The dissolution behavior of copper and gold from their pure state and from Au/Cu alloys in cyanide solutions has been investigated using a rotating disc. Oxygen was used as an oxidant. The effect of the composition of the alloys, stirring speed of the disc, concentration of lixiviants and temperature on the overall dissolution kinetics has been studied.
Theoretical Background
As a noble metal, gold remains unattacked by most aqueous solutions. However, gold can readily be dissolved in cyanide solution as a cyanide complex, Au(CN)2. The dissolution of gold in cyanide solution is an electrochemical process, as described by Equations 1, 2 and 3:
4Au + 8CN- = 4Au(CN)2- + 4e………………………………………………………….(1)
O2 + 2H2O + 4e = 4OH-…………………………………………………………………..(2)
4Au + 8CN- + O2 + 2H2O = 4Au(CN)2- + 4OH-………………………………….(3)
4Cu + 12CN- = 4Cu(CN)3²- + 4e…………………………………………….(4)
O2 + 2H2O + 4e = 4OH-………………………………………………………..(5)
4Cu + 12CN- + O2 + 2H2O = 4Cu(CN)3²- + 4OH-…………………………………..(6)
The dissolution of gold and copper alloys in aerated cyanide solution can be described in the same manner as the individual metals:
2AuCu + 10CN- + O2 + 2H2O = 2Au(CN)2- + 2Cu(CN)3²- + 4OH-………………………………(7)
