Table of Contents
Silver is an economically important component of many complex, massive sulfide ores, and often is produced as a byproduct during recovery of base metals from sulfide deposits. About two-thirds of the world’s silver resources are contained in base-metal sulfide deposits (Reese, 1991). These ores are beneficiated almost exclusively by flotation. Silver recovery during flotation, however, often is low, especially in the complex, fine-grained ores being developed in Alaska.
Despite the economic importance of silver, little is known about the flotation behavior of silver-bearing minerals. Mineralogic examinations of several silver-bearing ores indicated that the silver occurs in several mineral forms. To provide a sufficiently narrow focus for this research, two silver-bearing mineral samples were chosen for this study. A lead-zinc ore from Alaska and silver-bearing tetrahedrite (nominally Cu11.3 Fe1.4 Sb4, S13) were chosen as representative samples for the flotation study.
The focus of this research was to compile fundamental information on the flotation of silver-bearing minerals. The data reported in this paper represent a preliminary effort to understand the fundamental flotation behavior of silver minerals. Tests were conducted in a microcell flotation apparatus to observe trends in flotation response.
Electrochemical techniques were used to elucidate the fundamental phenomena occurring in the flotation process.
Several tests were conducted in sodium sulfate solution in conjuntion with the usual sodium tetraborate electrolyte to improve understanding of flotation in conventional processing mills. Xanthate consumption also was measured using ultraviolet spectrophotometry to better understand the interaction between the collector and the mineral surface.
Experimental
Mineral Samples
Specimen-grade tetrahedrite was obtained from Ward’s Natural Science Establishment, Inc. In addition, a complex, silver-bearing lead-zinc ore from a flotation mill in Alaska was obtained for testing. The ore had been crushed to -48 mesh. This ore was gravity concentrated on a riffle table before using in this study. Chemical compositions of these materials are reported in table 1.
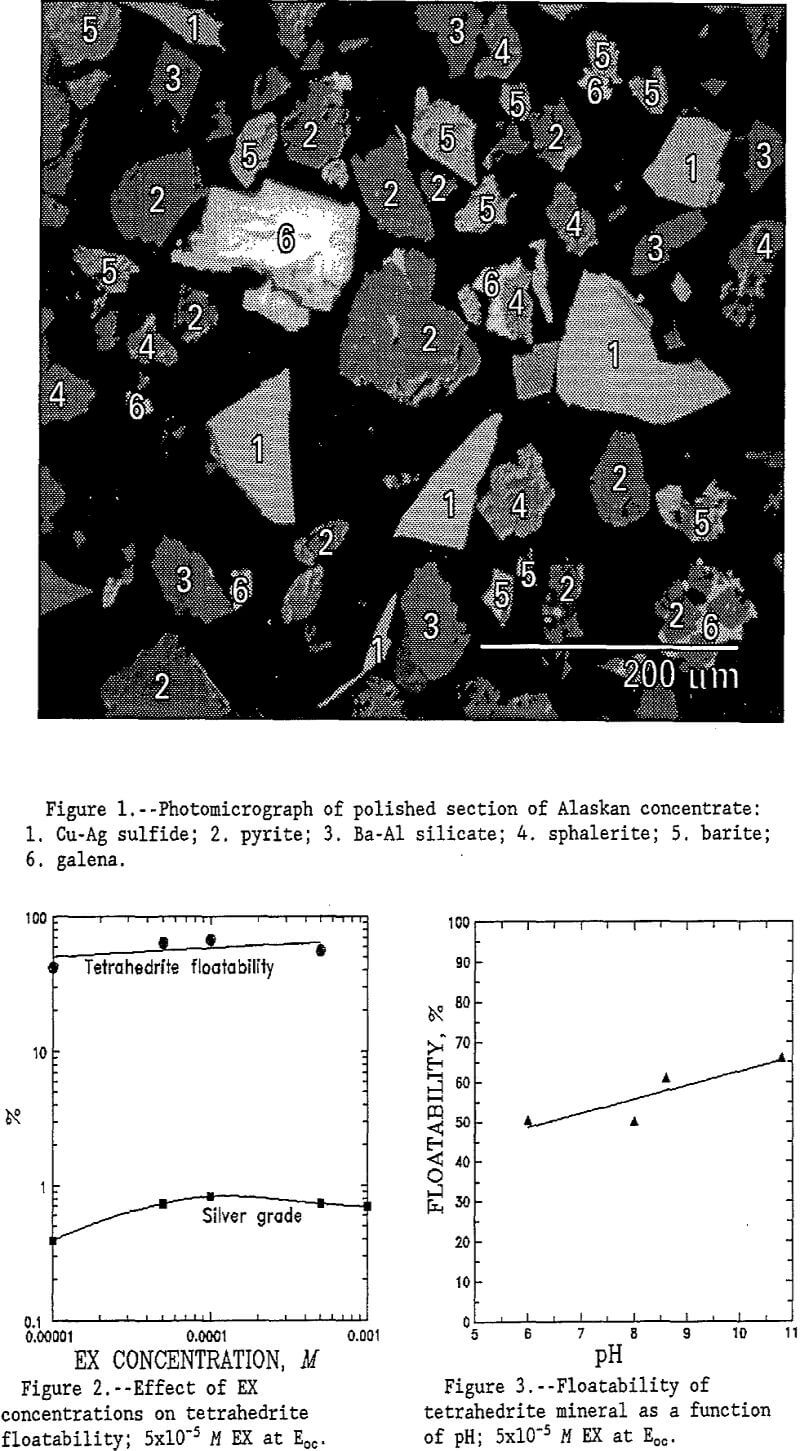
Both samples were examined in the scanning electron microscope (SEM) using energy dispersive x-ray (EDX) spectroscopy to characterize mineralogy. The tetrahedrite sample contained 50% to 60% tetrahedrite, 10% to 15% pyrite, 10% to 15% galena, and 20% to 30% quartz in intergrown grains. The tetrahedrite phase contained no silver; rather, separate phases of copper-silver sulfide and native silver occurred as inclusions within the tetrahedrite grains. Typical grain size of the silver-containing phase was approximately 40 µm; thus, the silver typically is locked within the tetrahedrite.
