Table of Contents
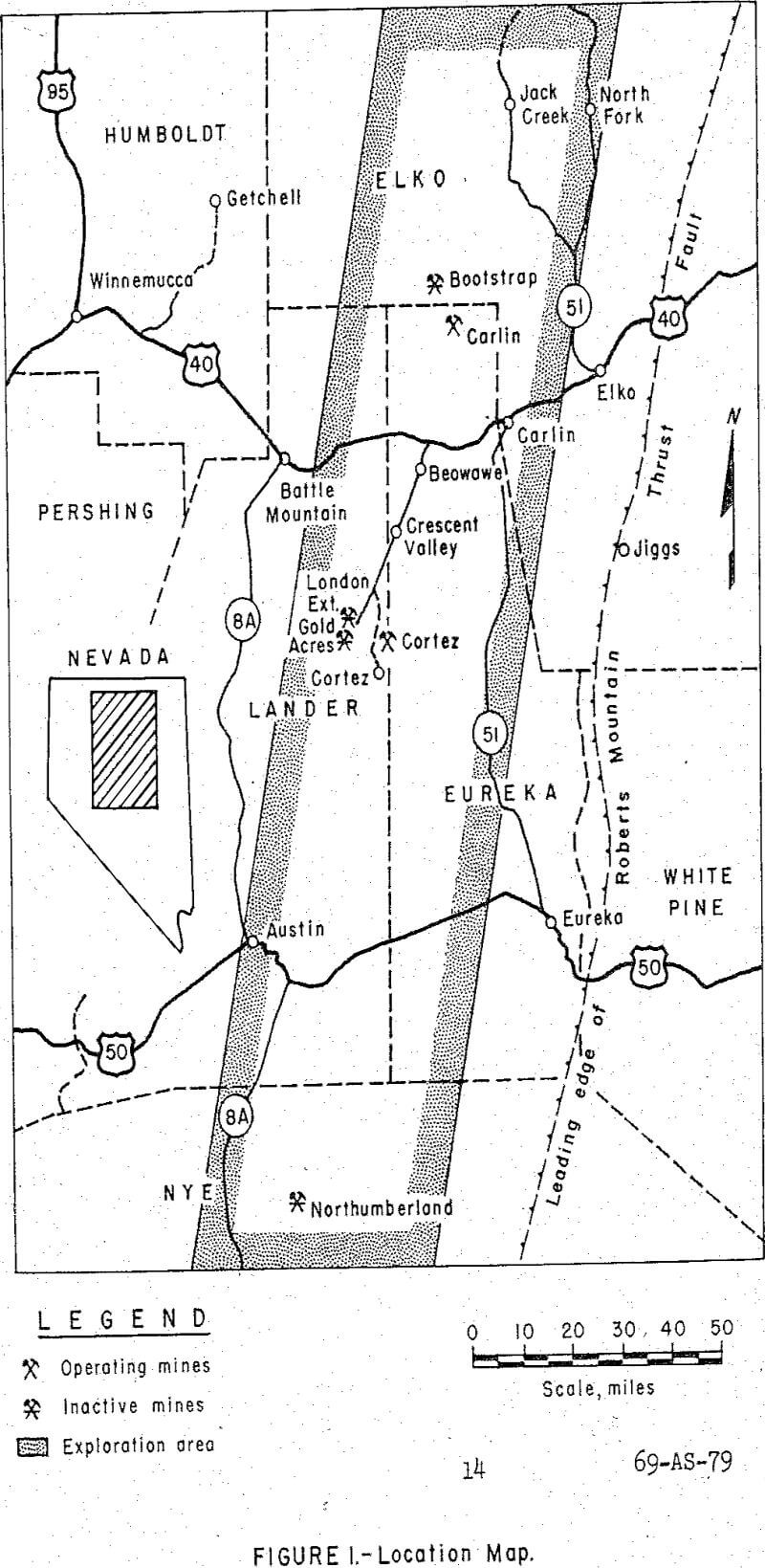
Heap leaching shows promise of being an attractive method for treating large tonnages of oxidized gold ores from the sedimentary beds of northeastern Nevada which are not economically amenable to treatment by conventional milling methods. The ores are unique, with the gold occurring as sub-micron particles in a porous host rock. The preliminary test work on representative samples from the region shows that a satisfactory recovery of the gold can be obtained by heap leaching the ores with cyanide at a relatively coarse size. Capital expenditures and operating costs for this method of treatment will be much less than conventional cyanide milling. The method could apply to deposits that are either too small or of too low a grade to warrant construction of a conventional mill, and for low grade portions of deposits where simultaneous operation of existing or proposed mills and heap leaching could increase treatment capacity and revenue from the operation at low additional capital and operating costs.
