Table of Contents
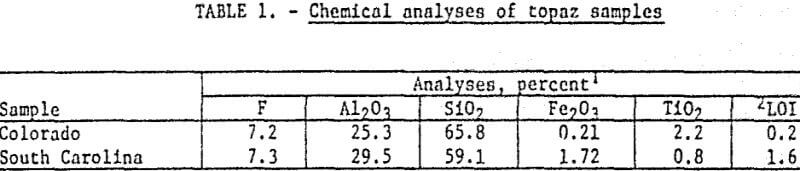
Samples of a topaz-bearing gneiss from Jefferson County, Colorado, and a topaz-bearing schist from Chesterfield County, South Carolina, were obtained for the investigation. These are the areas outlined by USGS as containing probable commercial quantities of topaz. Partial chemical analyses of the samples are shown in table 1.
Mineralogical examination of the Colorado gneiss showed it consisted primarily of topaz and quartz with some muscovite and small amounts of rutile and zircon. The topaz was essentially liberated in fractions finer than 100 mesh.
The South Carolina schist contained primarily a topaz-andalusite mixture with quartz, and minor amounts of pyrite, muscovite, magnetite, and feldspar. The topaz occurred as both cleavage fragments and as fine-grained aggregates. The fine-grained aggregates outnumbered the clear cleavage fragments by a factor of approximately five to one. In the fine-grained aggregates it was virtually impossible to distinguish between topaz and andalusite. Both these minerals were intermixed in the fine-grained matrix material and apparently did not possess a completely ordered lattice. Many of the aggregates were heavily iron-stained. Most of the topaz was liberated at 65 mesh.
Beneficiation Studies
Initial considerations of concentration techniques to be studied indicated that flotation procedures should be emphasized. Although gravity techniques had been proposed by earlier investigators of South Carolina topaz, the particle size of liberation on these samples showed that gravity techniques would probably be economically marginal. Cursory studies were made using laboratory gravity techniques such as tabling; the results proved to be unsatisfactory. The next technique considered was flotation. Studies were made to determine if topaz would respond to the flotation techniques used for concentrating kyanite, a member of the same mineralogical family. Results of the initial tests were extremely encouraging and led to the development of successful techniques for the selective flotation of topaz.
Fatty Acid Flotation
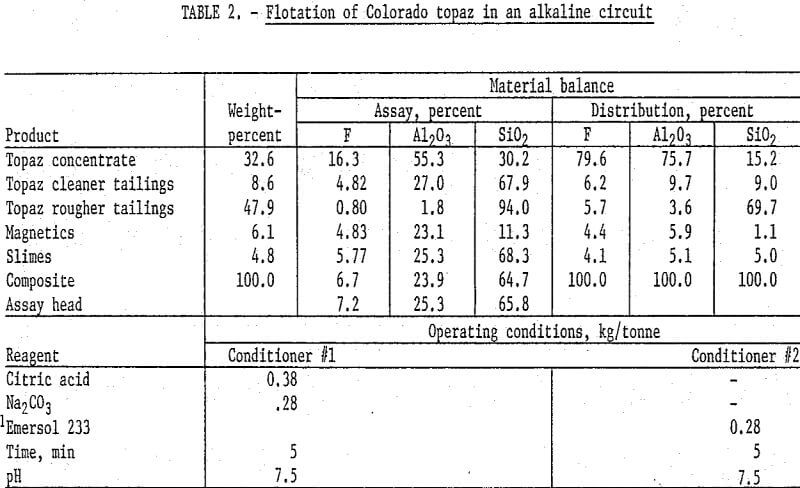
One of the kyanite flotation techniques that was studied utilized a fatty acid collector in an alkaline circuit. In this procedure, 250-gram samples of Colorado topaz were ground in stages through 100 mesh, deslimed at 10 micrometers, and magnetically separated in a wet high-intensity matrix-type unit to remove iron-bearing materials. The nonmagnetic pulp was conditioned with sodium carbonate and citric acid for pH control and quartz depression, respectively. The pulp was then conditioned with oleic acid, and a topaz rougher concentrate floated. The rougher concentrate was cleaned four times. The final concentrate contained 16.3 percent F and 55.3 percent Al2O3 and recovered about 80 percent of the fluorine and 76 percent of the alumina. Detailed results and operating conditions of this test are shown in table 2.