The Alaskan concentrate obtained from tabling contained 5% to 10% copper-silver sulfide with stoichiometry approximating CuAgS, 20% to 25% pyrite, 10% to 15% galena, 10% to 15% sphalerite, and 40% to 50% quartz and barite. Silver was not found in any other phase. The mineral grains in this sample were well liberated as shown in figure 1.
Reagents
Purified sodium ethyl xanthate (EX) was obtained by dissolving crystalline EX in acetone and crystallizing with ether three times (Harris, 1988). Fresh EX solution was prepared daily to ensure minimum decomposition. Sodium sulfate and sodium tetraborate electrolyte solutions were prepared using 18 MΩ deionized water and reagent-grade salts. The pH of the sodium
sulfate solution was adjusted with reagent-grade sodium hydroxide or sulfuric acid. Sodium tetraborate provides natural buffer of 9.2.
Procedures
Floatabilitv tests:
Floatability and electrochemical measurements were made in a modified Hallimond cell (Gebhardt, 1991). Because these tests were performed with limited agitation and without frothers, the results from Hallimond tests have been termed “floatability” in this publication. The cell had a platinum current collector supporting the mineral bed. The reference electrode connected to the cell through a capillary tube near the particle bed. The counter electrode was constructed of platinum gauze and was separated from the mineral bed with a fritted glass disc.
Floatability and mineral potential measurements were made in a single procedure. The minerals were crushed in a mortar and pestle, and dry sieved to 65×325 mesh immediately before use. The Hallimond cell was filled with 85 mL air-saturated solution sodium tetraborate or sodium sulfate. To this was added 2 g of sized mineral and a predetermined aliquot of EX solution.
Potential in the cell was controlled using a PARC Model 273 potentiostat/galvanostat linked to a computer. The mineral bed was conditioned for 10 minutes and floated with air in the absence of frother for 10 minutes. This flotation time was chosen to maximize the sensitivity of the tests. Floatability and silver recovery were determined on the basis of chemical analyses.
Xanthate Consumption Study:
EX consumption was determined using a UV-visible spectrophotometer (Woods, 1986; O’Dell, 1986; Walter, 1984). A peristaltic pump circulated the solution through the mineral bed in the electrochemical cell and through a flow cell in the spectrophotometer. Absorbance at 302 nm was used to calculate the EX concentration in the solution. EX consumption was calculated as:
Consumption = 100 x (Ci – Cf)/Ci
where Ci = initial EX concentration;
Cf = final EX concentration.
