The results were surprisingly reproducible in view of the complex reactions, and the scatter of data was small.
Rank of coal exerted the greatest effect on gasification. Tests with only three ranks of coals do not permit firm conclusions about the effect of rank. However, work in progress reveals the same trend for other ranks of coal. The difference in rate of carbon conversion between subbituminous B and subbituminous C coal (12 and 25 pct. carbon gasified per second) is large, in view of the relatively small difference in rank.
Just as Orning found that the reactivity of coals toward oxygen increases as volatile matter increases and decreases with geologic age, Bureau results show that the reactivity of coal toward steam also decreases with geologic age.
The effect of residence time on conversion was more pronounced at higher temperatures and for lower ranks of coal. A longer, wider reaction tube and higher pressures might enhance this effect; that is, lead to higher carbon conversions. However, a few tests at the same residence time and pressures up to 150 p.s.i.a. indicated that pressure inhibits the reaction slightly. Decreased slurry throughput would also increase conversion, but, at the same time, reduce reactor capacity. Although increased steam-coal ratios markedly improved conversion, quantities of steam larger than the theoretical amount might not be advisable. From an economic standpoint, increased conversion may not compensate for the cost of heating more steam than the theoretical minimum amount-approximately 1.2 lb./lb. of dry coal-unless the excess steam is needed for subsequent processing.
Gas composition was relatively constant for wide ranges of operating variables: (1) Residence time, 0.6 to 1.1 sec.; (2) carbon conversion, 30 to 90 pct.; (3) steam-coal ratio, 1.7 to 10.8; and (4) temperature, 1,660° to 1,900° F. In test 69 (tables 1 and 3) with subbituminous C coal, carbon conversion was 88 pct., the highest achieved in this series. About 41 cu. ft. (32° F. and 14.7 p.s.i.) of gas were produced per pound of dry coal. The gas contained 6.3 cu. ft. of CO, 8.0 CO2, 23.9 H2 and small amounts of CH4 and unsaturated hydrocarbons. Synthesis gas with such a high H2-CO ratio (about 3.8 to 1) can be used to produce methanol, Fischer-Tropsch products, or CH4 for high-B.t.u. pipeline gas.
In industrial high-temperature carbonization of low-rank coals, about 10 pct. coal is converted to liquids, such as tar and light oil. In this work much less tar and oil were obtained because of thermal cracking and reaction with steam. The small amount of tar and oil adhered to the walls of the collection vessels, making their recovery difficult. As shown in table 3, the calculated values for unreacted residue are larger than the measured values. The difference is probably due to tar vapors that escape thermal cracking and reaction with steam but are difficult to recover. Small amounts of solid occasionally collected from the recovery train were identified spectrographically as naphthalene.
The fact that all 70 tests were made intermittently in one reactor for a year, without repairs, proves its durability. Tests lasted only a little more than 1 hour at most, but water was pumped through the tube for several hours to flush it and establish steady-state conditions. This probably contributed to the wear of the tube. From a process standpoint, the heat resistance of the alloy steel used in the tube determines the upper temperature limit. At higher reaction temperatures, around 1,960° F., the life of the tube is considerably reduced. This temperature cannot be exceeded for commercially available high chromium and nickel alloy-steels amenable to fabrication if long operating life is desired.
The same method of gasification is currently being applied in larger scale apparatus to develop a gasifier for the utilization of heat from nuclear fission. Gasification of coal-water slurries in a tube, to which heat is supplied by recycled helium from a nuclear reactor, is discussed in another publication.
The technical feasibility of gasifying powdered, noncoking, low-rank coal with steam in an externally heated tube has been demonstrated. The tube, 112 ft. of alloy-steel tubing shaped into a helix, served as an electrical conductor of electricity used for heating.
The effects of the following variables were studied: Residence time of coal in reactor, average reaction temperature, steam concentration, and rank or geologic age of coal. Reaction pressure was maintained in the range 19-26 p.s.i.a.
High carbon conversions and large yields of synthesis gas were obtained with low-rank coal. In one test, for example, about 88 pct. of the carbon in a subbituminous C coal was gasified at 1,910° F. with a residence time of 1 sec. The gas contained 58 pct. H2, 19 CO2, 15 CO, and 3 CH4; the yield was 30 cu. ft. (at 32° F. and 14.7 p.s.i.) of synthesis gas (CO + H2) per pound of dry coal.
A geologically younger (subbituminous C) coal was gasified more readily and completely than older (subbituminous B) coal. Carbon conversion (percent of carbon gasified) for subbituminous C coal was a linear function of residence time between 0.5 and 1.3 sec. at all temperatures and steam concentrations used. For the same range of residence times, a linear relationship was noted between conversion and residence time for various ranks of coal at the same temperature and steam-coal ratio. Although carbon conversion increased with residence time for a given coal, the gas composition was unaffected. However, gas made from anthracite varied considerably from that produced from the lower rank coals; gas from anthracite appeared to have resulted chiefly from thermal decomposition rather than from the steam-carbon reaction.
For subbituminous C coal, conversion increased linearly from 37 to 85 pct, as temperature increased from 1,660° to 1,900° F. At the same time, CO, CH4, and unsaturated hydrocarbons in the product gas decreased linearly, and the concentrations of H2 and CO2 increased linearly. However, in the temperature range 1,570° to 1,660° F., changes in carbon conversion and gas compositions were rather abrupt, which probably indicates the beginning of the steam-carbon reaction.
Total carbon conversion also increased as steam concentration increased. For subbituminous C coal, this increase was rapid up to 4 lb, of steam per lb, of dry coal; above this ratio, carbon conversion increased more slowly. Although total conversion was a parabolic function of steam concentration, the rate of conversion of subbituminous G coal (25 pct. carbon per second) remained unaffected. The gas composition was slightly affected by changes in the steam concentration: CO, CH4, and unsaturated hydrocarbons diminished, and CO2 and H2 increased somewhat as steam concentrations increased.
The fact that the temperature of the tube was maintained between 1,500° and 1,960° F. for 1 yr. with 70 intermittent tests indicates good resistance of the alloy steel to severe operating conditions.
