The Relation Between the “ Effective Hardness ” of Corundum and its Content of Water: A specimen of corundum from Acworth, Ga., which was reputed to be of markedly inferior quality for the manufacture of corundum-wheels, was received by the Geological Survey of Georgia, with the request that it be analyzed, in order to ascertain whether this inferiority was associated with any peculiarity of composition. In its appearance there was nothing uncommon, though it seemed to be rather easily scratched with a knife, while, at the same time, it scratched agate easily. An analysis gave the following results:
- Alumina, 94.58;
- silica, 1.77;
- magnetic oxide of iron, 0.69;
- calcium oxide, 0.44; and
- water, 2.51 per cent.
There is nothing, unusual in this composition, except the amount of water, which is usually, in southern corundum at least, from 0.3 to about 1 per cent.
Concerning this corundum, the Mineral Resources of the United States, pp. 676 and 677, says: “It is supposed that this variety of corundum contains a little water, as it is somewhat less hard and more easily cleavable than the common variety known as sand-corundum.”
A similar opinion, that the presence of water indicates softness, is expressed in a private communication by Dr. T. M. Chatard, who says : “ If the corundum gives more than 1 per cent, of water on heating to redness, I should think its abrasive quality doubtful.”
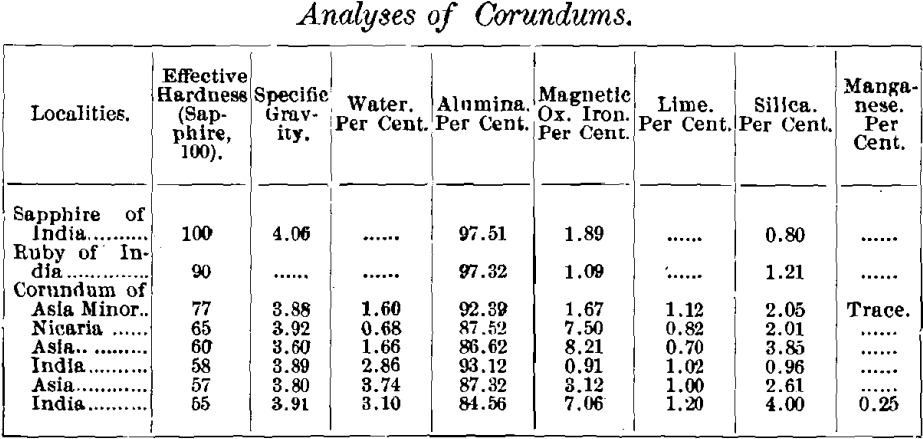
Also, Dr. J. Lawrence Smith, in connection with the table of his results, given below, says: “ And it would appear that, other things equal, those containing the least water are the hardest.”
In view of the prevalent opinion that water indicates softness, and at the suggestion of Dr. David T. Day, of the U. S. Geological Survey, this investigation was undertaken, to ascertain if any relation could be traced between the amount of water and the abrasive efficiency of corundums.
At the present time, the only recognized method of determining abrasive efficiency is that of Dr. J. Lawrence Smith, described in the Am. Jour. of Science and Arts. It consists in grinding a weighed amount of the mineral to an impalpable powder, on a weighed glass plate, with an agate surface. The loss of weight of the glass is considered a measure of the efficiency of the corundum, or, as termed by Dr. Smith, the “ effective hardness.” In the same journal, he gives complete analyses of several corundums the effective hardness of which he had also determined. These analyses are as follows :
The method of determining the water was to weigh the corundum into a platinum crucible, completely surround the latter, with quartz powder in an earthen crucible, and heat it in a furnace to bright redness for from half an hour to an hour. This method does not preclude the possibility of reducing-gases gaining access to the mineral, and thus, to a greater or less extent, reducing the peroxide of iron, a reaction which may perhaps account for discrepancies in the results.
It appeared advisable, therefore, to make the determination of water directly, by driving it off in a combustion-furnace, and catching it in a weighed calcium-chloride tube. The corundums for the test were kindly furnished by Mr. Francis P. King, then Assistant Geologist of the Georgia Survey. They were carefully freed from decomposition-products and gangue, then pulverized, as described in Dr. Smith’s paper, in a diamond mortar, by striking a few heavy blows, then shaking out the powder through an 80-mesh sieve and repeating until enough had passed through the sieve for the purposes of the test. The powder was freed from iron by very dilute nitric acid, washed, dried at 100° C., and placed in weighing-bottles.