Spectra were measured in borate and sulfate solutions containing 5×10 -5 M EX. Changes in EX concentration were studied as a function of the externally imposed potential, and as a function of pH.
Reversibility of EX adsorption also was investigated spectrophotometrically. Spectra were taken at the open circuit potential (Eoc), -0.155 V, 0.245 V, and 0.445 V using fresh tetrahedrite at each potential; the potential was then reduced to -0.345 V, and the spectrum again measured following 0, 5, 10 and 20 minutes of conditioning.
Results and Discussions
Oxidation potential is known to strongly influence the flotation behavior of sulfide minerals. Distinct differences in flotation response result through changes in the dissolved ions in solution and changes in the flotation gas (Cheng, 1992; Woods, 1984). In an attempt to simulate the slurry chemistry of commercial flotation circuits, flotation was carried out in air-saturated slurries containing sulfate ions. Tests also were conducted in borate solutions (pH 9.2 buffer solution) to aid in interpreting the electrochemical data.
Tetrahedrite Floatability
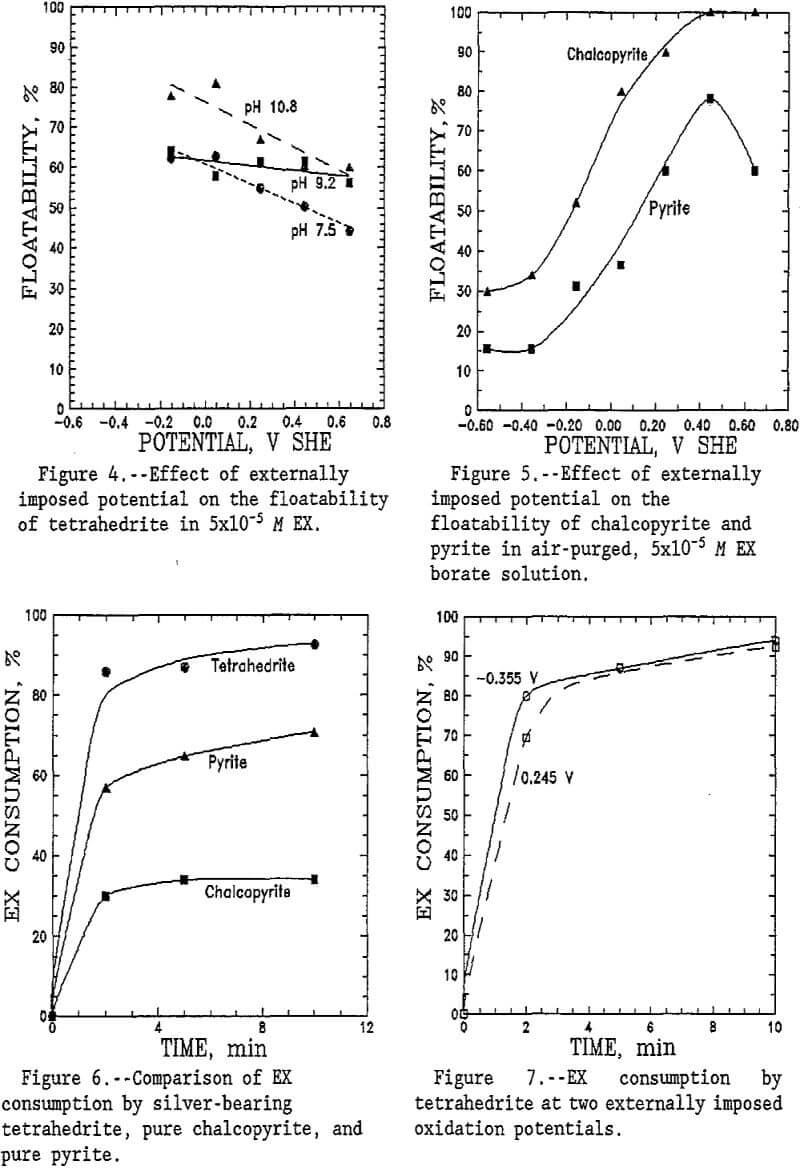
Hallimond cell tests were conducted to determine the floatability of silver-bearing tetrahedrite. Tetrahedrite floatability in borate solution increased with increasing EX concentration, as shown by figure 2. Tetrahedrite floatability reached 67% with 1×10 -4 M EX at pH 9.2 (borate buffer solution). The effect of pH on tetrahedrite floatability in sulfate solutions is shown in figure 3. The highest tetrahedrite recovery in 5×10 -5 M EX was 66% at pH 10.8 (sulfate solution), but the effect of pH was small. Silver grade in the floated product increase slightly to 1×10 -4 M EX, then remained essentially unaffected by increasing collector concentration.