The results of this work can be used in two ways: First, because the apparatus permits precise measurements, the effects of operating variables on the cost of making synthesis gas can be determined. Optimum overall conditions for the production of synthesis gas can thus be established. For example, higher reaction temperatures increase the rates of gasification but reduce the life of the reactor. Thus, the optimum economic operation for a given alloy steel could be determined.
Secondly, gasification of coal in externally heated tubes is well suited for the utilization of nuclear heat. The Atomic Energy Commission is now building a process-heat reactor (called Turret Reactor) to heat helium at 500 p.s.i. to 2,400° F, for recycling as a heat-transfer medium. The Federal Bureau of Mines is developing equipment to pass hot helium over metallic tubes to supply the heat needed for gasifying coal-water slurries.
Introduction
During the past 2 decades, the Federal Bureau of Mines has conducted research and developed processes to convert coal to fluid fuels. For complete conversion of coal to fluid fuels, at least part of the processed coal must be gasified. The search here and abroad for economical methods of making a coal-derived gas suitable for further processing has resulted in the development of several processes using oxygen to burn part of the coal within the reactor to supply heat for the reaction of steam with carbon. A process supplying the required heat from outside the reactor has the following potential advantages; (1) Elimination of the oxygen plant, (2) lower content of carbon dioxide in gas, and hence (3) savings in gas purification.
A process using external heat to gasify lignite with steam was patented by Reyerson and Gernes. Because it was thought that some water gas was formed at temperatures as low as 1,200° F, in this process, Parry and others gasified noncoking low-rank coals in alloy steel or alloy-coated tubes through which coal was passed downward, countercurrent to steam. Heat-transfer rates were relatively low-not more than 6,000 B.t.u./hr./sq. ft.
Bituminous Coal Research, Inc ., in 1948 reported work on a somewhat similar process, for gasifying pulverized coal with steam in externally heated vertical chromium-nickel cast alloy-steel tubes. Because the life of the alloy-steel tubes was relatively short, use of refractory material, such as silicon carbide, was suggested. At high temperatures, refractories would show better resistance to wear, but their thermal conductivity would be lower.
In 1955 developmental work was begun by the Bureau of Mines on a laboratory-scale steam-coal gasification process. Two distinctive features of the process are: (1) Feeding powdered coal in a water slurry, and (2) using a tube coil as a reactor. The coal-water slurry is pumped through an externally heated, coiled, metallic tube and flashed into a suspension of coal particles in steam. A rapid increase in the velocity of this suspension causes some size degradation and considerable turbulence, both contributing to a rapid reaction between steam and coal. Consequently, rate of reaction and yield are high, despite a relatively brief residence time. This process is described here, including a study of the effects of temperature, residence time, rank of coal, and steam concentration on the gasification of coal.
Gasification of carbonaceous components (volatile matter and fixed carbon) in coal will be described in a second publication on this experimental work.
Description of Apparatus
Figures 1 and 2 show two views of the apparatus, and figure 3 is a flowsheet. The apparatus consists of (1) a feeding system, (2) an alloy-steel coiled tube, and (3) a product-recovery system.
The feeding system includes a slurry pump, a water reservoir, and a mechanically agitated slurry feeder. A Sigmamotor pump transports 2 to 9 lb./hr. of slurry at pressures up to 30 p.s.i.g. with adjustable flow rates and against variable pressures. For higher pressures and flow rates, piston pumps were more expedient.
The slurry feeder is a cylindrical vessel about 2 ft. high and 4 in. in diameter. A propeller-type electric stirrer agitates the slurry and maintains enough turbulence to keep the particles in suspension. A distilled-water reservoir is near the slurry feeder. The lines from the slurry feeder and water reservoir join and lead to the pump through a T-connection, which vents air entrapped in the slurry.
The coiled tube progressed through two earlier designs. For compactness, a helix was used in all stages of development. The reactor itself was the conductor of the electricity used for heating. This arrangement resulted in uniform heating of the slurry without creating hot spots; it also eliminated transfer of heat through a gas film on the outside of the tube.
The two early reactors failed one after 4 hours and one after 2 weeks owing to short circuits in the turns, oxidation and spalling of the outer surface upon overheating, and excessive inside pressures due to plugging by coal particles.
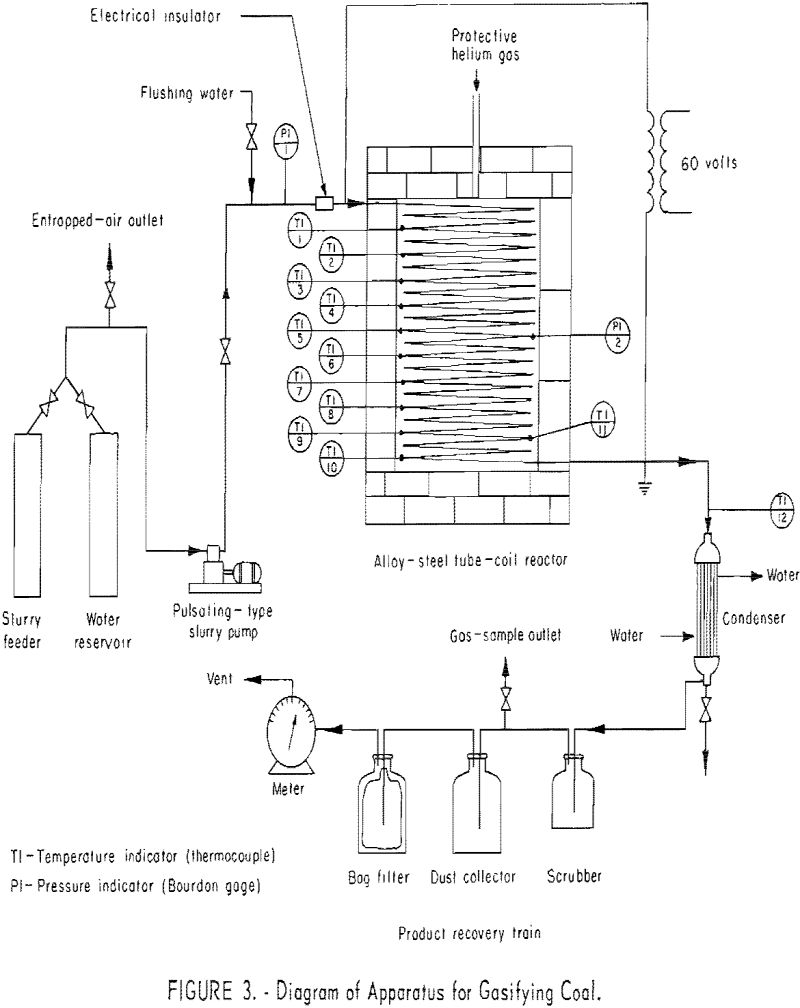
The present reactor is a 112-ft., 0.315-in. ID, 0.375-in. OD type-310 alloy-steel tube, coiled into a 20-in.-diameter helix. To prevent shorting, turns are separated with ceramic rings and supported by two columns of insulation firebrick. The firebrick wall around the coil is covered with a 3-in. coating of asbestos lagging, sealed with temperature-resistant paint. Helium is passed into the almost gastight furnace chamber to maintain a nearly inert atmosphere around the tube, thus prolonging its life. Ten chromel-alumel thermocouples, for measuring metal wall temperatures, are located at intervals along the tube, as indicated in figure 3. Each is welded to the surface of the tube (see table 1). Table 1 also shows the locations of two chromel-alumel thermocouples (11 and 12) for measuring gas temperatures; that is, the temperatures of coal and ash particles suspended in steam and product gas. A multipoint indicator, calibrated within ±5° F. against a standard potentiometer before each experiment was used for temperature measurements. One Bourdon-type pressure gage (60 p.s.i.g.) was connected to the tube inlet and another was connected halfway along the tube to measure average reaction pressure.
