All forms of “lime” are used in the steel plant. Calcium hydroxide is used for water treatment, acid neutralizing, and as a lubricant for wire drawing. All the other limes are used as fluxes in the sintering plant, blast furnace or steel producing furnaces.
In the production of self flux or super-flux sinter, high calcium and dolomitic limestone fines, are used. Where quicklime fines are available, they may be added to the sinter mix also.
The blast furnace uses both high calcium and/or dolomitic limestone. A few trials have been made using iron oxide stabilized dolomitic lime in an attempt to reduce coke consumption and to increase the production rate. The trials were very successful but would not be economical, except in periods of coke shortages, with resulting extremely high coke cost and exceptionally high hot metal demand.
This paper will deal principally with high calcium quicklime (Hi Cal Lime) and dolomitic quicklime (Dololime) as fluxes for open hearth, electric furnaces, Argon-Oxygen-Decarburisation (AOD) furnaces and basic oxygen furnaces (BOF, LD, LD-AC, and OBM or Q-BOP).
If all the coal burning electric power plants are forced to lime scrubbing to remove SO2, a huge market will develop for both limestone or quicklime. Here again, most electric generating plants are built on rivers, if the river is navigable, then water transportation will be extremely desirable. Otherwise unit trains will be utilized. The final decision on SO2 scrubbing may change the picture overnite, should other, more economical methods be discovered to reduce the sulfur in the fuels. It is hard to believe that lime scrubbing is the best answer to eliminating SO2 pollution. No one has considered how much SO2 is released by burning high sulfur fuels in the lime kilns that produce lime to scrub stack gases of all of our coal burning industries.
The reserves of high quality limestones are enormous but in most areas in the United States the deposits are very deep and cannot be mined economically or are too far from the point of usage. Much of the reserves of high quality limestone will be depleted in areas of high consumption in the next 20 years and lower quality stone will probably be used to manufacture lime rather than ship stone or lime long distances by rail. Some silica in flux lime or limestone, as much as 1-5% or more may be found helpful in developing good steel furnace slags. Higher sulfur lime may be used without undue harm also. These two subjects will be covered later in this paper.
A very high percentage of flux lime for steel-making is calcined (burned) in rotary or rotary hearth kilns. Some flux lime is calcined in shaft kilns. The shaft kiln produces very low reactive (LoR) lime and since only gas or oil firing can be used, the shaft kiln will soon be replaced by other types of kilns. Shaft kilns and other kilns wish preheaters cannot consistently and economically produce low sulfur lime, unless the lime is dead burned. The low sulfur specs demanded by the steel plant forces the lime producer to use open kilns with no recovery of heat from the waste gas, resulting in very low fuel efficiencies.
The shortage and high cost of gas and oil fuel has presented a serious problem to lime producers. Nearly all gas or liquid fired rotary kilns are being converted to powdered coal firing. With the shortage and high price of low sulfur coal, more problems arise for the producer. Stone preheaters cannot be used on the kilns because of the sulfur pickup in the lime and stone. One of the major costs of producing lime is fuel. The use of preheaters could lower the fuel consumption and the cost considerably.
Pure oxygen for combustion in rotary time kilns has been examined only superficially. Only two lime plants in the United States are using combustion oxygen. In areas where cheap pipeline oxygen is available, it is almost a certainty that the increased fuel efficiency and production increase will more than pay for the cost of oxygen. If liquid oxygen is available from a nearby source, its use may prove economical. It must be remembered that the development of combustion oxygen usage in the open hearth furnace was opposed by operators who feared that the use of oxygen would result in burning down the furnace. The resistance fell to near zero when it was discovered that the furnace roof temperature dropped, the production jumped 30% or more, the fuel consumption, per ton of steel, dropped from about 4, 5 to 3.6 million 3TU/T if steel. Only then was combustion oxygen generally accepted by open hearth operators. A tape recording of their objections to oxygen use in the late forties would be identical to one made today by a lime plant operator.
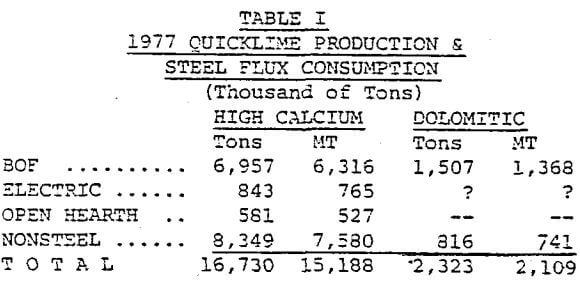
Table I lists the tons and metric tons of lime used as flux in the steel industry and the total lime produced. No figures are available on the limestone used in sinter, the blast furnace and other furnaces.
The very short oxygen blowing time for the BOF heats (15-20 min) presents continuing slag control problems, some of which are created by varying lime qualities. The Q-BOP has its own peculiar slag problems as does the AOD. The dying open hearth and the growing electric furnace have minimum slag problems. However, as the electric furnace power is increased to the point of electrocuting the scrap with a big “bang”, the slag practice may become more important and possibly critical. The Q-BOP is U.S. Steel’s name for the OBM (Oxygen-Boden-Maxhutte) furnace, developed by Maxhutte in Germany, replacing the basic, bottom blown, Thomas Converter for refining high 1.8% phosphorus iron. Flux lime, pulverized to over 90% thru 150 mesh, is blown thru the tuyeres with the oxygen stream.