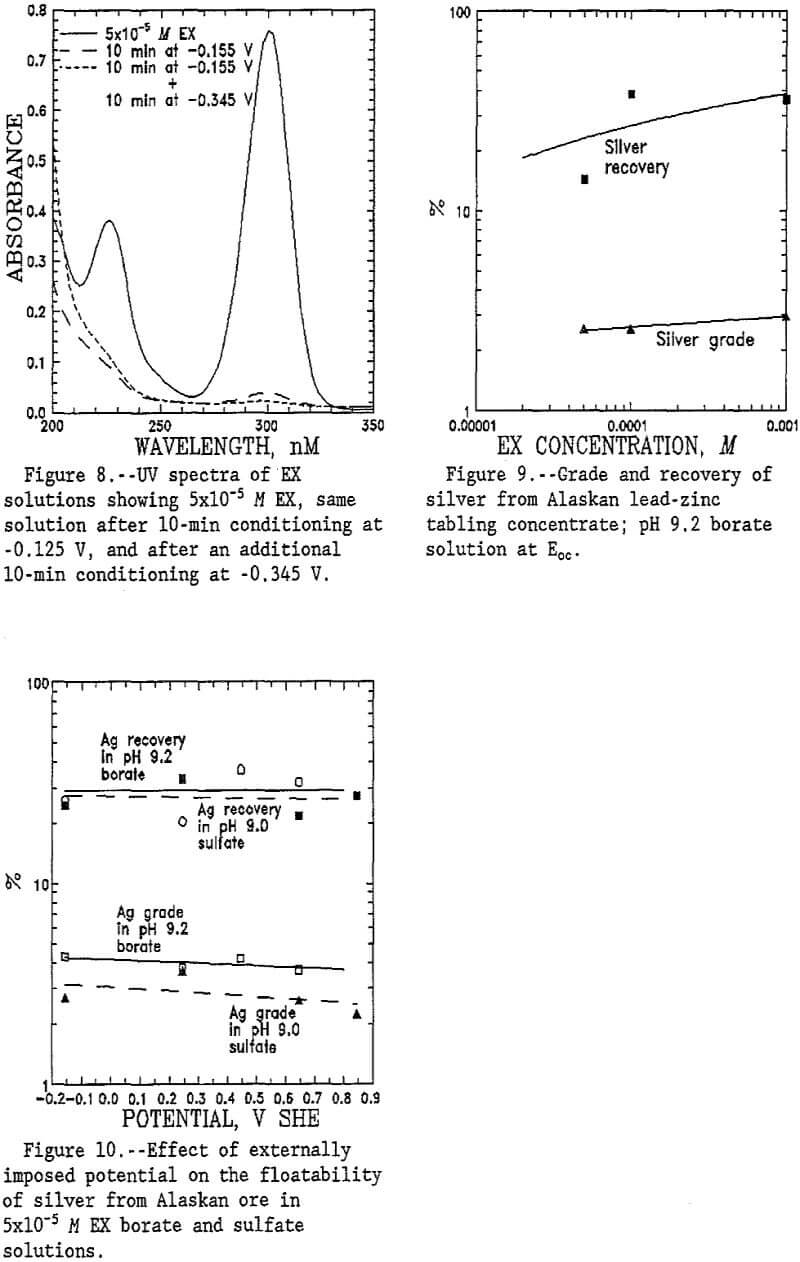
Flotation of sulfide minerals often is strongly tied to the oxidation potential of the mineral in the flotation slurry. The floatability of tetrahedrite is notable in that floatability improves at reducing potentials under potentiostatic control as demonstrated in figure 4. This is significantly different from the floatability of chalcopyrite and pyrite in that the floatability of chalcopyrite and pyrite is strongly potential dependent as shown in figure 5. Floatability of both chalcopyrite and pyrite tends to increase with increasing potential; in contrast, tetrahedrite flotation decreases with increasing potential. Thus, flotation at alkaline pH and reducing potentials may provide the basis for better separation of silver-bearing tetrahedrite from chalcopyrite and pyrite in complex sulfide ores.
