulp the lower critical pH is lowered with increasing collector concentration and is also dependent on the hydrocarbon chain-length of the collector.
Correlation of Adsorption Data with Flotation Conditions
Adsorption results may be used to supply quantitative information on the extent of the collector coating at the critical pH. In the past only the critical conditions within the solution phase which determine the floatability of the mineral could be determined. It should be realized, however, that the flotation system is characterized by a three-phase contact. The adsorption data obtained in this investigation refer only to the solid-liquid interface. Complete analysis of the flotation process will require, in addition to these data, a knowledge of conditions at the solid-gas interface.
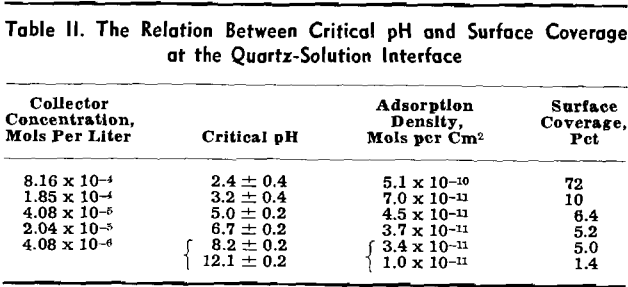
Too much significance should not be attached to the actual numerical values for collector concentration, adsorption density, or surface coverage and critical pH as quoted in this analysis. The order of magnitude of these values, however, should be noted. Gaudin and Chang found that barium-activated quartz could be floated by sodium laurate at a surface coverage of 4.5 pct as compared to the proposed 5 pct coverage of this mineral by dodecylammonium ions.
A Physical Model of the Quartz-Solution Interface
The above analysis of the experimental results has shown that it is possible to correlate adsorption data with the flotation behavior of quartz. However, this correlation is still only empirical in nature; a causal relation is lacking. In view of the close relationship between colloidal phenomena and flotation processes, the application of the well-known electrical double layer model to this system might be a step in the desired direction.

