Recently, details of a study optimizing gallium and germanium extraction from zinc residues using H2SO4 leaches was published. In the process, filtrates containing gallium and germanium along with other impurities such as iron, copper, zinc, and cadmium are produced. The focus of this paper is the recovery of gallium from these solutions.
It has been reported that in an H2SO4 system, di(2-ethylhexyl) phosphoric acid (DEHPA) can be used to extract gallium, but commercial production has not been attained. Musto Explorations, in St. George, UT, tried extracting gallium from an H2SO4 leach filtrate of Apex Mine ore with DEHPA; however, processing problems prevented the operation from becoming economically viable. A major obstacle in using DEHPA is that the pH of the leach filtrates, which range from 0 to 0.5, must be raised by neutralization to a range of 2 to 3 for high gallium extraction. Neutralization increases the cost and time required for processing. Thus, the goal of this research was to investigate solvent extractants to recover gallium from low pH H2SO4 solutions.
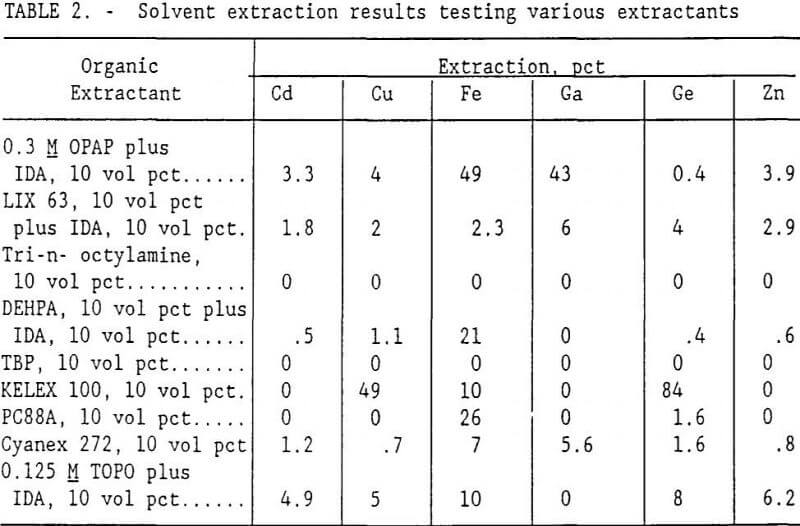
Table 2 shows that 0.3M OPAP plus 10 vol pct IDA in kerosene (this combination will subsequently be referred to as OPAP) extracted the most gallium. Therefore, OPAP and DEHPA were studied in more detail for gallium extraction. Although preliminary results using DEHPA for gallium recovery were not encouraging, its use was not eliminated at this point because other researchers have found it to be successful in recovering gallium. DEHPA and OPAP are both organophosphate extractants. The commercially prepared OPAP used in this study was obtained from Albright, Inc., and is reportedly a 60-40 mixture of monoctylphenyl acid phosphate and dioctylphenyl acid phosphate.
Gallium Stripping
Batch solvent extraction tests conducted on a wrist-action shaker showed that gallium could be stripped from OPAP using 1M to 3M-H2SO4 solutions. Figure 3 reveals that stripping with a 1.5M-H2SO4 solution removed 98 pct of the gallium in 10 min. A McCabe-Thiele analysis of the equilibrium stripping isotherm for a 1.5M-H2SO4 strip solution and a 0.3M-OPAP organic containing 0.32 g/L Ga, indicated that a strip liquor containing 1.3 g/L Ga could be produced at an A:O ratio of 1:4 with four stages (fig. 5).
Solvent extraction was tested for recovering gallium from H2SO4 filtrates produced from leaching zinc processing residue. The following conclusions were made.
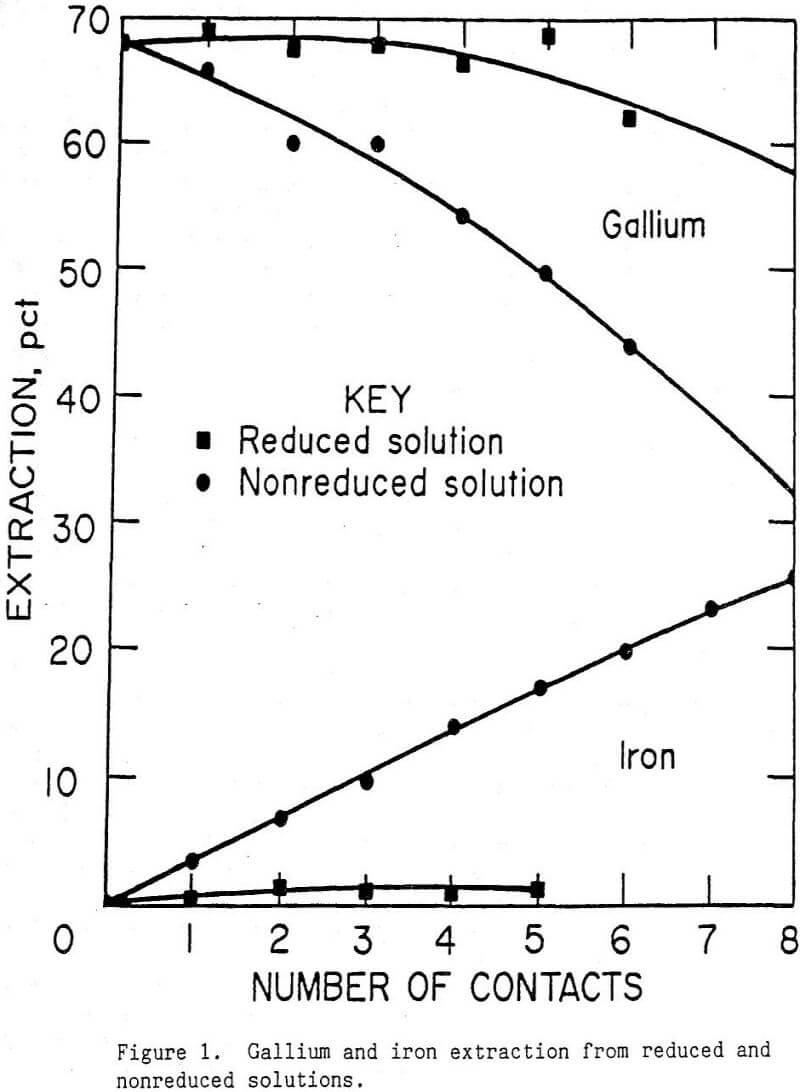
- Solvent extraction with the organophosphate, OPAP, proved very successful for recovering over 98 pct of the gallium from solutions with pH values as low as 0.
- Gallium extraction using OPAP was hindered when ferric iron was present in solution. Therefore, it was necessary to maintain the iron in the filtrate in a reduced state. This was accomplished by contacting the solution with metallic iron.
- Rate and isotherm tests were completed for gallium extraction with OPAP. From this data, McCabe-Thiele analyses were performed and a continuous gallium solvent extraction circuit was built and operated. When a leach solution containing 0.32 g/L Ga and an organic containing 0.3M OPAP was used, the continuous system effectively extracted 95 pct of the gallium onto the organic. Stripping the organic with a 1.5M-H2SO4 solution recovered 94 pct of the gallium.
- A 50-pct-phosphoric acid strip effectively reduced the amount of ferric iron remaining on the OPAP to below 0.5 g/L, thus enabling the continuous system to maintain high gallium recoveries.