AuCu3 + 11 CN- + O2 + 2H2O = Au(CN)2- + 3Cu(CN)3²- + 4OH-…………………………….(8)
Au3Cu +9CN-+ O2 +2H2O = 3Au(CN)2- + Cu(CN)3²- + 4OH-…………………………………..(9)
Experimental
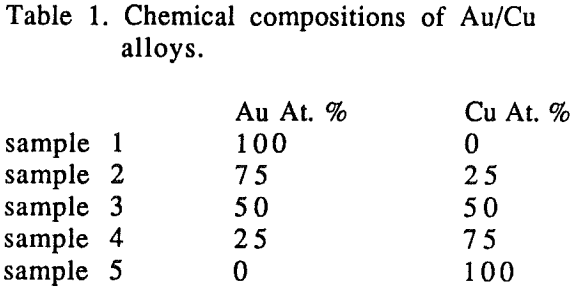
Batch-leaching experiments were carried out in a 2000 ml pyrex vessel using a 0.95 inch diameter disc of Au/Cu alloy. Leaching solutions were prepared by dissolving a known amount of sodium cyanide in distilled water. The pH of the solution was adjusted by addition of sodium hydroxide.
Results and Discussion
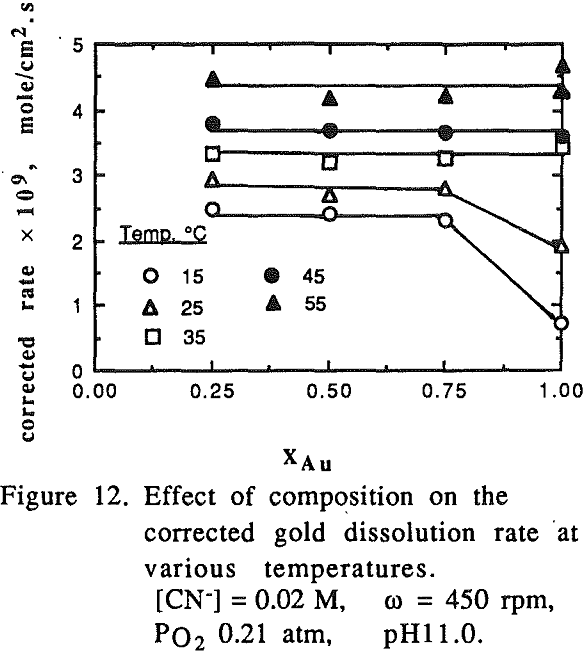
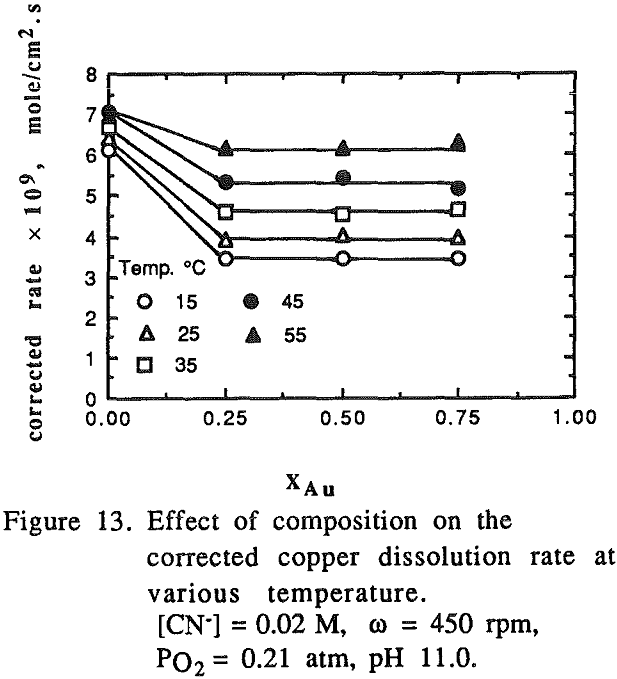
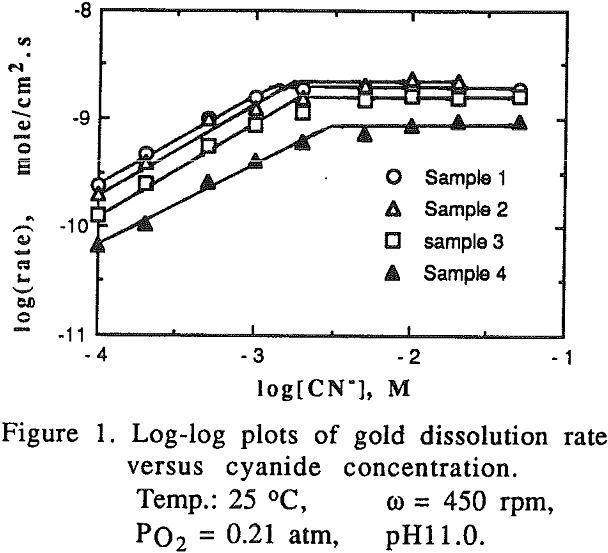
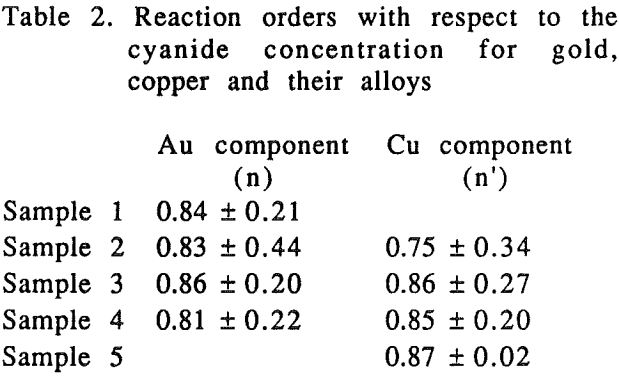
The effect of cyanide concentration on the dissolution of gold and copper from their pure metals and their alloys was investigated. As seen from these figures, the dissolution rate increased linearly with the cyanide concentration until the maximum rate was reached, above which the rate was independent of the cyanide concentration.
There was no preferential dissolution observed for the alloys. The amount of gold and copper dissolved from the alloys was directly proportional to the volume percentage of those metals in the alloys.