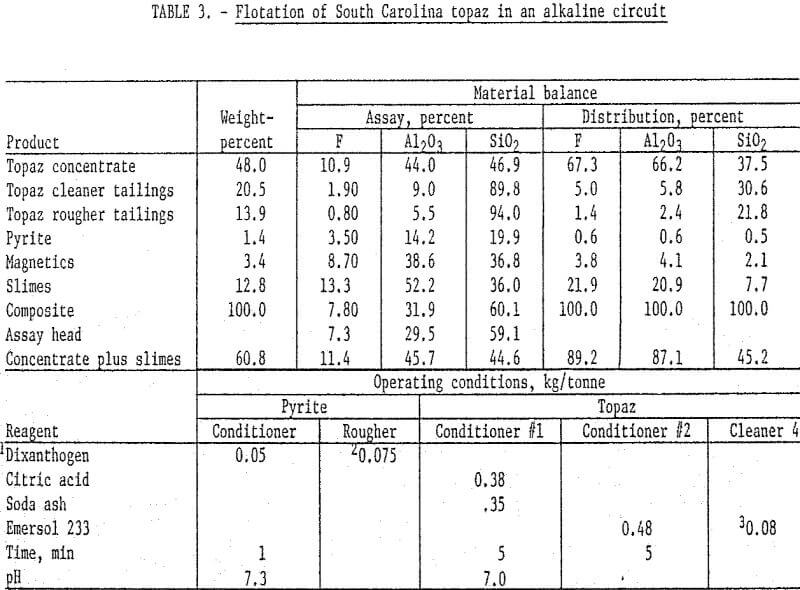
The South Carolina topaz was treated in a manner similar to the Colorado sample except that the material was ground to pass 65 mesh and deslimed at 5 micrometers. Also a sulfide flotation step was included after magnetic separation. The nonmagnetic pulp was conditioned at natural pH with dixanthogen, and a pyrite product floated. It was necessary to stage-add collector during flotation to insure complete pyrite flotation. The sulfide underflow was then conditioned first with sodium carbonate and citric acid and then with oleic acid. A topaz rougher concentrate was floated and cleaned four times. It was necessary to add a small amount of oleic acid to the fourth cleaner to maintain satisfactory flotation conditions. The final concentrate analyzed 10.9 percent F and 44 percent Al2O3 and recovered about 66 percent of the fluorine and alumina. The slimes contained an appreciable amount of fluorine.
X-ray diffraction analyses of the slimes showed them to consist of topaz and quartz with possible traces of hematite and andalusite. Topaz is extremely hard and would not normally be expected to be in the slime fraction; however, the topaz in the slime may result from the breaking of the aggregates noted in the petrographic examination. Consequently, the slimes were composited with the concentrate, which not only improved recovery of flourine and alumina but also increased the grade. Summarized results of this test and the operating conditions are detailed in table 3. The concentrate plus slimes contained excess quartz, and even after defluorination the mullite would contain quartz.