The product recovery system includes a water-cooled-shell tube-type condenser , a 4-liter scrubber bottle, a gas collector and two 20-liter bottles to collect dust. The condenser is a single-pass counterflow heat exchanger, 18 in. long, composed of eight 3/8-in. copper tubes within 2-in. schedule 40 steel piping. The gas collector (based on mercury displacement) is located between the scrubber and first dust collector. Larger dust particles are removed in the first dust collector due to the sudden decrease in gas velocity; fine dust is removed by a fiberglass bag filter in the second collector. The gas is metered with a wet-test meter.
The power system includes a variable transformer with a maximum alternating-current output of 112 a, at 0-230 v., a wattmeter, an ammeter, and a voltmeter.
Operating Procedure
The unit needed little maintenance or repair after difficulties during early development were overcome. Proportional weights of coal and distilled water were charged to the agitated slurry feeder. Distilled water from the reservoir was pumped through the reactor while the slurry was being prepared in the feeder. When the reactor reached steady-state conditions with respect to flow rate, pressure, and temperature, the flow of water was discontinued, and pumping of coal-water slurry was begun. At the time of initial entry of slurry to the reactor-recorded as the beginning of the run-the temperature dropped, owing to the endothermic reaction of steam and carbon. The power input was then increased to restore the preset temperature.
Water in the slurry was flashed to steam in the first few feet of tubing; the resulting suspension of coal particles in steam was then heated to reaction temperature in the first 20 ft. and reacted with steam in the remaining 90 ft. of tubing under nearly isothermal conditions. After leaving the gasifier, unreacted steam was condensed, and the gas was scrubbed, filtered, metered, and vented. Temperature, pressure, gasflow, and electrical data were recorded at intervals during each test. Tests lasted 20 to 80 min. Four gas samples were taken at 5- to 15-min. intervals, depending on the length of the run. Volume of total gas and duration of tests were recorded. At the end of each test the slurry remaining in the reactor was flushed into the condenser with distilled water. Temperature and pressure readings were averaged over the entire run.
Unreacted residual char collected in the recovery train and slurry collected in the feeder were filtered separately, dried at 105° C, weighed, and stored for subsequent analysis.
Coals Gasified
Coals gasified included (1) subbituminous C from the Healy bed, Lake de Smet mine, Lake de Smet, Johnson County, Wyo.; (2) subbituminous B from Adaville No. 1 bed, Elkol mine, Kemmerer Coal Co., Lincoln County, Wyo.; and (3) anthracite from the middle bench Bottom Ross seam, Glen Alden Coal Co., Wilkes-Barre, Pa. Table 2 shows the analyses of these coals.
The coals were crushed in a jaw crusher, pulverized in a laboratory-type disc grinder, then screened one-half hour with a standard mechanical shaker to pass a 70-mesh U.S. Standard screen. Aliquots of the 70-mesh coal were prepared and stored in airtight containers for subsequent analysis.
Results
As shown by sample calculation in the Appendix, carbon conversion or carbon gasified me an s the fraction of carbon in coal converted to carbon-bearing gaseous products ; namely, CO, CO2, CH4, and unsaturated hydrocarbons. It does not include other gases, such as hydrogen or nitrogen. Steam-coal ratio is the weight-ratio of steam (moisture in the coal plus water used in preparing the slurry) to dry coal. Temperature was calculated by averaging the temperatures of the nearly isothermal reaction zone; the individual temperatures were weighted by the length of tubing between adjacent thermocouples. Residence time was obtained by dividing the volume of the reaction zone by the average volume flow rate; the average volume flow rate was the average of the flow rates of input steam and product gas plus steam leaving the reaction zone.
Effects of Variables on Carbon Conversion
Residence Time
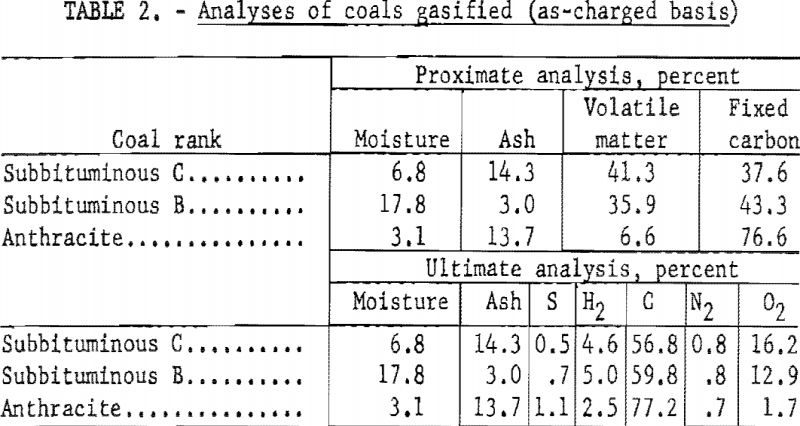
Figure 4 shows the effect of residence time on carbon conversion of subbituminous C coal at 1,760° F. and a steam-coal ratio of 3.29. Carbon conversion increased linearly with time within the indicated time limits. This was not anticipated because the complex reactions during gasification would not be expected to proceed at the same rate. Extrapolation to zero residence time yields an intercept at about 36-pct. carbon conversion, so that linearity must cease at some residence time less than 0.5 sec. This linearity is a property of the system rather than the reaction, because the large quantity of steam makes the calculation of carbon gasified insensitive over comparatively narrow ranges of residence times.