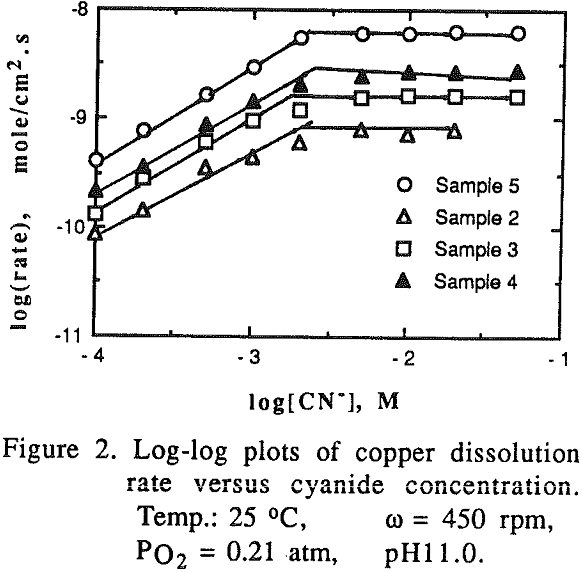
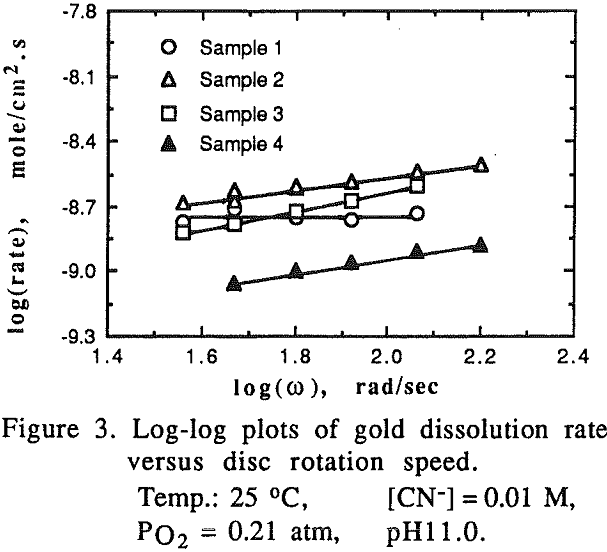
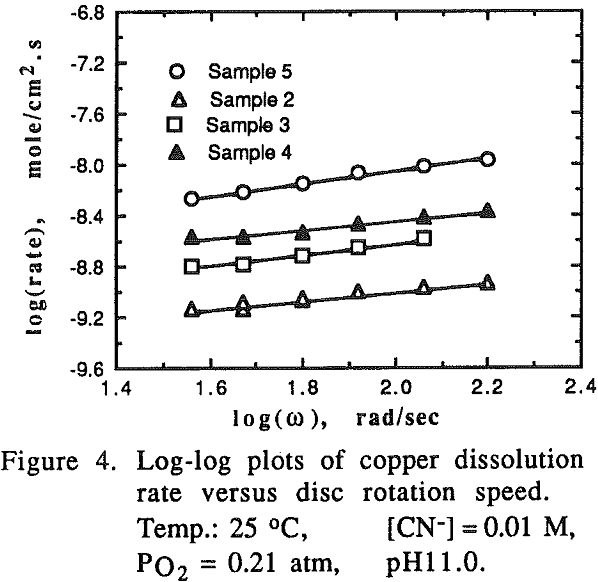
The effect of oxygen partial pressure on the dissolution rate was studied over the range of 0.105 to 1.0 atm PO2 with other experimental conditions being constant.