Petroleum Sulfonate Flotation
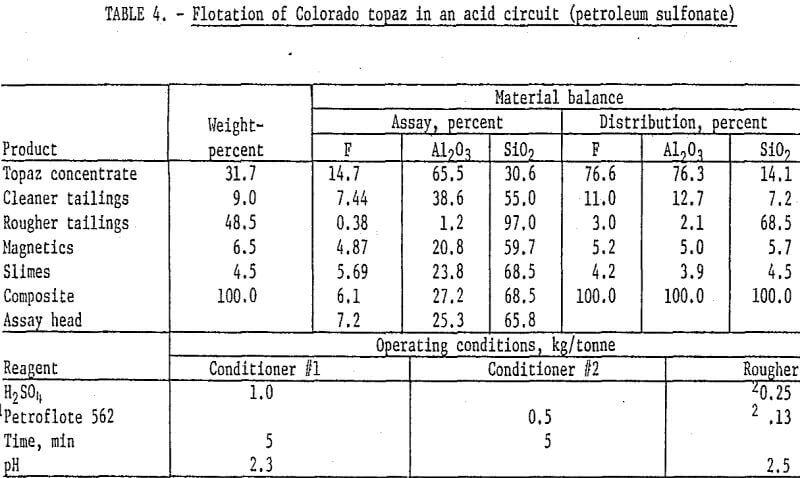
The second technique studied for the flotation of topaz was the use of petroleum sulfonate in an acid circuit. This procedure has been widely used to float kyanite and related heavy minerals. Samples of the Colorado topaz were stage-ground through 100 mesh, deslimed at 10 micrometers, and magnetically separated, and the topaz was floated with Petroflote 562 in an acid circuit at pH 2.5. It was necessary to stage-add both collector and acid during the rougher step to maintain flotation conditions. The rougher concentrate was cleaned twice, and Petroflote 562 was added at the start of the second cleaning to produce a concentrate grading 14.7 percent F and 65.5 percent Al2O3. Fluorine and alumina recoveries were about 76 percent. Results and operating conditions are shown in table 4.
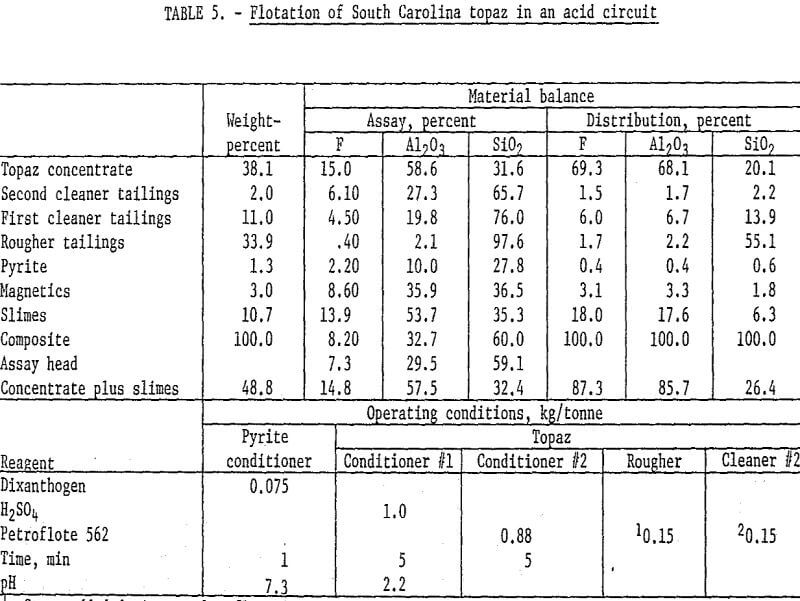
Samples of the South Carolina topaz were stage-ground to pass 65 mesh, deslimed at 5 micrometers, and magnetically separated. The pyrite was removed by flotation with dixanthogen in a neutral circuit, and a rougher topaz concentrate floated with Petroflote 562 in an acid pulp and was cleaned and recleaned. Petroflote 562 was stage-added to the rougher circuit and at the beginning of the recleaner step. The recleaner concentrate analyzed 15.0 percent F and 58.6 percent Al2O3 and recovered about 68 percent of each. Addition of the slimes to the recleaner concentrate changed the grades only slightly but increased fluorine and alumina recoveries by 18 percent. Operating conditions and results are shown in table 5.