EX Consumption
Tetrahedrite is notable in that collector consumption is greater than for either chalcopyrite or pyrite as indicated in figure 6. This is particularly interesting in view of the smaller surface area of the tetrahedrite sample as determined by BET analysis.
EX consumption is essentially complete after 2 min of conditioning. This same trend was observed in floatability tests, where floatability remained constant when conditioning was extended longer than 2 min.
Many investigations have demonstrated the correlation between oxidation potential and absorption of xanthate collector onto sulfide minerals (Woods, 1986; O’Dell, 1986; Pritzker, 1984). However, externally imposed potentials produced little effect on EX consumption by the tetrahedrite as indicated by figure 7. This agrees with the floatability data showing little effect of imposed potential.
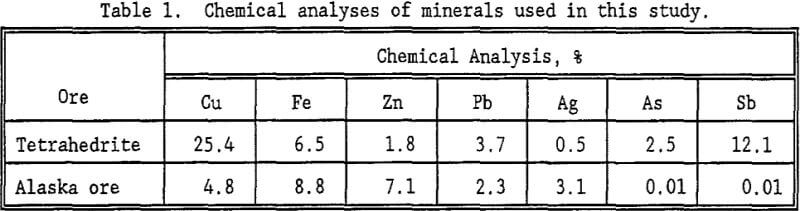
The reversibility of EX adsorption on tetrahedrite was investigated spectrophotometrically in borate and sulfate solutions. As was shown in figure 5, EX adsorption by most sulfides is favored under mildly oxidizing potentials. In adsorption reversibility tests, the tetrahedrite was conditioned first at -0.155 V; EX is stable against oxidation and reduction at this potential. Typical results are presented in figure 8. No increase in EX concentration was observed following conditioning at -0.355 V. This suggests that absorption of EX is irreversible.
Silver Recovery from Alaskan Ore
Survey tests were also conducted to determine the flotation behavior of silver in the complex silver-bearing concentrate from Alaska. The objective of the testing was to achieve better control over flotation of the silver-bearing minerals. Many of the trends observed with tetrahedrite were observed in these silver recovery tests. Silver recovery increased with increasing EX concentration as shown in figure 9. Maximum silver recovery was 38%. As shown in figure 9, increased EX concentration had a small effect on the silver grade. Microscopic studies suggested that the silver minerals were adequately liberated; thus, poor liberation does not appear to have contributed to the low silver recovery. The low silver recovery probably resulted from the complex composition of the ore and the inherently poor separations obtained in the Hallimond cell due to poor agitation. Thus, the Hallimond tube tests are useful to indicate flotation response, but are poor at predicting ultimate selectivity in flotation.
As noted above, the flotation of sulfide minerals often is strongly tied to the oxidation potential of the mineral in the flotation slurry. The behavior in both borate and sulfate solution was determined. The floatability of silver from the Alaskan ore was unaffected by potentiostatic control of potential as demonstrated in figure 10. The grade of silver in the floated product, however, declined as the applied potential became more oxidizing.
Summary
The focus of this research was to investigate the flotation properties of silver-bearing minerals. Specimen-grade, silver-bearing tetrahedrite and a tabling concentrate from an Alaskan lead-zinc mill were chosen as typical sources of silver. Floatability of tetrahedrite increased with increasing EX concentration to a maximum value of 67% in 1×10 -4 M EX. Floatability slightly increased with increasing pH. Most notably, however, tetrahedrite displayed floatability at highly reducing potentials in contrast to most sulfides. Adsorption of EX on tetrahedrite also was found to be irreversible.
Silver recovery from an Alaskan concentrate increased with increasing EX concentration; however, recovery did not exceed 38% in these tests. The grade of silver in the floated product declined as the applied potential became more oxidizing.
This research has indicated tetrahedrite floatability is good over a wide range of potential and not subject to the strong effects of potential observed in the flotation of chalcopyrite and pyrite. The research suggests, however, that maintaining a reducing potential during flotation may improve rejection of chalcopyrite and pyrite (and possibly copper-activated sphalerite). Further testing in conventional flotation equipment will be required to assess the usefulness of these findings in plant practice.