Steam Concentration
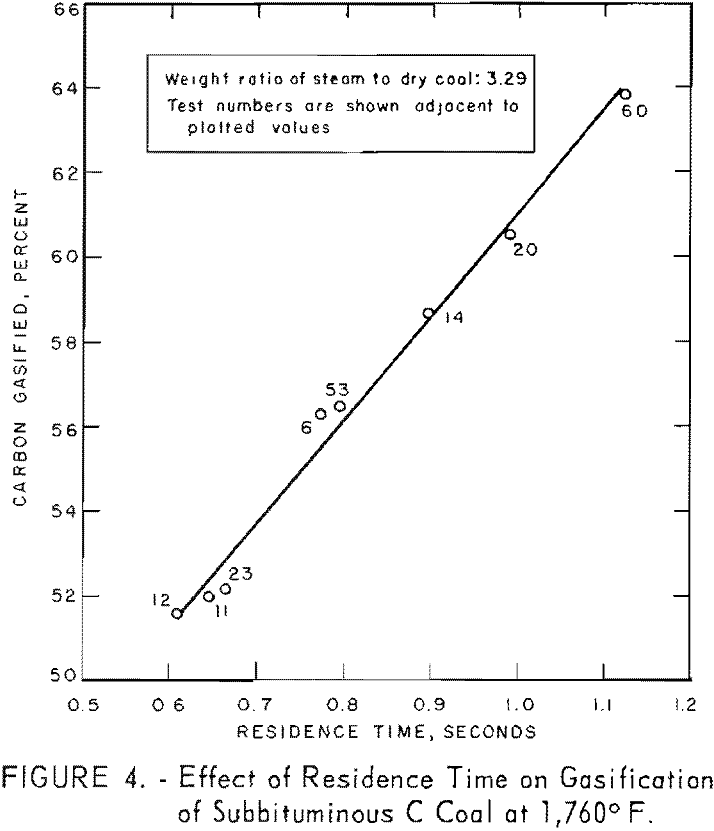
Figure 5 illustrates the combined effects of residence time and steam concentration. Although the extent of carbon conversion increased as steam- coal ratios for a given residence time were increased, the rate of conversion (25 pct./sec. carbon) was the same for all steam concentrations.
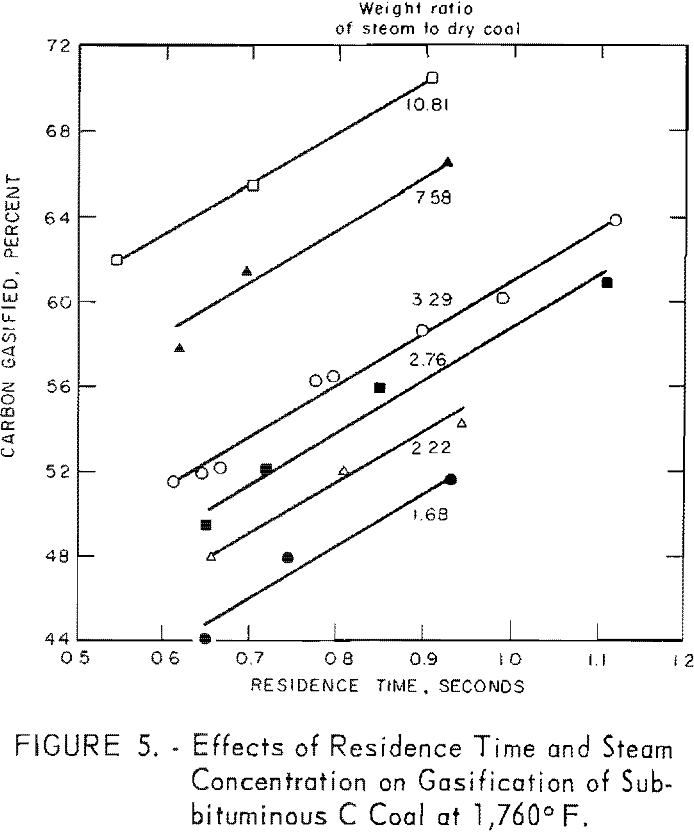
Data for one residence time, as derived from figure 5, are presented in figure 6 to illustrate the effect of steam concentration on carbon conversion, At 1,760° F., conversion increased from 51 to 70 pct. as the steam-coal ratio was increased from 1.7 to 10.8. However, figure 6 b, shows that conversion was a logarithmic function of steam concentration within the indicated limits. The lower limit of steam concentration, corresponding to 37.3 pct. solids represents the most viscous slurry that could be pumped at these low flow rates. Such limitations can be overcome in larger apparatus.