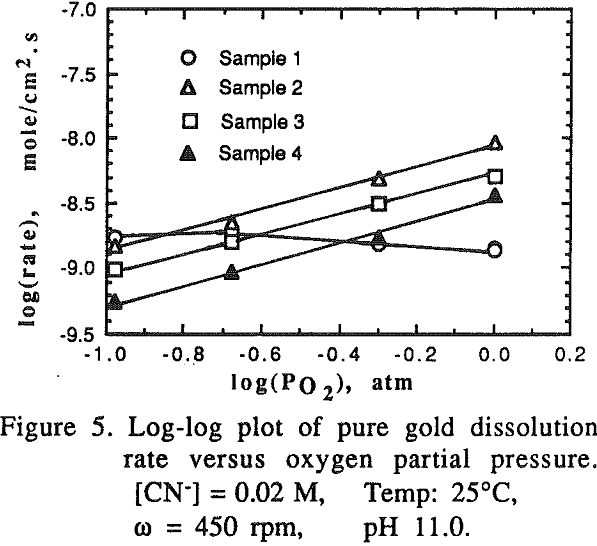
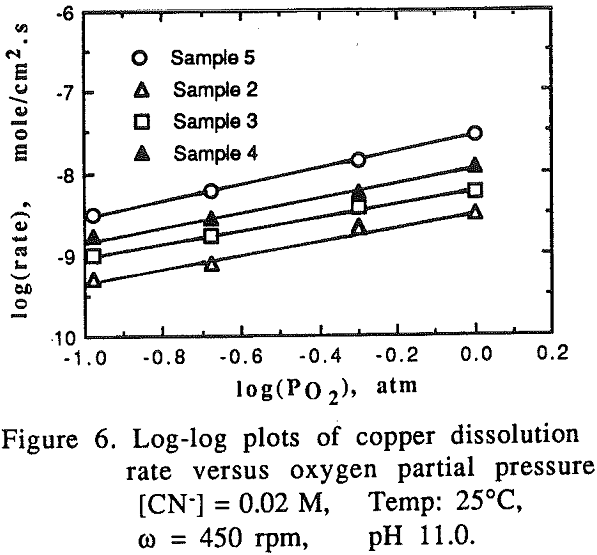
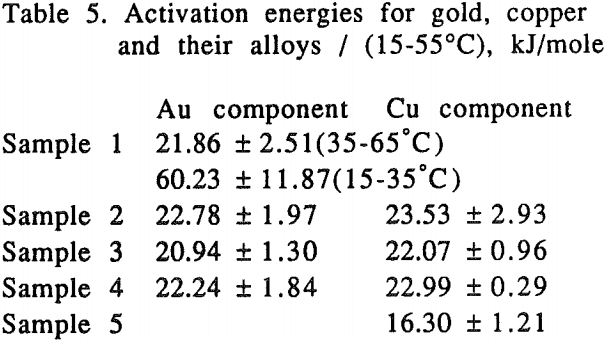
For pure gold, it appears that two mechanisms are responsible for the temperature range studied. At low temperatures (15-35°C), the activation energy for gold was found to be 60.23 kJ/mole (14.41 kcal/mole) which is a typical value for processes controlled by chemical reaction.
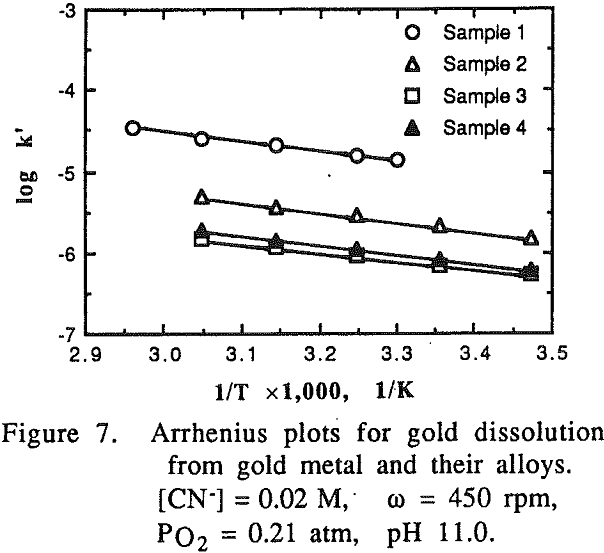
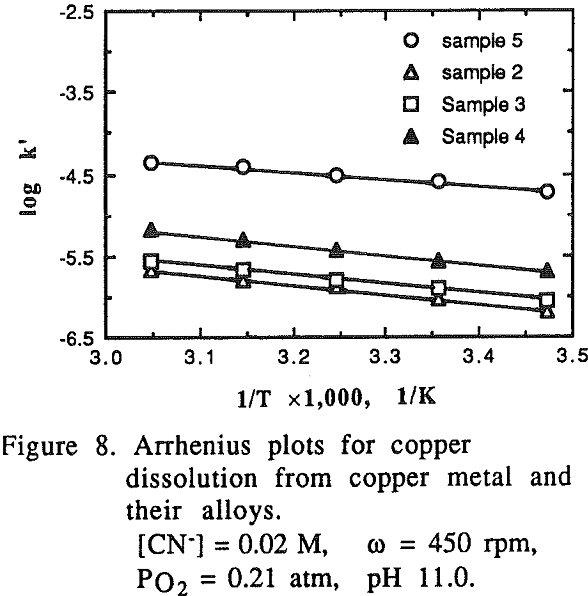
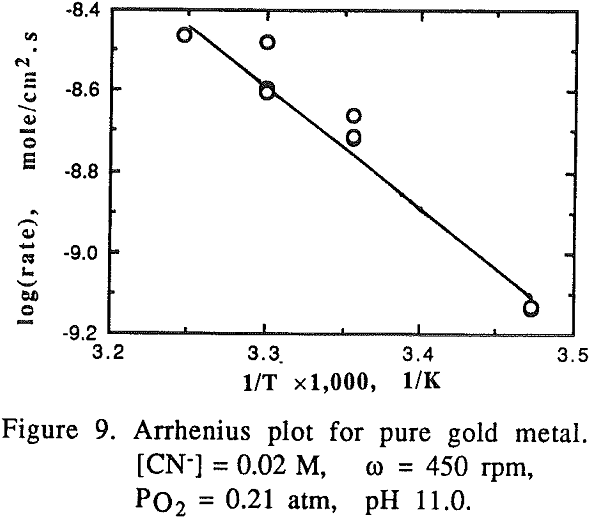
It was noted that at high temperatures no change on the gold surface was observed during the dissolution, while at low temperatures changes on the gold surface were observed during the dissolution. The gold surface became