Froth flotation is usually effected by the addition of a collector agent and a frothing agent to an aqueous suspension of suitably comminuted mineral ores. The action of collectors is to adsorb onto the surfaces of minerals to be separated, sensitizing them to bubble adherence. The action of frothers has, in the past, been accepted as that of froth formation only, brought about by a lowering of the air/water interfacial tension. Substances capable of producing froth are classed according to their relative capacities for production of froth-volume and froth stability in the simple frother-water system.
Many instances of antagonistic effects of certain mixtures of frothers (or collectors and frothers) on flotation froth have been known to flotation operators and have been reported in literature. Taggart and Cooke give several examples of incompatibility of certain ratios of frothers and collectors, e.g., oleate and long-chain sulphates, pine oil and soaps.
The degree of association between two or more types of surface active agents is very sensitive even to small changes in electric (dipole) moment of the polar groups of the amphipathic molecules as influenced by magnitude and position of neighboring ions or dipoles, their size, concentration, and stereochemistry. In addition, the molecular association is greatly influenced by concentration and type of inorganic salts in the substrate, by its pH, and by temperature. The nonpolar groups of interacting molecules greatly affect the stability of molecular complexes. Progressive shortening of the aliphatic chain of one of the reacting molecules weakens (at an increasing rate) its tendency to form stable complexes. Similarly, introduction of a double bond of cis-form into one of the reacting chains, which changes the straight hydrocarbon chain into a kinked one, or introduction of a branched chain, reduces the stability of the associated complex.
Experimental procedure was based on: (a) interactions between an insoluble alcohol or sulphate monolayer, short-chain homologues of which are used as frothers, spread on the Langmuir trough, and a xanthate (typical collector) injected into the substrate; (b) determinations of the amount of frother abstracted from solution by mineral particles coated with collector; (c) measurements of contact angles formed by air bubbles and insoluble oils on solid surfaces in collector solutions; and (d) frothing and flotation tests using equivalent additions of collectors and frothers.
(a) Trough interactions. It can be readily shown that by monolayer penetration of long-chain alcohol films by long-chain xanthates, strong molecular associations exist between these two types of compounds. If a monolayer of hexadecyl alcohol (cetyl alcohol, C16 H33 OH) is spread on N/100 KCl solution at pH 11 and is held at a constant area and at a surface pressure of 10 dynes per cm, and ½ mg of potassium lauryl xanthate (C12 H23 xanthate) is then injected into the underlying solution of 400 cc, an immediate rise of surface pressure of the alcohol film from 10 dynes per cm to 52 dynes per cm takes place, although the potassium lauryl xanthate solution itself shows initially only 5 dynes per cm surface pressure.
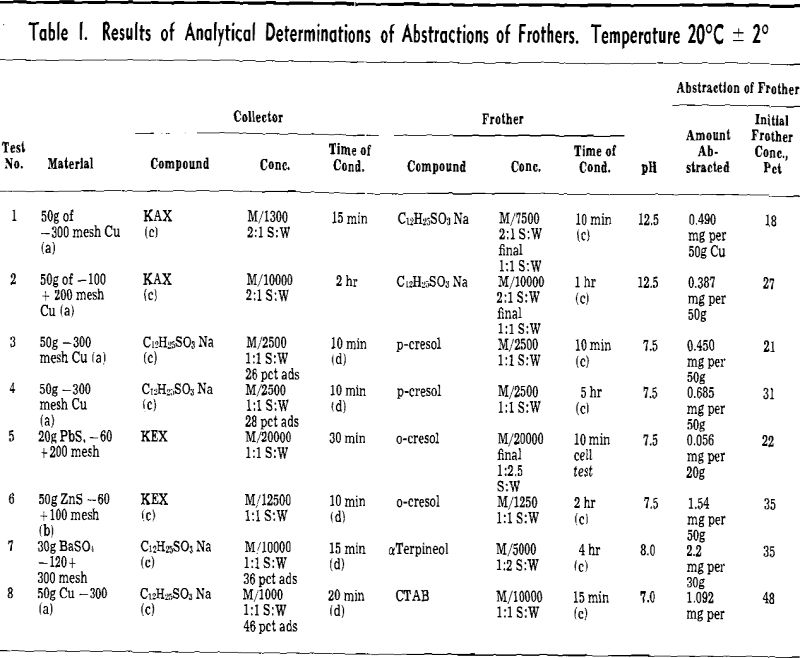
(b) Abstraction of Frothers by Collector-Coated Solid Particles (Table I). Procedure: A quantity of air-atomized Cu powder or pure mineral particles was shaken in a cylinder with a solution of collector. Then a solution of frother was added either directly to the cylinder or after prior removal of collector solution by decantation.