Temperature
Figure 7 shows a large in-crease in carbon conversion as temperature increases. Reaction- zone temperatures were estimated temperatures of the thermocouples in the coal-laden gas stream, as described in the appendix, and were 15° to 25° F lower than the average measured temperatures of the metal wall; the estimates are probably higher than the actual reaction temperatures. Estimates were made because rapid deterioration of bare thermocouples makes measurement of
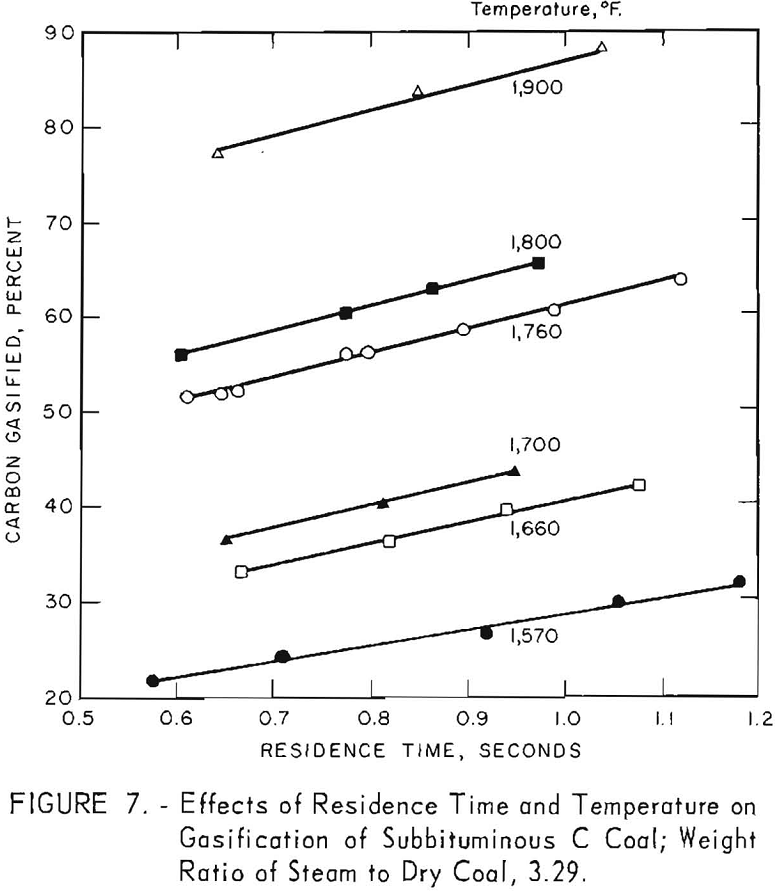
temperature of a suspension of coal in steam and gas very difficult. However, the metal-wall temperatures were well defined, being determined from 60 to 110 measurements during each run.
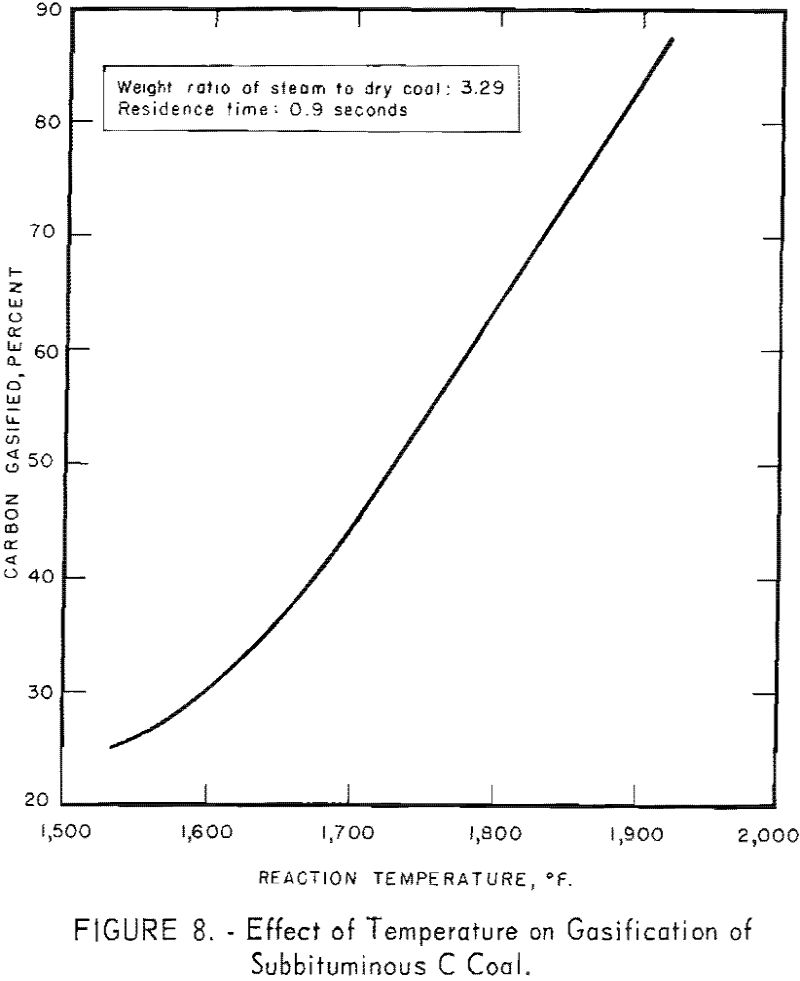
Figure 7 also indicates a possible increase in rate of conversion (the slope of each line) as temperature increases from 1,570° to 1,660° F. A similar increase in conversion rate was noted between 1,660° and 1,900° F. Figure 8 presents data for 0.9 sec. residence time, as derived from figure 7. Carbon conversion increases linearly with increasing temperature between 1,660° and 1,900° F. The curvature at the lower temperatures indicates that steam-carbon reaction begins around 1,660° F. A change in gas composition (see below) gives the same indication.
Rank of Coal
Coals of various ranks, when gasified under identical conditions, differ widely with respect to yield of gas and rate of carbon conversion
To compare ranks, three non-coking coals were gasified. (Coking coals, without pretreatment to destroy their agglutinating property, invariably plugged the preheating section of the reactor.)
Each sample was mixed with three times its weight of water, but owing to the moisture already in the coal, the weight ratios of steam to dry coal were 3.29 for subbituminous C coal, 3.87 for subbituminous B coal, and 3.13 for anthracite. Because the sum of ash and moisture was nearly equal for each coal, the ratios on a dry, ash-free basis were 3.88, 4.02, and 3.65, respectively.
Figure 9 shows that subbituminous C coal gave the highest total conversion and the highest conversion rate (slope of the curve); subbituminous B was intermediate; a negligible amount of carbon in anthracite was gasified, and that at a very low rate. For a residence time of 1 sec., conversions at 1,760° F. were 61, 38, and 4 pct. Generally, the conversion rate increased as volatile matter increased in the coal. The slopes of the lines in figure 9 indicate that the conversion rate of the subbituminous C coal was 25 pct. carbon gasified per second, that of the subbituminous B coal was 12, and that of anthracite was negligible.
Effects of Variables on Gas Composition
Table 3 shows the composition of the gas produced in each test. The values are averages of analyses of two samples collected by mercury displacement and analyzed with a precision Orsat modified for the analysis of fuel gases. Accuracy is generally better than 1 pct although in some instances nitrogen, determined by difference and representing an accumulation of errors in sampling and analysis, may be several percent in error.