Technical and Economic Considerations of Heap Leaching
The technical and economic feasibility of applying the heap leaching process to the oxidized ores of northeastern Nevada offers great promise because of a number of favorable factors, including the following:
The nature of the gold occurrence. The ores are unique in that the gold particles are extremely fine (in the sub-micron range) and in a porous host rock. The gold occurs in the interstices of porous microcrystalline silica and along the fracture planes of relatively impervious siliceous rocks. Present knowledge indicated that in both cases the gold is amenable to cyanidation and recovery by crushing the ore to about 2-inch size, or coarser.
Rapid solubility in cyanide. Once contacted by cyanide solution the gold dissolves almost instantaneously and with virtually no loss of cyanide, due to the very low cyanide contents in these materials.
Past experience. Two classes of dissimilar ore were coarsely crushed and leached in vats, and the results indicated that heap leaching might be possible.
Lower capital investment. Large capital expenditures are required for conventional cyanide milling, and it is conservatively estimated that the minimum-sized orebody that would warrant construction of a conventional countercurrent cyanide plant should contain 4 million tons of ore, have a favorable stripping ratio, and contain about $10 per ton in gold. Such an orebody would warrant a mill with a capacity of 1,500 tons per day. Mills with less capacity would entail too high a capital expenditure per ton of capacity and would result in higher unit operating costs. Thus, smaller mills would be economically feasible only for higher grade ore deposits. The economics of a smaller operation would have to be studied for each particular case, taking all factors in account
Lower cut-off grade for ore. Milling costs of a large operation (3,000 tons per day) require that the cut-off grade of the ore sent to the mill be set at or not much below 0.06 ounce gold per ton, depending upon the free market price of gold. Material below this grade, which is placed in the waste dump, might be segregated for heap leaching. In the case of smaller operations the cut-off grade may rise to 0.15 ounce gold per ton, or above.
Where there is an existing mill, the development of an economic method of heap leaching of low-grade ore would allow the available mill capacity to be utilized for the more profitable higher grade ore. In the case of a proposed mill, a smaller conventional cyanide mill could be constructed than would normally be designed for a particular size of orebody. The result in both cases would be to increase the cash flow and accelerate the return on the investment in the mill.
The interesting possibility exists that the development of a heap, leaching technique might eliminate the need to build a conventional cyanide mill, even though the percentage recovery of the gold might be somewhat less. (The cost of a conventional mill might range from $2 million for a 500-ton-per-day plant to $7 million for a 3,000-ton-per- day plant. The amortization of this mill cost, plus the high operating costs, might make the acceptance of the lower percentage recovery possible or even attractive, when all factors are considered.)
The development of an economic heap-leaching technique would stimulate exploration in the district. Present efforts must be limited to the identification of the larger and better grade deposits that will warrant the installation of a conventional cyanide mill. The attainment of lower capital expenditures and operating costs by heap leaching would justify the more intensive exploration required to locate and delineate the smaller and lower grade deposits. Some of these deposits are already known, in some instances, in some detail; in other cases exploration was suspended when the preliminary findings indicated that the deposit would probably be small, or of low grade, or both. (Historically, in almost all gold mining regions, there have been relatively few large mines, but a large number of small mines. However, the aggregate production of the small mines has been a significant portion of the total production of the region)
Deposits amenable to heap leaching will be near the surface and will have reasonable stripping ratios. Costs for stripping and mining will be moderate, about $0.20 to $0.25 per ton of material moved, including overhead. The cost of site preparation, including an asphalt pad, will be $2 per square yard of heap area. Assuming a 40-foot depth of ore on the pad, the capital expenditure would be $0.11 per ton of ore treated. Cost of coarse crushing (if required) and placing the material in the heap will probably be moderate, as to both capital expenditure and operating cost. Direct operating costs for treating the ore, once it has been placed in the heap, are estimated at about $0.20 to $0.25 per ton of ore.