Abstraction results: Cuming and Schulman have found that adsorption of sodium dodecyl sulphate onto copper powder is possible in the narrow range of pH 5.5 to 9.0 where the insoluble basic copper sulphate soap (C12 H23, SO4 (OH) Cu) is formed. In the more alkaline solution the surface of Cu powder is incapable of adsorbing sulphate, but xanthates are still strongly adsorbed.
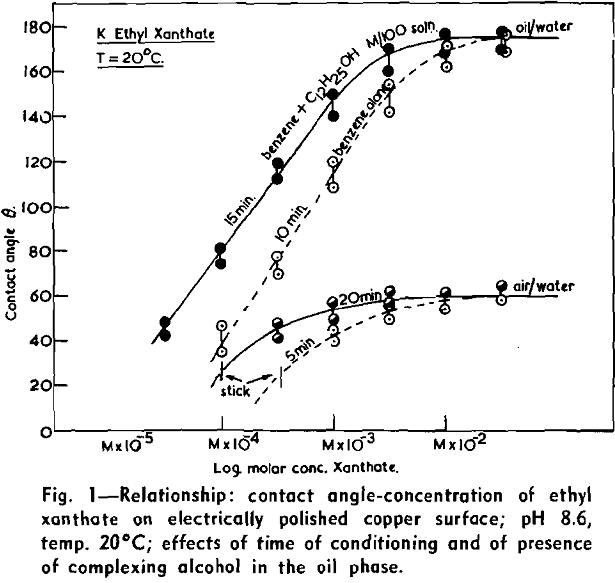
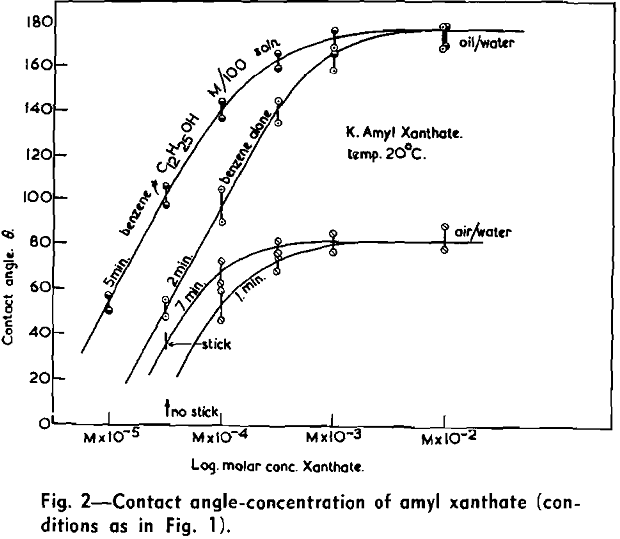
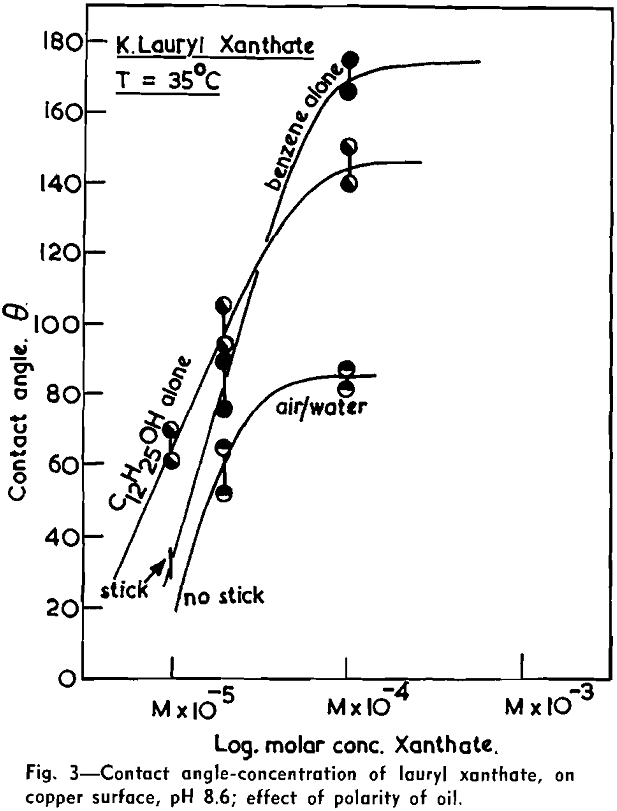
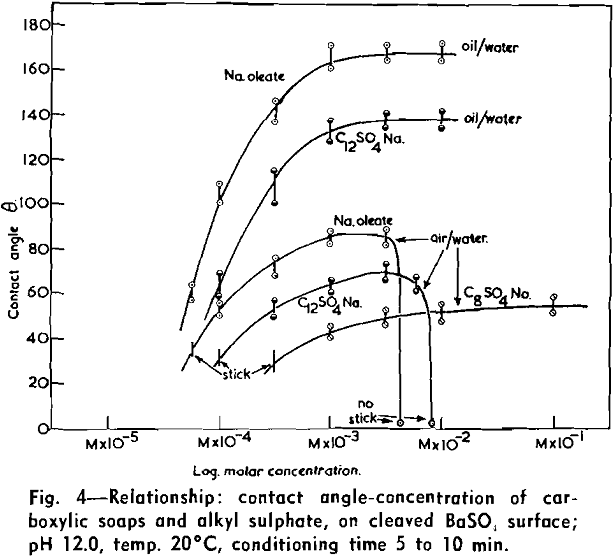
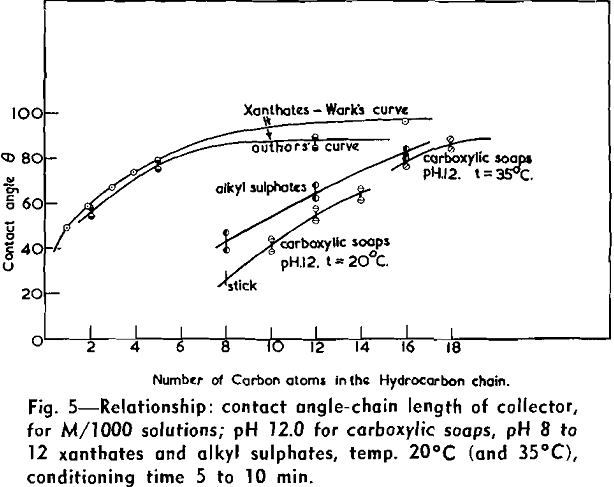
(c) Contact Angle Measurements. Procedure: A horizontal telescope equipped with cross wires and protractor was used; contact angles were always measured across the water phase. In addition to air bubbles, drops of various oils such as benzene, alcohols, and nujol were also employed.
Solid surfaces used for contact angle measurements consisted of freshly electrolytically polished specimens of copper bar (anodic polishing in 55 pct H3PO4 solution) and freshly cleaved faces of BaSO4 crystals. Conditions for measurements were standardized with regard to time of conditioning, time of bubble-contact, and aging of the reactive surfaces.
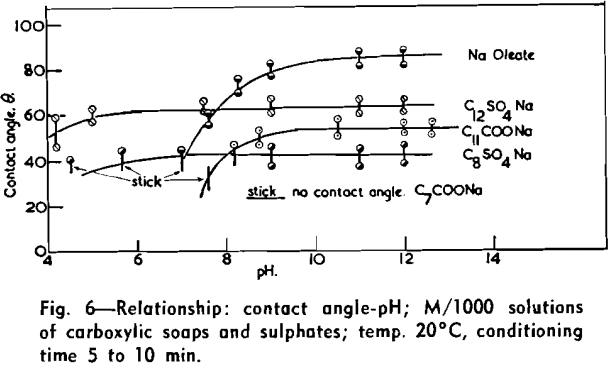
The immediate effect in flotation of the formation of micelles by carboxylic soaps, alkyl sulphates, and also amines is the nonadherence of air bubbles to the solid surface coated by these agents in solutions of high concentrations. The proof that the surface is then still coated by the surface active agent is furnished by the development of full contact angles at the oil/water/solid interfaces. Oil is capable of penetrating the physically bound hydrophilic double layer on the solid and gives as high a contact angle as the fully hydrophobic single layer.
(d) Frothing and Flotation Tests. Procedure: Effects of additions of collectors on the froth-volume of frothers were studied in a frothing setup similar to one used by Sun. Flotation tests were conducted in a 100-ml all-glass cell with a sintered disc partition, aerated by compressed nitrogen. Rate of aeration was measured by a flowmeter and was kept constant for a series of tests.
The froth of individual alcohols used as frothing agents is greatly affected by the addition of xanthates, the effect being shown by the variation of froth-volume and by the changes in the character of the resultant froth. The results show that when a constant amount of ethyl or amyl xanthate is added the froth volume at first decreases and then increases, with increasing chain length of alcohol. The detailed tracing of effects of frothing of mixtures is complicated by the simultaneous changes in the character of the froth.