In determining the water, a gas-combustion furnace was used, with a porcelain tube glazed inside and out. A weighed amount of the mineral was heated in a platinum boat for one hour, with a slow current of dried air passing over it, under the pressure of a constant head of water. At the expiration of this time the boat was transferred to a desiccator, and the calcium-chloride tube to the balance, and, when cool, was weighed. As soon as the boat had been weighed, the corundum was transferred to a platinum crucible and heated for five minutes with the blast-lamp, care being taken to avoid any reduction of per-oxide of iron. There was always a slight loss of weight on heating with the blast-lamp, which indicated that the temperature of the combustion-furnace was not sufficient to remove the water completely. That this was the case, was evidenced by heating some corundum for one hour in a porcelain crucible with a Bunsen burner, then determining the loss of weight, and then heating with the blast-lamp (when a further slight loss was observed), and, finally, in a platinum crucible, without further loss of weight.
The tests extended through three days, and on each day a blank charge was run, to ascertain whether the air was completely freed from moisture. There was always a slight gain in weight of the calcium chloride, amounting to 0.0015, 0.0012 and 0.0016 grammes on the first, second and third days respectively. The average of these, 0.0014 grammes, was subtracted from the gain in weight of the calcium-chloride tube in all cases. The following results were obtained :
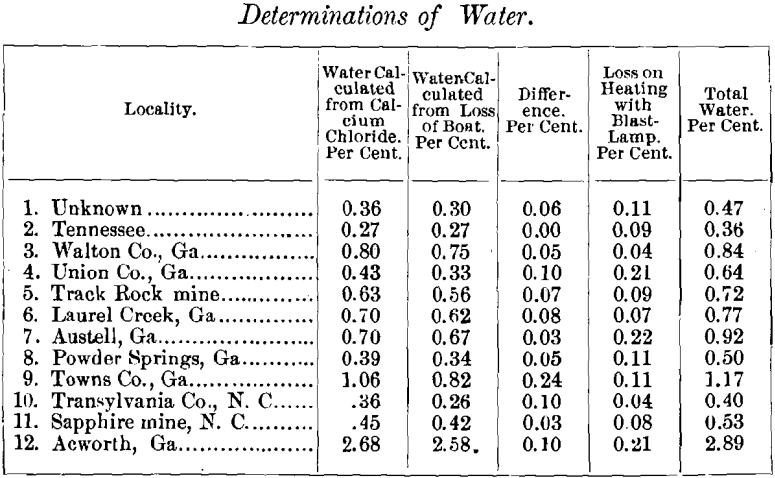
The water driven off by the blast-lamp in Nos. 1 and 9 was not determined; the average of the other determinations was taken for these. The last column is the sum of the gain in weight of the calcium-chloride tube, and the loss on heating with the blast-lamp.
It will be noticed that the gain in weight of the calcium chloride exceeds the loss in weight of the boat in every case, except No. 2, in which they are the same. The average difference is 0.07; and in only one case does it exceed 0.1 per cent.
In making the tests for effective hardness, it was found impracticable to pulverize the mineral until it ceased to abrade the glass; and the following procedure was adopted, after some preliminary tests, which showed that any further pulverization would not change the relative values appreciably :
Of the original sample, 0.7 gramme was weighed and divided roughly into four equal parts; and each part was pulverized for twenty minutes on a weighed glass plate, with the bottom of a small agate mortar which had been fitted with a handle. The plate was then washed and weighed, and its loss in weight, divided by the weight of the corundum, was taken as the effective hardness.
A separate plate was used for each corundum, though all the plates were cut from the same sheet. This was believed to be better than to use the same plate for all, as the increasing concavity of the glass, together with its possible variation in hardness at different depths, would probably cause as much error as could arise from variations in different pieces.
Repetitions of the test with the same corundum indicated that the resultant values were occasionally liable to vary as much as 10 per cent.
The results obtained, arranged in the order of their efficiency and compared with the percentage of water, are as follows:
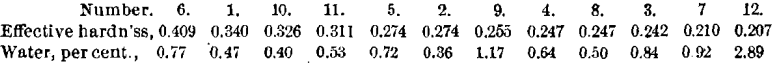
From the above table it will be seen that No. 8, among the lowest in effective hardness, is among the lowest in water, and that No. 6, much the highest in effective hardness, is among the highest in water. Nor is there, in the other cases, much indication that low effective hardness is closely connected with a high percentage of water, or vice versa. At the same time it will be observed that, of the four samples highest in water, three occupy the lowest places in effective hardness, and that, of the four lowest in water, three occupy the highest places (save one) in efficiency.