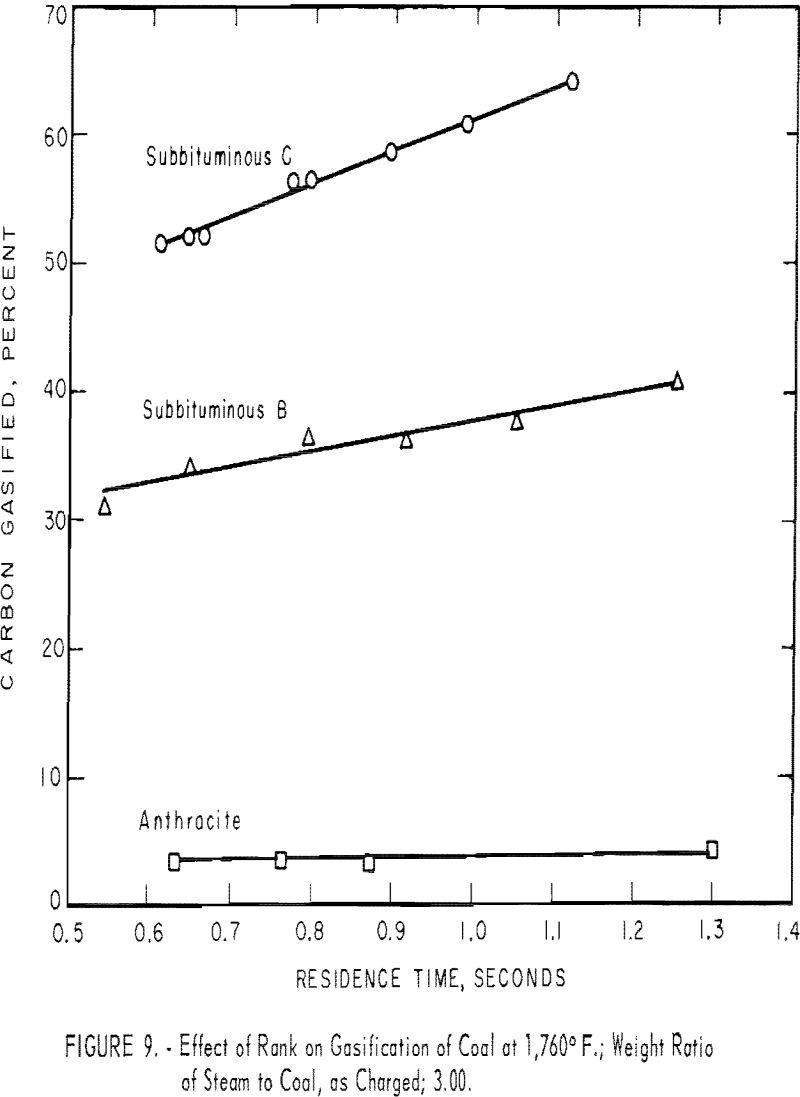
Figure 10 shows that the composition of gas, produced from gasification of subbituminous C coal at 1,760° F. with a steam-coal ratio of 3.29, remained virtually constant at residence times from 0.6 to 1.1 sec. This constancy was an expected result because adding increments to a percentage already large has little effect on the value expressed as percentage. In the second paper, discussing gasification of carbonaceous components (volatile matter and fixed carbon) in coal, the quantity of individual components of the gas per pound of coal will be plotted as a function of each variable instead of percentages. The incremental increases in each gas with residence time will then be shown.
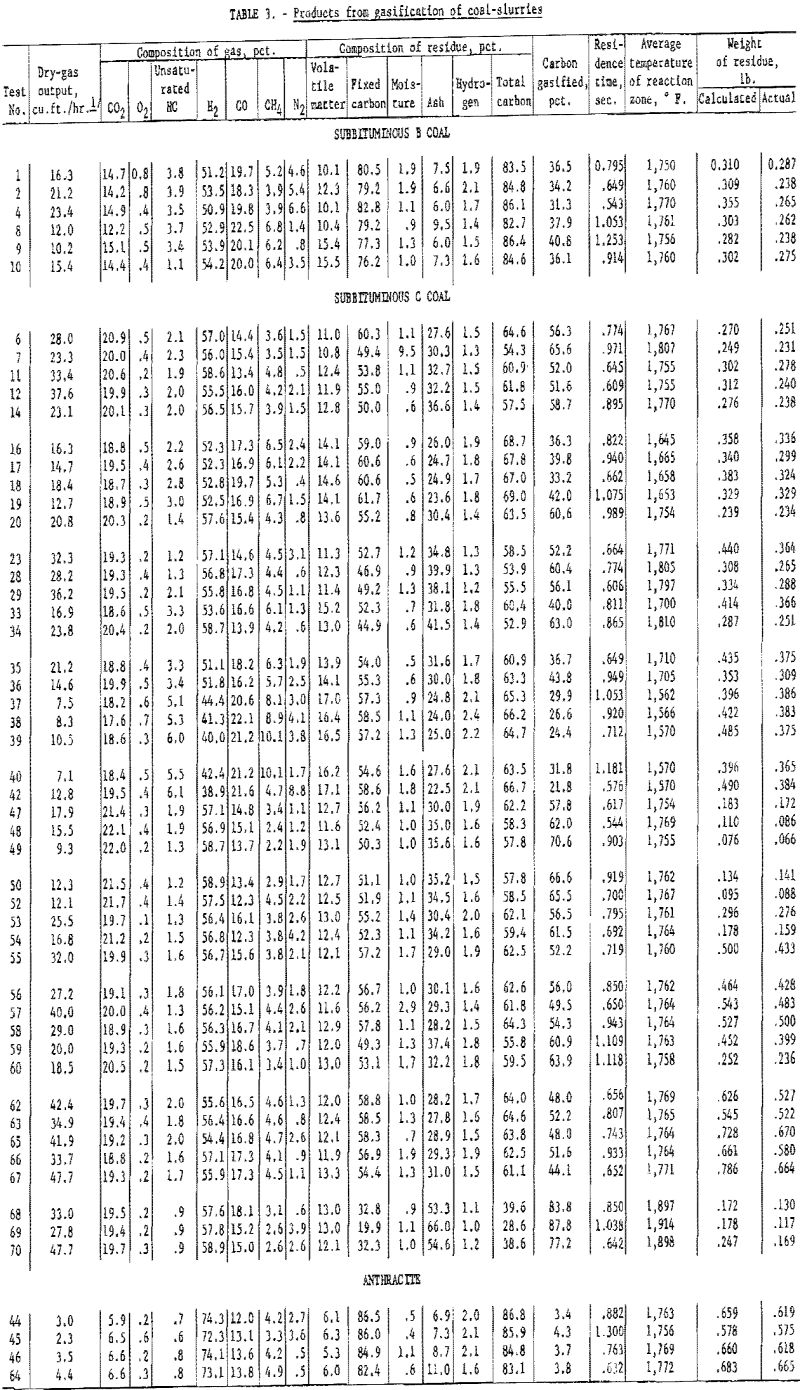
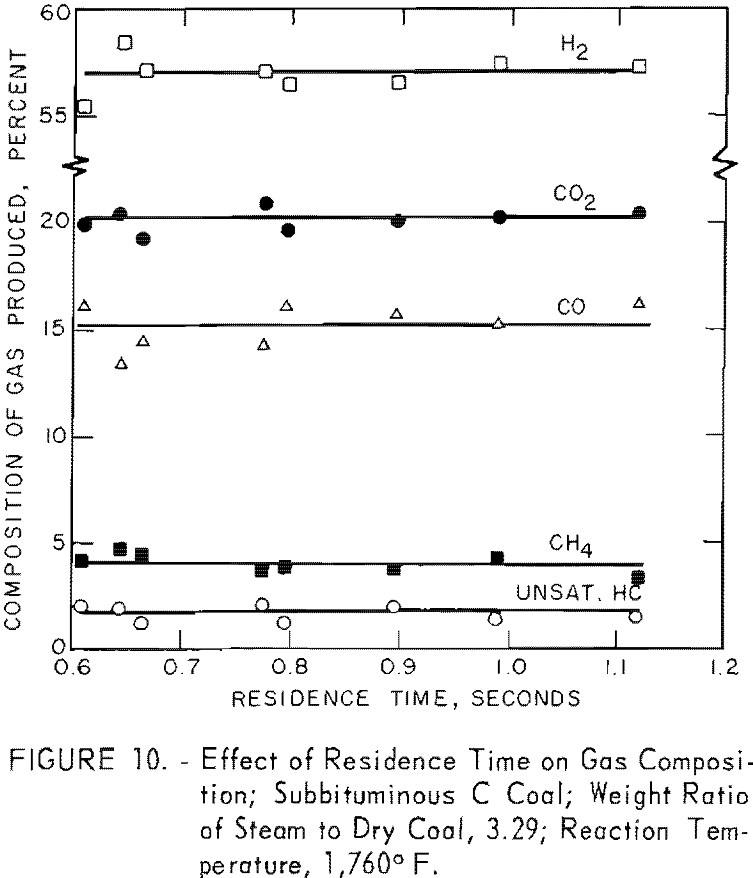
Table 4 Illustrates the variation in composition of gases produced from coals of different rank. The steam-coal ratios are close enough to assume that