The concept of molecular interactions between collector and frother molecules makes it possible to visualize the process or mechanism involved in dynamic attachment of air bubbles to solid particles. Fig. 8a shows the distribution of collector and frother molecules at interfaces and in bulk solution at optimal flotation conditions, as an air bubble and a particle approach each other. Both at the air/water interface and at the solid/water interface diffuse monolayers of associated and unassociated collector and frother molecules are present, temporarily in equilibrium with molecules in bulk solution.
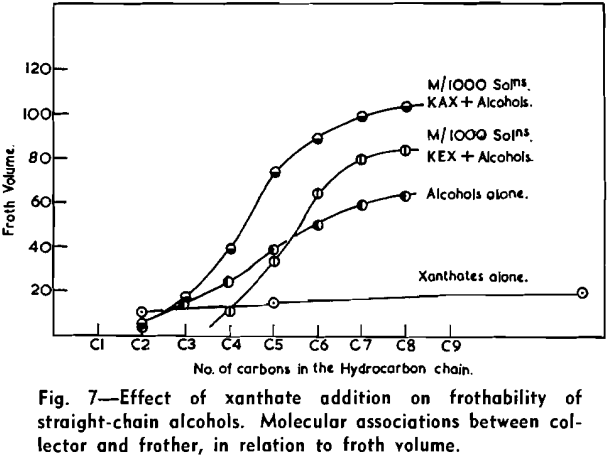
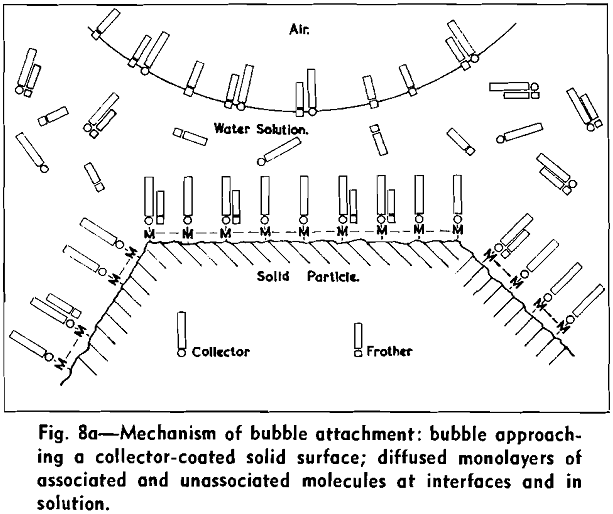
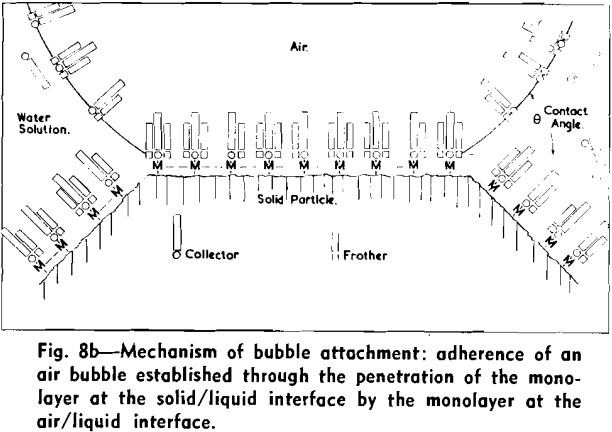
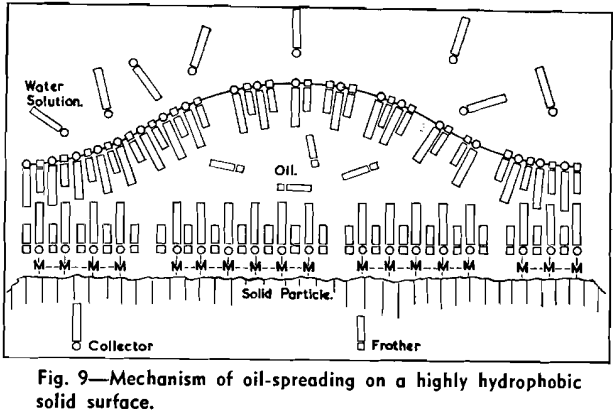
The strongly hydrophobic nature of densely collector-covered surfaces which prevents penetration by frother molecules causes further (a) adherence of particles to one another (flocculation, due to van der Waals forces) and (b) spreading of oils (derived from flotation reagents and from mining operations) and of the degradation products, such as dixanthogens.