The conclusion to be drawn from the above results appears to be that, while there is some relation between the amount of water and the effective hardness, it is not intimate enough to permit the estimation of one from the other, though it would appear, from Dr. Smith’s results and those given above, that where water is very high, say over 2 per cent., the effective hardness is likely to be very low.
It was thought worth while to test the effective hardness of the corundums after they had been deprived of their water, to ascertain whether heating had affected this property.
The following are the values thus determined, before and after heating :
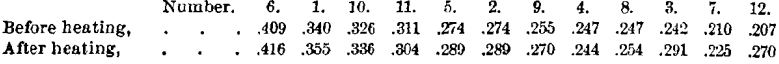
It will be noticed that the values after heating are higher in all cases, excepting Nos. 11 and 4. The differences are nearly all within the limits of possible error. But from the fact that they are nearly all in the same direction, it seems probable that heating to a high temperature slightly increases the effective hardness.
Smith’s Test as a Means of Determining the Abrasive Efficiency of Corundum
It has been already observed that Smith’s test is the only recognized method of testing corundum for abrasive purposes. But while the method unquestionably gives the relative values of corundums used in the loose state, its accuracy where the corundum is fixed, as in a wheel, has been called in question. Mr. T. Dunkin Paret, President of the Tanite Company, in an elaborate article in the Journal of the Franklin Institute, Nos. 5 and 6, points out the differences between the conditions of Smith’s test and those of the ordinary methods of using abrasives, and contends that Smith’s test is valueless as a means of testing emery for such purposes. Dr. H. S. Lucas, the well-known manufacturer of corundum-wheels, in a private communication expresses a similar opinion as to corundum.
There seems to be good ground for doubt, at least, on this point, since in Smith’s test the corundum is pulverized by crushing, while, if it be fixed, the force is applied in an entirely different way, and the relation between the resistances to stresses so differently applied may not be the same in different corundums. At the same time, no experimental evidence having been presented to support the objections thus urged, it seemed desirable to determine by experiment whether or not different corundums would give the same relative values with Smith’s method and with a method in which the corundum was tested in a fixed form. The following is an outline of the method of testing adopted for this purpose:
A shallow brass box was fitted to the top of the rotating spindle of a centrifugal machine so that the bottom should be horizontal. A steel plate ¼ inch thick and 5 inches in diameter was placed in this and wedged tight. The test-piece of corundum, a small cylinder 5/8 inch in diameter and about 0.8 inch long, made by cementing corundum-powder, was pressed upon the surface of the plate with a weight of 3.25 pounds, and the plate was rotated with a speed of 300 revolutions per minute. The test-piece was moved at intervals, so as to grind nearly the whole surface of the plate. At the expiration of a certain time the plate and test-piece were weighed, and the loss of weight of the plate, divided by the loss of weight of the test-piece, was taken as the efficiency of the corundum.
The selection of a satisfactory cement proved to be a matter of considerable difficulty. As a criterion of the effectiveness of the cement, a piece of emery-wheel, supposed to have been a “ vitrified ” wheel, was ground down to the same size as the test-pieces, and compared with them. In most cases, it was found that the cement did not hold the particles of corundum firmly enough to permit their being torn to pieces, but rather that the grains were torn from the cement, without much comminution. Finally a cement was prepared, which, with the corundum used, gave an efficiency about twice that of the emery-wheel test-piece, and was consequently employed in the experiments.
The materials of this cement were water-glass and a strong solution of the mixed chlorides of calcium, magnesium and iron. The water-glass contained 10.2 per cent, of sodium oxide and 27.4 of silica, making a 37.6 per cent, solution of, approximately, Na2O, 3SiO2. The solution of the chlorides contained 40 grammes of ferric chloride, 35.5 of calcium-chloride, and 42.6 of magnesium chloride in 200 c.c. of the solution. This solution contains about equal weights of the three metals, and does not, in standing, appreciably evaporate or take up moisture from the air.