It is believed that proper site selection and preparation and the use of adequate monitoring and control systems will assure against loss of solution and gold in the process. Furthermore, proper precautions would preclude environmental problems that could be caused by the use of cyanide solutions in the leaching operations. The design criteria and operational methods used by past and present operations in the storage of mill residues and plant cyanide solutions in tailings-dam areas have proved effective in preventing pollution.
Past Applications of Vat Leaching in the Area
Results obtained from past vat leaching operation in the region lend validity to the proposal that heap leaching would be applicable to ores from the area because vat leaching has been used on several distinct and widely differing types of oxidized ores from the area.
Prior to the recent recognition and development of the region as a major gold producer, four gold mines were operated for various periods of time between 1935 and 1961. From these mines gold valued at $11,725,000 was recovered from 2,835,000 tons of ore for an average yield of $4.14 per ton milled, which was profitable during this period of low capital and operating costs. Additionally, three of the mines employed simplified milling methods.
Two of these mines were operated profitable because they simply crushed the ore to minus 2 inches and leached this product in vats. The ores from these operations were dissimilar. In one operation the ore was porous, with the gold occurring in the interstices of micro- crystalline rocks. In the other mine the ore was a relatively impervious siliceous rock with the go Id apparently occurring along fracture planes. In both cases, however, satisfactory recovery was obtained by employing a relatively short leaching cycle time.
At another operation the ore was reduced to minus 3/8 inch size, a sand-slime separation made the coarse portion treated in vats, and the slime treated in a conventional countercurrent plant. The size to which the ore was ground was dictated more by the material handling problems associated with filling and discharging the vats, and the leaching cycle time requirements, than the need to grind to this size for metallurgical recovery. A coarser grind would have required mechanical loading and discharging of the vats rather than the cheaper hydraulic methods employed, and would have required additional vats for the longer time cycle that would have been needed to obtain an equivalent recovery.
These three operations used treatment methods intermediate between conventional countercurrent cyanidation at a fine mesh-of-grind and heap leaching at 2-inch size or coarser, and demonstrated the practicability of treating the oxidized sedimentary bed ores at a relatively coarse size. The methods employed have been considered for further application in the region. However, because of current construction and operating labor costs, it is probable that both of the above, methods would have limited application at this time for the following reasons:
- In the case of a small vat leaching operation, the unit construction costs of a mechanized plant would be excessive. On the other hand if not highly mechanized the labor costs for operation would be excessive.
- In the case of a large operation employing direct vat leaching, proper design would obtain reasonable operating costs similar to those of large vat leaching operations in the copper industry. However, because of the long leaching and washing cycles, it is probable that the capital expenditure requirements would approach those of a conventional cyanide mill.
- In the case of either a small or a large operation, a mill designed for coarse grinding and a sand-slime separation would cost more to build and to operate than a conventional countercurrent cyanide mill.
Requirements for the Heap Leaching Process
The requirements for effective leaching are a heap that remains permeable during the relatively long leach cycle and fine size gold in a porous ore essentially free from cyanide-consuming impurities. In heap leaching, the coarsely crushed ore is piled on an impervious pad and the leach solution either distributed on top of the pile or injected within the heap. After percolating through the heaped ore, the solution is collected and recycled until the ore is adequately leached. The pregnant solution may be treated continuously or intermittently to recover the gold.