Amine Flotation
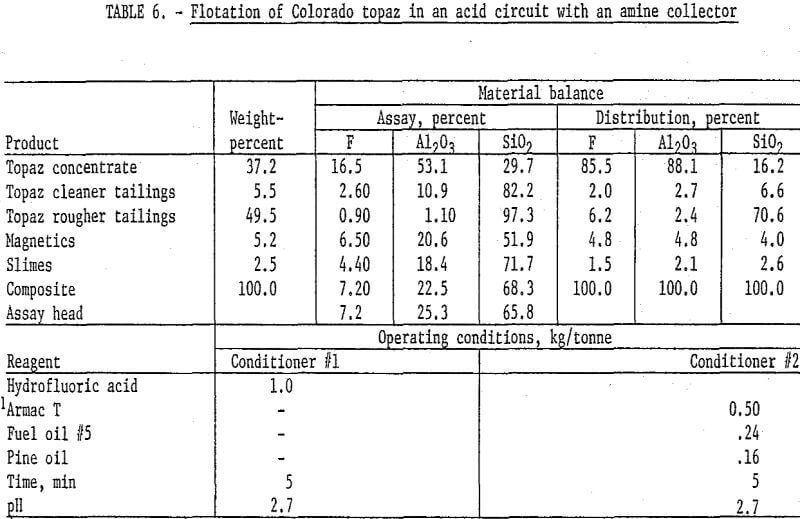
The mineralogical composition of the topaz-bearing samples suggested that it might be possible to adapt feldspar flotation techniques to the concentration of topaz. Flotation of topaz from the Colorado gneiss sample with an amine (Armac T) in an acid circuit produced the best results obtained on this material. The Colorado topaz was stage-ground to pass 100 mesh, deslimed at 10 micrometers, and magnetically separated; the pulp was then acidified with hydrofluoric acid, and the topaz was floated with Armac T, fuel oil, and pine oil. The rougher flotation concentrate was cleaned four times to result in a product that recovered 86 percent of the fluorine and 88 percent of the alumina at grades of 16.5 and 53.1 percent, respectively. Details of this test are shown in table 6. The South Carolina sample did not respond to this technique.
Defluorination
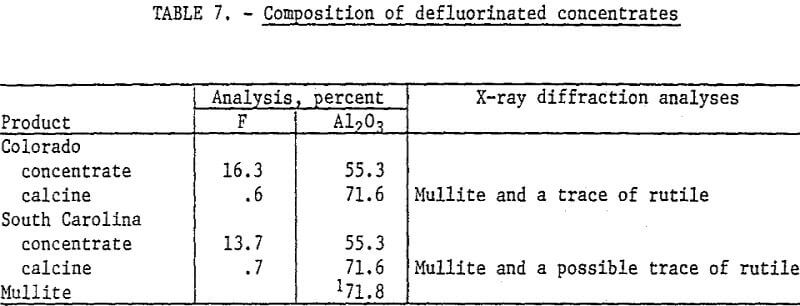
Selected concentrates were defluorinated to determine the composition of the calcines. The samples were calcined at 1600° C for 1 hour. Although topaz can be defluorinated at lower temperatures, 1600° C was used to insure that conversion to mullite was complete. Data in table 7 show the composition of the concentrates and calcines. The Colorado topaz concentrate was obtained by fatty acid flotation in an alkaline circuit. The South Carolina topaz concentrate was obtained in preliminary tests with a petroleum sulfonate collector in an acid circuit. X-ray diffraction analysis of the calcine showed that mullite was produced from the flotation concentrates.
Conclusions and Discussion
The results of this investigation show that topaz can be effectively concentrated by froth flotation. The topaz concentrates, when defluorinated at 1600° C, produced a mullite.
To effectively compare the various reagent schemes would require larger scale testing than utilized in this study. These larger scale tests would also be useful in providing material for optimizing defluorination conditions and fluorine recovery.