All the test-pieces were prepared, as nearly as possible, at the same time, as follows :
The corundum, selected nearly free from decomposition-products and gangue, was powdered in a steel mortar, then passed through a sieve of 80 meshes to the inch, and caught on one of 100 meshes. Of this powder, 10 grammes was weighed into a No. 16 paper shell, which served as a mould, the cap-hole being closed by a cork. To 3 c.c. of the chlorides 5 c.c. of the water-glass solution was added, and the mixture was ground in a mortar till thoroughly incorporated, and then poured into the shell on the corundum, and mixed with it by means of a wire. The corundum was then shaken down by tapping the bottom of the shell, and the excess of the cement was drawn off with a glass tube. A piece of bolting-cloth was then laid on the end of the shell, and forced down with a perforated cork. The corundum was thus compacted by pressure on the cork, the bolting-cloth permitting the excess of cement to pass, but retaining the corundum. The shells were next put in a water-oven over night, then heated for one hour in an air-bath to 130° C., and then for half an hour at 230° to 240°. The moulds, which had partially charred, were trimmed off with a knife; care being necessary, as the pieces were fragile. They were next wrapped in platinum-foil to prevent sticking, and heated in a sand-crucible to the full heat of a brass-furnace for two or three hours. The test-pieces, at this point, were fairly coherent, but not sufficiently so ; and after being ground down to the proper size for the holder, they were placed in a water-glass solution to which a little water had been added (1 c.c. of water to 12 c.c. of the solution), under the receiver of an air-pump (the air being exhausted); then exposed to atmospheric pressure for half an hour, and then put under the air-pump again. Finally, they were again heated in the brass-furnace as before, and were then strongly coherent.
It is, very likely, not necessary to use water-glass and chlorides of this particular strength ; and it may not be necessary to use all three chlorides, or to apply them in this particular way. But in the preliminary experiments it was found that a test-piece made with water-glass alone was not satisfactory. Such a test-piece was then placed in calcium-chloride solution in an exhausted air-receiver, removed and heated, and was still unsatisfactory. A similar experiment was made with ferric chloride, with like result. This test-piece was next placed in water-glass solution and then heated, but without improvement. It was finally placed in a solution of the mixed chlorides of calcium and magnesium, heated and found to be very coherent, and for this reason the three chlorides were used. In the preliminary experiments, an excellent test-piece was made at a single heat by incorporating the water-glass and chloride solutions, mixing this compound with the corundum and heating ; but for some, reason the favorable conditions could not be hit upon again.
The apparatus used in testing is shown in Fig. 1. Rotation was imparted by means of a centrifugal machine, a, such as is used in physical laboratories, to which a brass plate, b, was secured by means of a stem perpendicular to its surface, so that it would be horizontal and remain so while turning. Brass strips, c, were screwed to the upper surface of the plate, so as to receive and hold firmly a shallow brass box, d, about 1 inch deep and 5 inches square, which received the steel plate, e, to be ground by the test-piece. The steel plate was ¼ inch thick and 5 inches square, with corners cut off, and a hole in the center 1.5 inch in diameter. It was fitted close in the box and wedged tight. The holder for the test-piece was screwed to the machine by means of a heavy wooden piece, f, passing underneath. To this a strong upright, g, was firmly secured, from
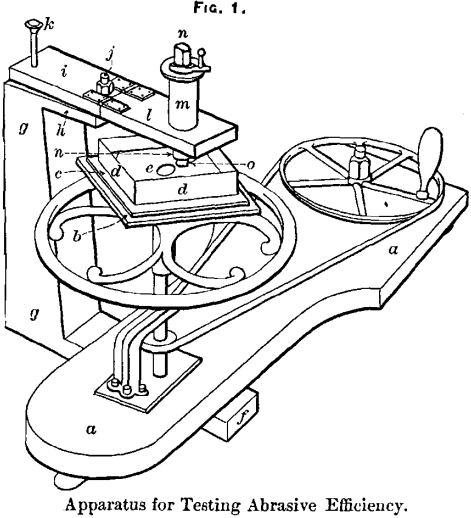
the upper end of which a broad arm, h, projected out to, and slightly above, the box. Upon this arm, another, i, of the same size, was placed and held by a bolt, j, passing tightly up through both pieces, with a thread at the upper end to receive a nut, by which the two pieces might be clamped together, or loosened to permit rotation of the upper piece about the bolt as an axis. By means of a pin, k, passing through both of these, the upper piece could be fixed in any one of three positions. To the upper piece, a similar piece, l, projecting over the box, was secured by two strong strap-hinges. A brass cylinder, m, about 1.5 inch in diameter, passed through this piece with vertical axis, and was secured to it by screws through a flange. A hole 0.75 inch in diameter was bored through the center of the cylinder and provided with a screw-thread of 102 revolutions to the inch, into which the holder proper screwed. This consisted of a cylinder, n, passing through the larger cylinder, and projecting an inch or so at either end. The upper end was squared to receive a small handle, by which to turn the screw.