Results of Heap Leaching Tests on Ores from the Region
The Bureau of Mines Metallurgy Research Centers at Reno and Salt Lake City are conducting heap leaching tests on oxidized ore from the region, studying three entirely dissimilar types of ore which present knowledge indicates will cover the full range of ore types that might be amenable to heap leaching. The tests to date, while only preliminary in nature, have been yielding promising results. Gold recoveries have been excellent and the economics of the process appear attractive.
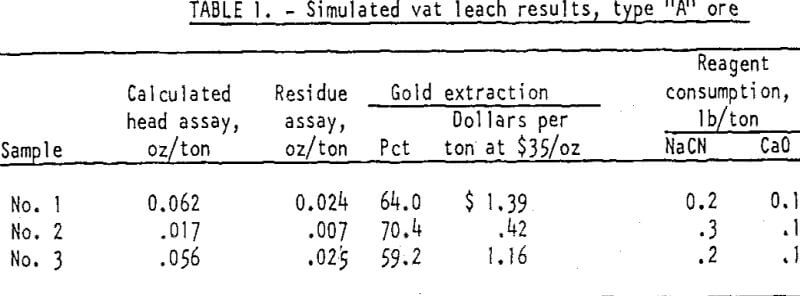
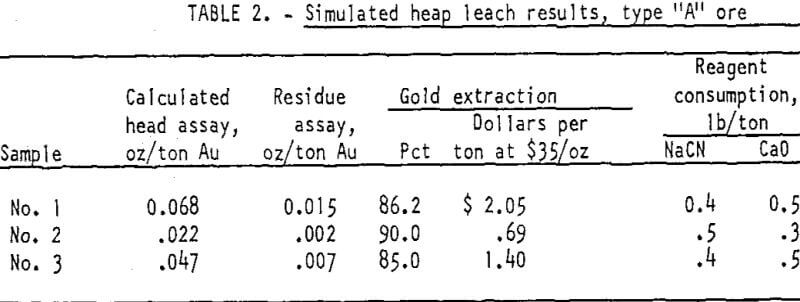
Type “A” ores are mineralogically described as fine-grained, silicified limestones, marked by horizontal streaks of limonite cut by veinlets of white crystalline calcite. The coarser fragments of the samples are seemingly hard, compact, and non-porous, but readily crushable with production of few extreme fines. Three samples of this type of ore were tested. Calculated head assays ranged from 0.02 to 0.07 ounce gold per ton.
The first series of column leach tests made on these samples simulated vat leaching practice wherein the ore bed was constantly flooded with leach solution that-after draining downward was recycled to the top of the column. The ores at minus 1-inch size were loosely packed to a depth of 2-feet and leached 6 days with a solution containing 0.1 percent sodium cyanide and 0.01 percent calcium oxide. The gold in the final pregnant solution was precipitated on activated carbon on completion of the 6-day leach cycle. The results indicated recovery of 59 to 70 percent of the total gold in the ores consumption of 0.02 to 0.3 pound of NaCN and 0.1 pound of CaO per ton of ore.
The second series of tests simulated heap leaching in which the solution is slowly distributed on the surface of the ore and allowed to freely drain downward through the ore. Minus 1-inch size ore in a loosely packed 2-foot-deep column was leached for 7 days with a solution containing 0.1 percent sodium cyanide and 0.1 percent calcium oxide. Pregnant gold solution from the leach column was continuously stripped of its gold content by passing it through a bed of activated carbon before adjusting the cyanide and lime strength and returning the solution to the leaching cycle. The test results showed that from 85 to 9.0 percent of the gold in the ores was recovered. Reagent consumption was 0.4 to 0.5 pound of NaCN and 0.3 to 0.5 pound of CaO per ton of ore treated. The results of these two series of tests are summarized in tables 1, 2, and 3 (sides 2 and 3)
Type “B” ores are mineralogically described as porous sedimentary rocks which are chiefly siltstone and limy shale, containing large amounts of clay. The gold occurs as submicroscopic particles. The calculated head assays were about 0.06 ounce gold per ton.