the comparison is made under similar experimental conditions. The two younger coals yielded similar gases as expected; however, the product from anthracite contained considerably more H2 and less CO2. This composition results from the lower reactivity of anthracite and indicates that the gas resulted largely from thermal decomposition.
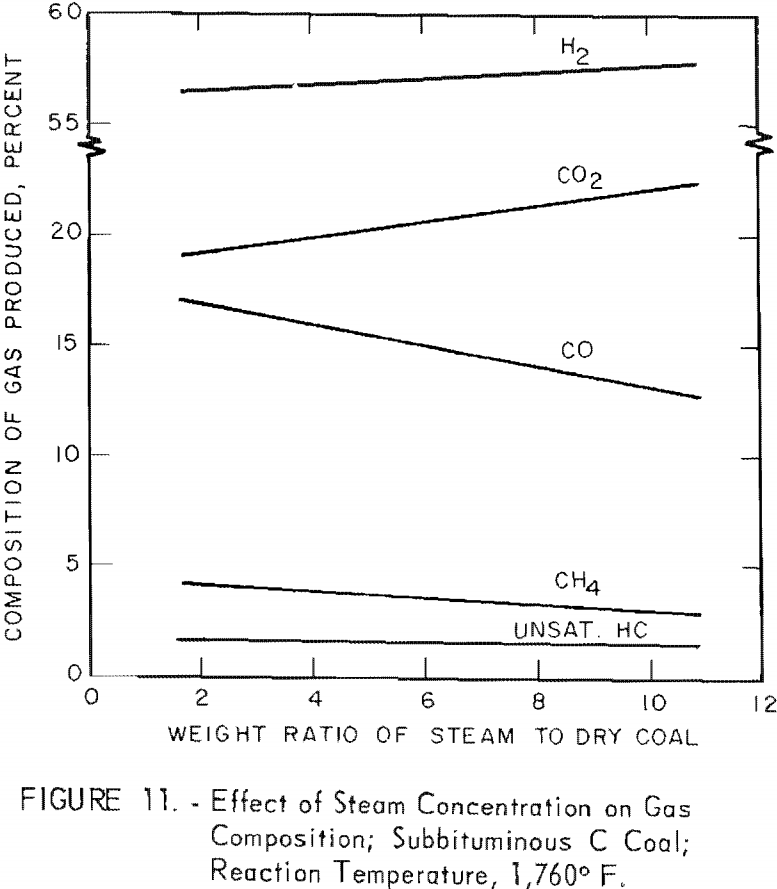
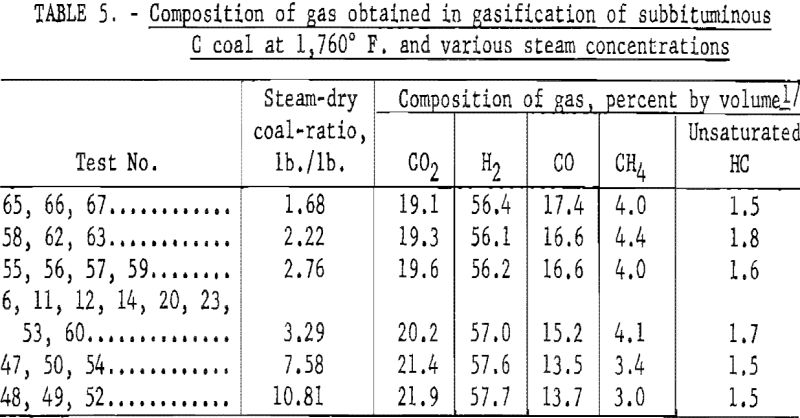
Figure 11 shows the effect of steam concentration on the composition of gas from a subbituminous C coal (see also table 5): Linear increases in H2 and CO2 were offset by linear decrease in CH4 and CO with increased steam concentration. Hence some of the CO from the steam-carbon reaction reacted with steam to form H2 and CO2. However, over a narrow range of steam concentrations, excess steam has little effect on the composition of the product gas. Slurries containing 30 and 40 pct. solids would be expected to produce essentially the same gas.