The handle could be fixed in any of four positions by means of a pin passing through a hole in it and into corresponding holes, 90° apart, on the upper end of the large cylinder. The lower end of the small cylinder was hollowed out to the depth of an inch to receive the test-piece, o, and was slit through on one side. The test-piece, with one or more turns of paper around it, fitted tightly into the holder, and could be further tightened by a nut on its outer surface.
The brass cylinders and the wooden piece to which they were screwed weighed together about 3.25 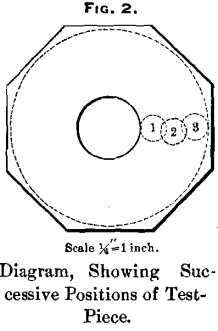 pounds. The material of the plate was a soft steel, as it was found that with hardened steel, under the weight applied, the test-piece would become glazed and would almost cease to be abraded. A similar result was obtained with glass. On the other hand, a copper plate wore down the test-piece very rapidly; but steel was used, because it was thought that its action was somewhat more uniform under the given weight.
pounds. The material of the plate was a soft steel, as it was found that with hardened steel, under the weight applied, the test-piece would become glazed and would almost cease to be abraded. A similar result was obtained with glass. On the other hand, a copper plate wore down the test-piece very rapidly; but steel was used, because it was thought that its action was somewhat more uniform under the given weight.
It may be observed here, as possibly of significance, that the plate, as a whole, became, during the test, never hot, but only slightly warm to the hand—I should say, on a guess, 40° to 50° C.
In making the tests, pieces of round cardboard were first placed in the holder, so as to cause the selected test-piece to project about 1/8 inch, and the test-piece was then forced firmly down upon the cardboard and the nut was tightened. As a further precaution, to prevent the test-piece from turning upon its axis, the inside of the holder was roughened by scratching it with a knife. The test-piece was then lowered upon the steel plate in the first or inner position (see Fig. 2) and adjusted perpendicular to the surface, and the plate was rotated at a speed of 300 revolutions per minute. At the expiration of a minute the test-piece was turned through one-fourth of a revolution, so as to dislodge pieces of steel which might tend to clog it, and, as was feared, to abrade the plate without corresponding abrasion of the test-piece. This was continued until a revolution had been completed, when the test-piece was slightly out of the vertical position, owing to the fact that the screw lowered it somewhat faster than it wore away. It was therefore turned backwards, so as to keep it nearly perpendicular to the surface. When the test-piece had been run for fifteen minutes in the first position it was moved to the second or middle position, where it was run for twenty minutes, and finally to the third or outer position, where it was run for twenty-five minutes. In this way the ground surface of the plate was kept nearly level. The steel plate was next washed, dried and weighed, and the test-piece was removed, brushed and weighed. The loss of the weight of the plate divided by the loss in weight of the test-piece gave the efficiency.
Duplicate test-pieces had been prepared from five corundums and one emery, and also a single test-piece from another corundum, of which there was hardly enough for one test, 7 grammes being used instead of 10. They were numbered as follows : 1 and 1a, location unknown; 2 and 2a, Acworth, Ga.; 3 and 3a, Iredell Co., N. C.; 4 and 4a, Macon Co., N. C.; 5 and 5a, near Sapphire mine, N. C.; 6 and 6a, Chester emery; 7, Laurel Creek, Rabun Co., Ga.; 8, test-piece made from a piece of emery-wheel.
On testing these it was found that the same test-piece, while tending to a particular value, was liable to give, in successive tests, results varying too widely to make a single-hour test reliable. It was determined, therefore, to get an average value by extending the test over a period of six hours, which could be accomplished in one day. The plate was washed, weighed and reversed at the expiration of each hour. The stick was also weighed and moved down in the holder as it wore away, so as to allow a variation of not more than 0.01 inch in the amount of its projection from the holder. The plate was likewise raised as it wore down.
The pieces were tested in the following order, with the results given:
On comparing the results of each pair, it will be seen that the later result is invariably higher. It was thought that perhaps some change was in progress, increasing the efficiency. On the following day, therefore, Nos. 1 and 1a were alternated. No. 1 gave an efficiency of 19.3 and No. 1a gave 18.4, which confirmed this opinion. The increase, it will be observed, is somewhat irregular, though it averaged about 5 per cent, a day.