Leaching experiments were conducted in apparatus consisting of a plastic barrel (27 inches high, 13.5-inch diameter) and a plastic pipe (27 inches long, 1.5-inch diameter) which was.centrally located inside the barrel. Provision was made at the bottom of the barrel for the leach solutions to be drained and collected. The pipe was dosed at the lower end and was perforated with four 0.25-inch-diameter holes spaced 90 degrees apart around the periphery at 4-inch intervals along its entire length. The pipe was placed in position, and the barrel was filled with approximately 200 pounds of 1-inch size ore.
The injection system of heap leaching was applied to the charge, since it minimized the mechanical difficulties often encountered in leaching clay-bearing materials. The leachant used for extraction contained 2 pounds of NaCN per ton of solution, and the quantity of solution used was 400 pounds per ton of material. No lime was added to the extractant since the water and the rock formed a pulp with a pH of 8. The solution was fed into the perforated pipe at a controlled rate to first wet the lower portions of the charge. Leaching occurred
as the solution percolated through the charge to the bottom of the barrel where it was collected. The solution was then fed back into the top of the plastic pipe and recycled several times during an 8-hour test period. The system was allowed to drain overnight while the charge was partially dried and aerated. Gold was removed from the leach solution by use of activated carbon. The gold-laden carbon was recovered by filtration, then the barren solution was recycled for another 8-hour period. The carbon was ashed, and the gold content was determined by fire assay of the resulting residue.
Each day, prior to beginning the extraction, the inner pipe was filled with sufficient mine waste to raise the level 2 inches. This blocked the lowest set of holes every second day and forced the solution into the bed at the next higher set of holes. This sequence was repeated, and after 12 days, the material in the pipe approached the top set of holes. This set of holes was never covered, and leaching was continued under these conditions for the duration of the 21-day test. At no time was the leachant poured directly on top of the bed.
Gold recoveries are shown in table 4 (slide 4). At the end of 10 days, the recovery was about 80 percent. In the remaining 11 days, an additional 10 percent of the gold was obtained for a total recovery of about 90 percent. The resulting tails assayed 0.006 ounce gold per ton.
Table 5 (slide 5) shows the distribution of gold in the cyanide tailings. As expected, the coarser fraction plus 1-3-inch retained the largest portion of the undissolved gold. About 19 percent of the gold lost in the tailings was water soluble gold not washed out of the column.
Reagent consumption was 0.4 pound NaCN per ton of feed. The final Strip solution had a cyanide content of less than 0.002 pound NaCN per ton of solution. The cost of the consumed reagent was approximately $0.07 per ton of ore treated.
Type “C” ores are mineralogically described as a breccia containing fragments of quartzite, and silicified and altered igneous flow rocks. Areas of clay, limonite, and hematite occur throughout the breccia, which is relatively permeable through cracks and fractures in the rock. No discrete ore-bearing minerals were identified.
The samples of ore representative of this type of material were of relatively high grade, averaging about 0.50 ounce gold per ton. The results obtained indicated that high-grade ores from the region might be amenable to heap leaching.
Tests that simulated heap leaching were made on ore crushed to minus-5-inch and loosely packed in a 2-foot column. Cyanide solutions containing 0.2, 0.1, and 0.05 percent NaCN, respectively, and 0.12 percent CaO each were tried in comparative tests. In each case the solution was distributed to the surface of the ore and allowed to
drain freely down through the ore. The gold in the solutions flowing from the columns was continuously stripped by passing the pregnant solutions through activated carbon columns before recycling. Cyanide and lime strength were adjusted as required to maintain leach solutions at initial concentrations. The best overall results were obtained by using 0.1 percent cyanide solution. Ninety-five percent of the gold in the ore was recovered in less than 24 hours leaching time, and 0.66 pound of NaCN and 2.7 pounds of CaO per ton of ore were consumed.