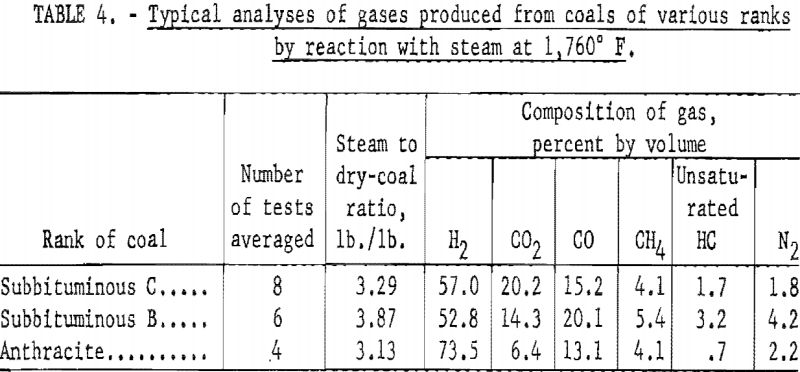
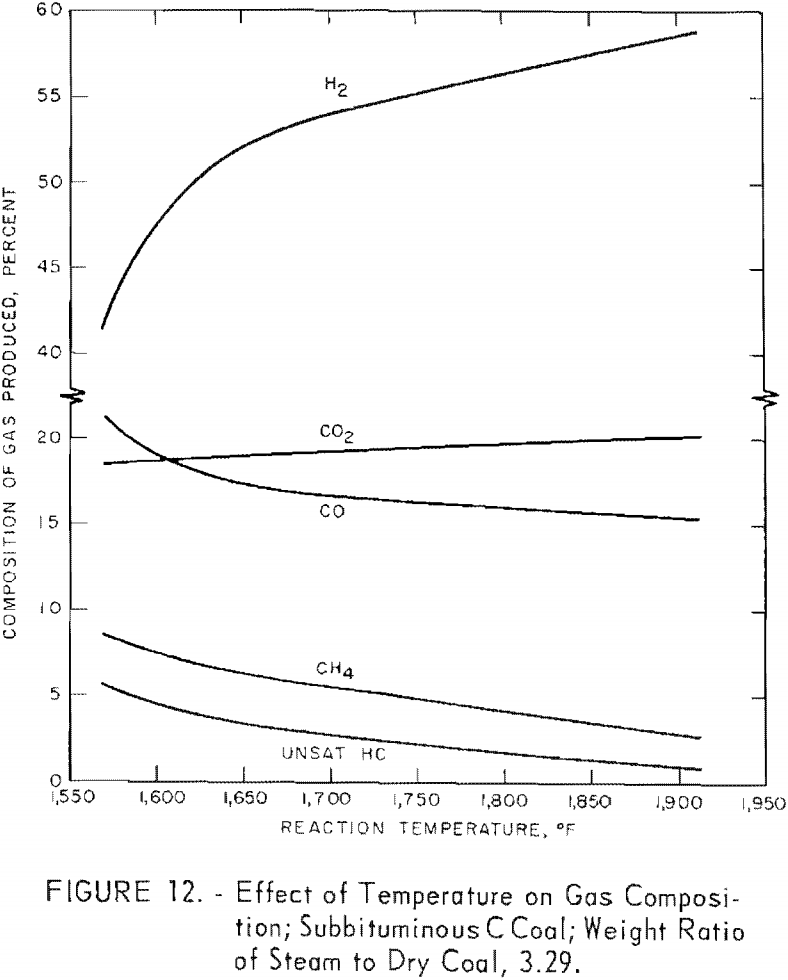
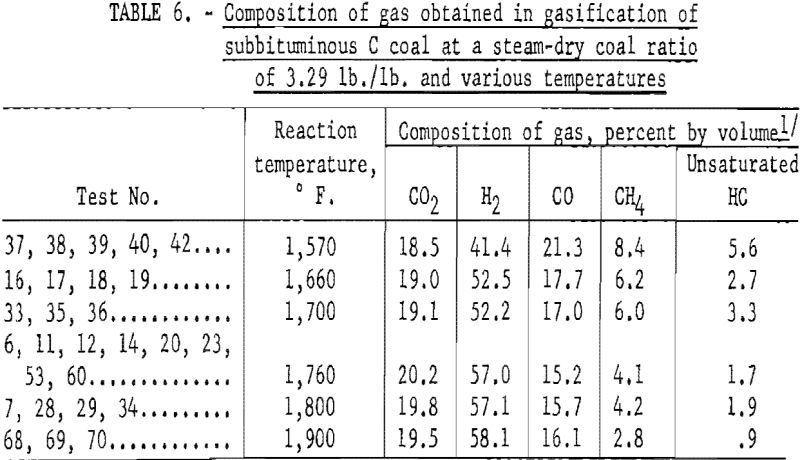
Figure 12 shows the effect of temperature on the average composition of gas from tests on subbituminous C coal at different residence times at each temperature (table 6); the steam-coal ratio was 3.29. Hydrogen and CO2 increased, and the other components decreased as the temperature increased. An inflection in most of the curves at about 1,660° F. probably indicates the start of the steam-carbon reaction; a similar indication was noted in figure 8, which showed the effect of temperature on carbon conversion.