rougher and the color was different from the original gold at low temperature. The phenomena observed may be attributed to the formation of a passive film on the gold surface at low temperatures.
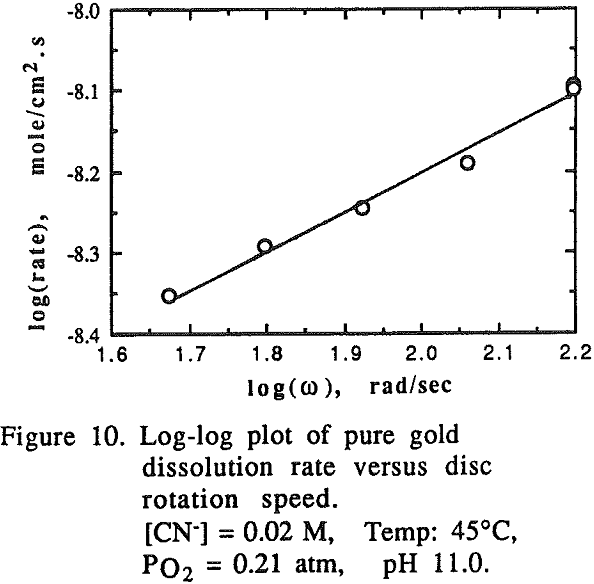
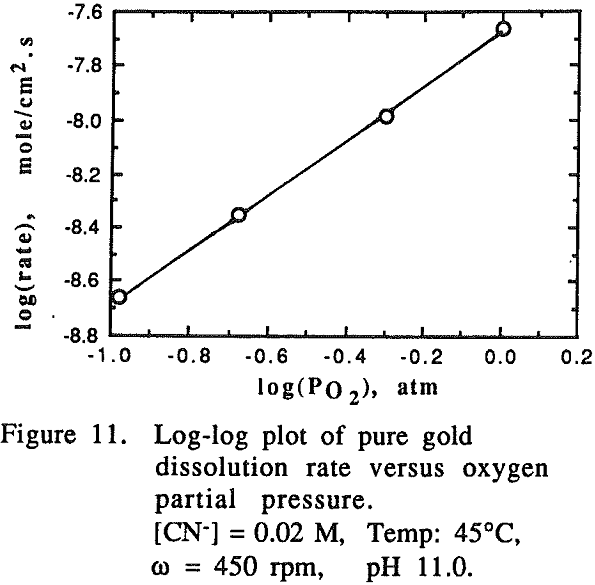
A direct comparison of the dissolution rate of an individual element from an alloy system with that of a pure metal system cannot be made. This is because the number of atoms per unit area in the alloy system is different from pure metal due to the difference in their composition. Therefore, it is necessary to correct the rate of dissolution of the element in question in view of the number of atoms per unit surface area (or per disc for the same dimensional discs).