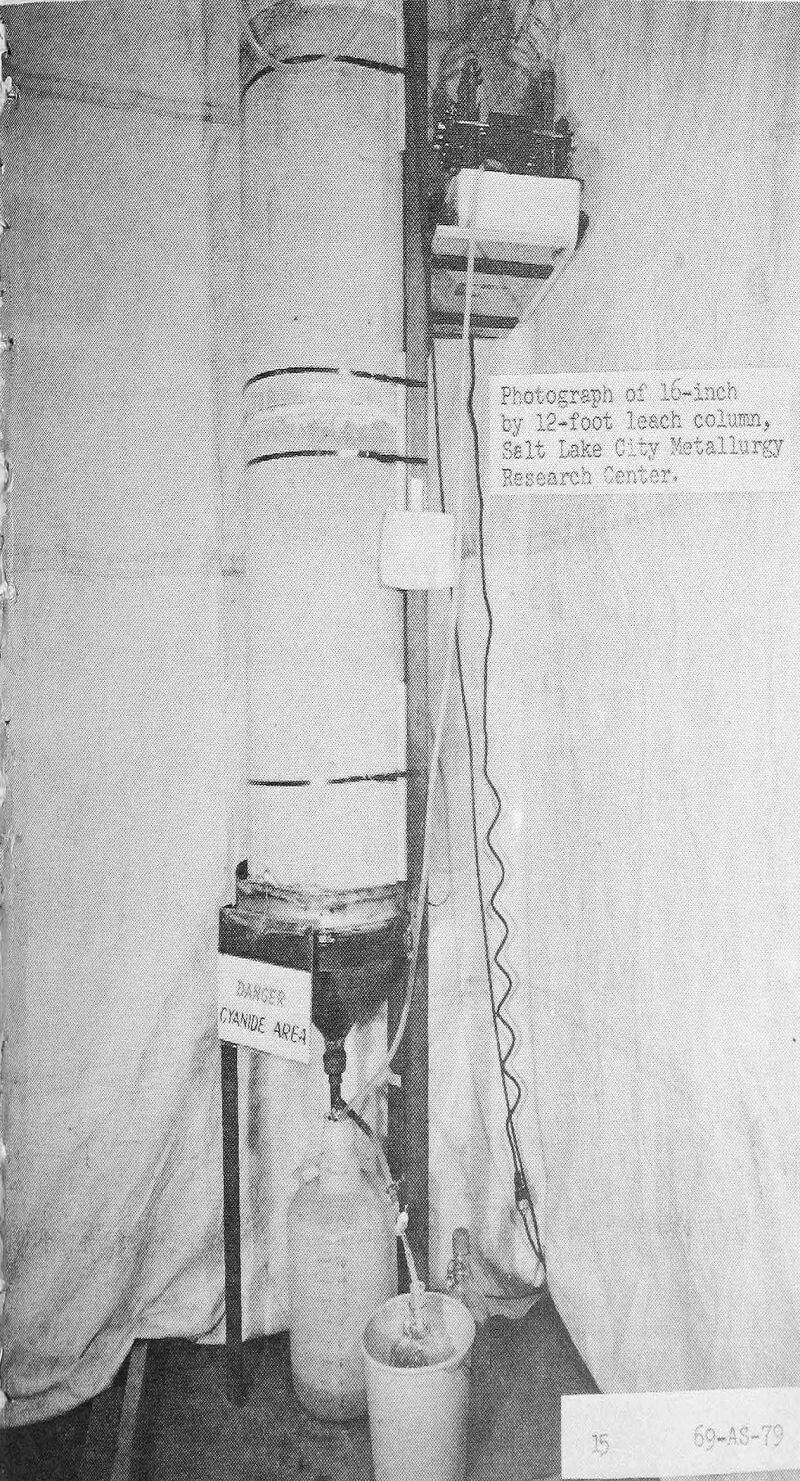
Additional leaching tests are in progress at the Bureau’s research centers to better define the process variables that control achieving maximum recovery at minimum costs. Among the factors being examined are the method of applying the leach solutions, influence of access of oxygen to the bed, rate of solution movement, the continuous versus intermittent removal of gold from the pregnant solutions, the gold loading on the carbon, the strength of the cyanide leaching solution, the time of the leach cycle, and the size of the ore fragments leached. The response of the ores to leaching in a deeper column is being studied in a 16-inch by 12-foot-high unit. (slide 6).