As it could not be assumed that this change was constant, it now became necessary to alternate the test-pieces; and, as the plate was becoming thin, it was thought advisable not to use more than three. So 1 and 1a, 2 and 2a, 3 and 3a were chosen. These were alternated over a period of two days, which gave only two hours to each test-piece. The results are shown in Table II.
The plate had now become somewhat less than 1/32-inch thick, and buckled so as to be unreliable. It had been about 1/6 of an inch thick when the tests in Table I. began, and had worn away about 0.01 inch per day.
As the plate became quite thin, the change in the efficiency
became more rapid. The test-pieces altogether in Table II. gave :

It had been observed that the exterior of the test-pieces was much more efficient than the portions just inside, though it was believed that this variation did not continue to the center, because the hard exterior abraded much less steel per hour than the softer inside, and if this variation continued, the amount of steel abraded per hour in Table II. should be greater than that abraded in Table I. by the same corundums, while in fact the difference is insignificant. As, however, this point was somewhat uncertain, the test-pieces were always ground down about the same amount before comparing them. The change in the efficiency noted above could hardly have been due to any change in the test-pieces, as the manner of testing would indicate. This was proved by alternating Nos. 1 and 1a, for half an hour each, on another plate, B, which had been worn down to the same thickness as that of the first plate, A, when the tests recorded in Table I. were begun. The results were :

The efficiency was less than half the value, 24.6, found on the former plate, A, when nearly worn away, and near the value, 12.4, found on it at a corresponding thickness.
The tests which follow were made in a slightly different manner from those above, as it was impossible to extend the test through a whole day, owing to other duties. The time on each test was reduced to 31 minutes (8, 10 and 13 minutes on the 1st, 2d and 3d places respectively), in order to be able to alternate six test-pieces in a half-day. Moreover the tests were not made daily, sometimes two days intervening. Alternated in this way, Nos. 4 and 4a, 5 and 5a, and 7 gave on Plate B:
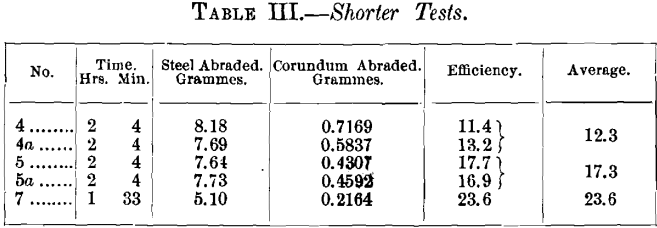
In order to compare these values with those previously found for Nos. 1, 2 and 3, Nos. 2a and 5a were alternated, Nos. 6 and 6a being also tested at the same time. The results obtained for Nos. 2a and 5a were as follows :

With the emery, the results were altogether unsatisfactory. The cement seemed not to be suited to it, as large pieces were occasionally torn away, and the edges cracked off, while the corundum, on the other hand, was finely ground, and the edges of the test-pieces remained fairly sharp.
It was expected that No. 5a would show an efficiency somewhat higher than its previous value, conformably to the experience with Plate A, but on the contrary the new value was lower. That this was not due to any irregularity of the test-piece was indicated by the fact that the ratio between the values of  Nos. 2a and 5a, found here, was about the same as had been previously found with Plate A, as the following comparison will show. The values of Nos. 2a and 5a in Table IV. have the ratio 1.21 to 1. Taking the values of Nos. 2a and 5a in Table I., and deducting 10 per cent, from the former for the estimated change in the two intervening days, we have the ratio 20.82 to 16.3, or 1.27 to 1. Also, taking the average of Nos. 5 and 5a in Table I., then the average of Nos. 2 and 2a, and deducting 2.5 per cent, from the latter, we have the ratio 23.063 to 19.6, or 1.18 to 1. As it is not probable that both test-pieces were irregular in the same way, we conclude that the variation was due to the plate.
Nos. 2a and 5a, found here, was about the same as had been previously found with Plate A, as the following comparison will show. The values of Nos. 2a and 5a in Table IV. have the ratio 1.21 to 1. Taking the values of Nos. 2a and 5a in Table I., and deducting 10 per cent, from the former for the estimated change in the two intervening days, we have the ratio 20.82 to 16.3, or 1.27 to 1. Also, taking the average of Nos. 5 and 5a in Table I., then the average of Nos. 2 and 2a, and deducting 2.5 per cent, from the latter, we have the ratio 23.063 to 19.6, or 1.18 to 1. As it is not probable that both test-pieces were irregular in the same way, we conclude that the variation was due to the plate.