Industry groups in the region are also active in evaluating heap leaching. One operator has completed the test work on a pilot heap. The method involved intermittent sprinkling of the leach solution on a 300-ton heap of uncrushed mine waste material. Encouraging results were achieved during the 1-month test period. Another company is treating a similar heap of low-grade material.
Conclusions and Discussion of Heap Leaching tests.
In tests simulating heap leaching practice with continuous recovery of gold from recycled solutions, from 85 to 95 percent of the gold was recovered from oxidized gold ores representative of three distinct types encountered in northeastern Nevada. The ores are characterized by adequate permeability, fine-sized gold rapidly soluble in dilute cyanide solutions, and freedom from cyanide-consuming impurities.
The maximum consumption in any of the tests was 0.6 pound of NaCN and 2.7 pounds CaO per ton of ore. Thus the direct cost of the leaching reagents consumed at current prices would be less than $0.15 per ton of ore. No attempt was made in any of the tests to achieve optimum gold loading in the activated carbon, which was burned to recover the gold. Industrial practice usually includes removal of the gold by leaching with a hot strong solution of cyanide and caustic (sodium hydroxide), electrolytic precipitation of the gold, and reuse of the carbon. In the case of small operations it might be preferable to ship the loaded carbon to a smelter. Plant experience suggests that total costs for employing the carbon precipitation method including regeneration should not exceed $0.10 per ton of ore.
Precipitation of the gold on activated carbon is indicated to be a feasible and economic method for recovering the gold from the low-grade solutions obtained from heap leaching. However, if heap leaching is practiced in conjunction with a conventional cyanide mill it may be preferable to interchange solutions with the regular mill circuit and recover the gold in the mill’s zinc precipitation system.
Certain limitations in using the results of laboratory percolation leaching tests to project their application in practice should be noted. For example, the tendency of certain ores and sub-ores to degrade in size, form slimes, and become more compact and impervious when piled in wet heaps cannot be fully explored even in tall laboratory columns. When carbon is used as the adsorbent, exact data on carbon losses, optimum loading, and handling are likewise difficult to obtain in small tests. Such information is best accumulated by the operation of pilot or larger scale test leaching operations in the field.
Planning for Proper Application of Heap Leaching
The successful application-of heap leaching to sedimentary gold ores will depend upon adequate test work, sound engineering, and thorough feasibility studies for each deposit. Basic criteria or technologies have been developed and are rapidly being improved for the heap leaching of copper and uranium ores. These basic criteria will be applicable to heap leaching gold ores; However, secondary criteria must be established for factors that are peculiar to the ores to be treated. Both basic and secondary criteria may vary widely from one gold deposit to another deposit in this area. Based on present knowledge of the problems that might be encountered, the following suggestions and comments are offered:
- Test work on representative samples should follow a sequence of bench- scale tests, experiments in tall columns, and operation of a small heap.
- The maximum size of material that can be leached effectively will be determined during the early stages of the test work.
- Reagent consumption due to chemical reactions can be determined during the course of the bench-scale tests. Mechanical losses will be partially determined during the course of column tests and finally determined during the operation of the test heap.
- The maximum allowable height of an operating heap can be determined reasonably well during the course of experiments in tall columns, and can be verified during the operation of the small heap.
- The construction of a substantial asphaltic pad is presently considered to be the preferred base for a heap, although the use of other materials should be considered.
- Various methods of placing the ore in the heap should be studied, with the objectives of preventing compaction and segregation, obtaining a uniformly permeable heap, and allowing the operation of a high heap.
- The effect of using various methods of applying the leach solution (flooding, spraying, and injection) should be studied during the course of operating the test heap.
- Methods for washing the treated heap should be studied with the objective of obtaining the maximum recovery of the cyanide and absorbed gold without undue dilution of the leach solution.
- The available methods for recovering the gold from the leach solutions should be investigated with the objective of determining the most economic procedure for the particular deposit.
- Close attention should be given to proper design and operating procedures so as to provide assurance against pollution either during, or subsequent to, operations.