No. 8 was next tested for 31 minutes, and gave: steel abraded, 2.02 grammes; corundum abraded, 0.2626 gramme; efficiency, 7.7.
The irregularity noted above had not been expected, and could not be explained, as care had been taken to maintain the conditions as nearly uniform as possible. Of course, the wearing away of the test-pieces and the plate, thus exposing new portions, were unavoidable changes. Besides these, it is believed that there was only one other condition which was not maintained constant, namely, that which was due to the thinning of the plate, which would thus yield more and more to pressure (see Fig. 3).
Reasons have been given above for believing that the test-pieces were nearly uniform after getting through the exterior.
There appeared to be, then, but three sources from which the changes might arise: (1) thinning of the plate; (2) change in the plate from exterior to interior, possibly due to cooling after rolling or cold-rolling; (3) the actual physical effect of “ cold work ” upon the plate, modifying its properties. In the first case it would be necessary to assume that a plate, 1/6 to 1/8-inch in thickness and 5 inches in diameter, would be appreciably deflected by a weight of 3.25 pounds ; that this yielding would increase the efficiency of the test-piece ; and that this effect was obscured in plate B by local irregularities, both plates having been cut from the same piece. The irregularity of plate B is brought out by the following table, giving the daily efficiencies in the tests made on it:
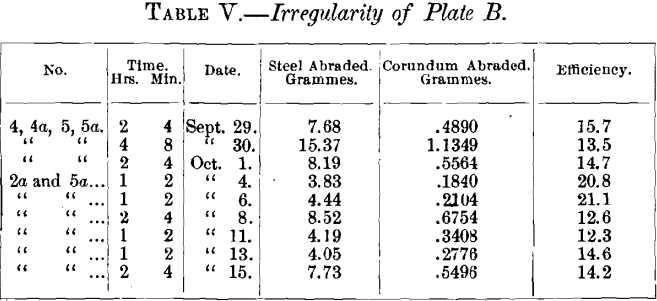
During this time Nos. 6 and 6a were being alternated with 2a and 5a, and No. 7 with 4, 4a, 5, 5a, so that the abrasion of the plate was greater than the table shows. The weight at the beginning of the tests was about the same as that of Plate A on beginning the tests of Table I., and at the end of the test it was about the same as that of Plate A at the end of September 14th, having lost about 100 grammes. Greater irregularities might have been expected in this table than in Table I., as the duration of the tests was shorter.
On the second hypothesis, we would have to assume, as observed above, that the effect was obscured in Plate B by local irregularities.
On the third supposition, we have to assume that continued cold work gradually weakened the metal, and that in Plate B this effect was obscured by irregularities; or else that the periods of rest permitted the metal to recover, and even exceed, its original strength.
That a period of rest had the effect of increasing the efficiency is suggested by the fact that the two first tests of the day in Table I., on the rested surface, gave an efficiency about 6 per cent, lower than the average for the whole day. The average efficiency from the whole of Table I. was 18.0; the average for the first 2 hours was 17.1. On the assumption that 5 per cent, was the daily change, this difference should have been 1.6. This is more strikingly shown by a comparison of the weight of steel ground from the plate per hour, for the first 2 hours and for the day:
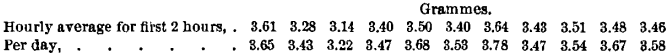
The rested surfaces, without exception, resisted abrasion better. Taken together, the first 2 hours average 3.44 grammes, while the average for the whole time is 3.55 grammes. The third and fourth hours averaged 3.58, the fifth and sixth 3.60 grammes.
If rest alone produced this effect, it would seem that “ fatigue ” should have increased the amount of steel abraded per hour. On comparing the amount of steel abraded by the duplicates in Table I., we see that, with one exception, the later result is slightly higher, and that the exception (No. 4a) followed a day of rest. Moreover, the amount of steel abraded in the second half of the table slightly exceeds that in the first half, even when No. 7, which is a little low, is omitted. The difference, however, is small. It has been noted above that the amount of steel abraded per hour in Table II. differed but little from that in Table I., for the same corundums. It should be said, however, that a half-day of rest intervened between Tables I. and II., and a full day of rest separated the two days of the test. The latter rest, being longer, may account for the smaller abrasion of steel on the second day.
In this connection, however, it should be pointed out that even when a day of rest had intervened, and the actual amount of steel abraded was smaller, the rise in efficiency still continued.
These details concerning the variation in efficiency have been presented thus fully in the hope that some one with a wider acquaintance with such facts may be able to give an explanation throwing light upon the matter.
Having obtained the relative efficiencies of Nos. 5a and 2a as above, we can now construct a table giving the relative values of all the corundums tested. No. 4, being the lowest, is taken as unity.
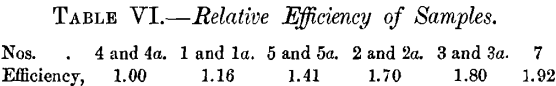
This table is constructed entirely from the values in Tables II. , III. and IV. Comparing it with the values in Table I., on the assumption that the change in efficiency was 5 per cent, a day, we get the same order, with the exception that Nos. 4 and 4a come above No. 1 by about 1.25, and Nos. 3 and 3a come very much lower. The values of the duplicates 3 and 3a agree fairly well in Table I., and still better in Table II. It appears, therefore, that the change in the plate had for some reason affected the efficiency of Nos. 3 and 3a much more than that of any other corundum. While the values given above cannot be claimed to be absolutely accurate, they give us at least the test-pieces very nearly, if not quite, in the proper order of their efficiencies at the time the tests were made.
The efficiencies of the corundums were next determined by Smith’s test, as described above, except that the powder used for abrasion was some of that prepared for the test-pieces, and was therefore of a fineness between 80- and 100-mesh. Moreover, the corundum was heated and cooled twice, as had been done in making the test-pieces. The results obtained were as follows :

Comparing this order of efficiency with that shown in Table VI., we have :
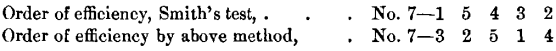
There seems to be no relation between the two. Throughout the tests described above, No. 2 showed itself at all times to be among the best, while No. 1 showed itself at all times as one of the least efficient. Under Smith’s test, on the other hand, No. 2 is lowest, while No. 1 is next to the highest, in efficiency.
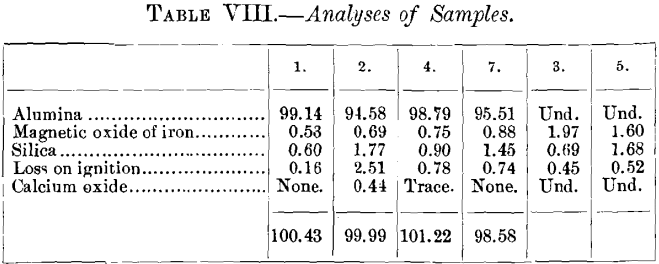
It was thought that perhaps the corundum, through differences in composition, had not acted alike chemically on the cement, and that the differences in values from those determined by Smith’s test might arise from variations in the tenacity with which the cement held the grains. Accordingly, a partial analysis of the corundums was made with the results shown in Table VIII.
In these analyses, the mineral was decomposed by fusion
with a mixture of equal parts of sodium and potassium carbonates. Perhaps it is not generally known to chemists how easily it can be decomposed in this way. From 0.5 to 0.75 gramme of the mineral was finely pulverized, mixed with from eight to ten times its weight of the mixed carbonates, and fused for about 20 minutes in a small platinum crucible, at the highest temperature obtainable with the blast-lamp. By the use of a strong blast and a flame about 2 inches in length, a portion of the lower edge of the crucible could be brought to nearly or quite a white heat. A small residue was invariably left, varying from 2 to 8 per cent, of the original mineral sample. This was readily and completely decomposed by a second fusion.
The efficiency appears not to be connected—at least not closely—with the composition. The analyses were not made with sufficient attention to details to ensure a high degree of accuracy, but the results obtained do not encourage the belief that more accurate analysis would reveal any important relations. We conclude, therefore, that the difference in values obtained by Smith’s test and that described is not due to chemical action between the cement and corundum, and hence must arise from the different manner in which the abrasive force is applied in the two methods of testing. As a corollary, we draw the conclusion that Smith’s test is valueless as a means of determining the efficiency of corundum, when applied in a fixed state, instead of a powder.
In the first part of this paper, attention was called to the fact that, in all corundums tested, the loss upon ignition was nearly equal to the amount of water; and we see from the tests of these corundums that the loss on ignition affords no evidence of close relationship between the amount of water and the efficiency by either method of testing.
