Table of Contents
The manufacture of stainless steel results in a variety of wastes; the number, quantity, and composition are dependent on the manufacturing method used, the alloys produced, and the manner and degree of finishing of the metal products. A single domestic plant using conventional electric arc furnaces in conjunction with the argon-oxygen process, and producing finished stainless steel items such as bars, plates, and structural shapes, generates four wastes: electric arc furnace dust, argon-oxygen furnace dust, mill scale, and centerless grinding swarf. Other plants, especially the larger ones, produce ferroalloys other than stainless steel; the dusts collected from the plants are mixtures of furnace dusts of varying composition and contain ferroalloy elements of high unit value.
The amount of dust or other waste produced at any one location is relatively small, but the cumulative quantity is large and represents an important loss of costly and scarce alloying element, especially nickel and chromium. Based on annual production of stainless steel as reported for the entire industry and assuming that 40 pounds of dust is generated for each ton of steel produced, over 24,000 tons of dust is generated each year. This represents several million dollars worth of alloying elements, including about 5 million pounds of chromium, more than 1 million pounds of nickel, and 90,000 pounds of molybdenum, all of which is currently wasted. The generation of mill scale and grinding swarf is not as readily defined; however, representatives of one plant indicated that these two wastes represent about 70 percent of the total waste generated by that plant. Because furnace dust is generated in all production of stainless steel, this material was the object of the major part of the research reported herein; however, other wastes were also shown to be amenable to the proposed process.
The Bureau of Mines undertook this investigation to demonstrate the feasibility of recovering the metals and producing a nonpolluting, easily disposable residual material. This would eliminate the disposal problem caused by these wastes, as well as alleviating supply problems associated with the metal.
Wastes
Furnace Dusts
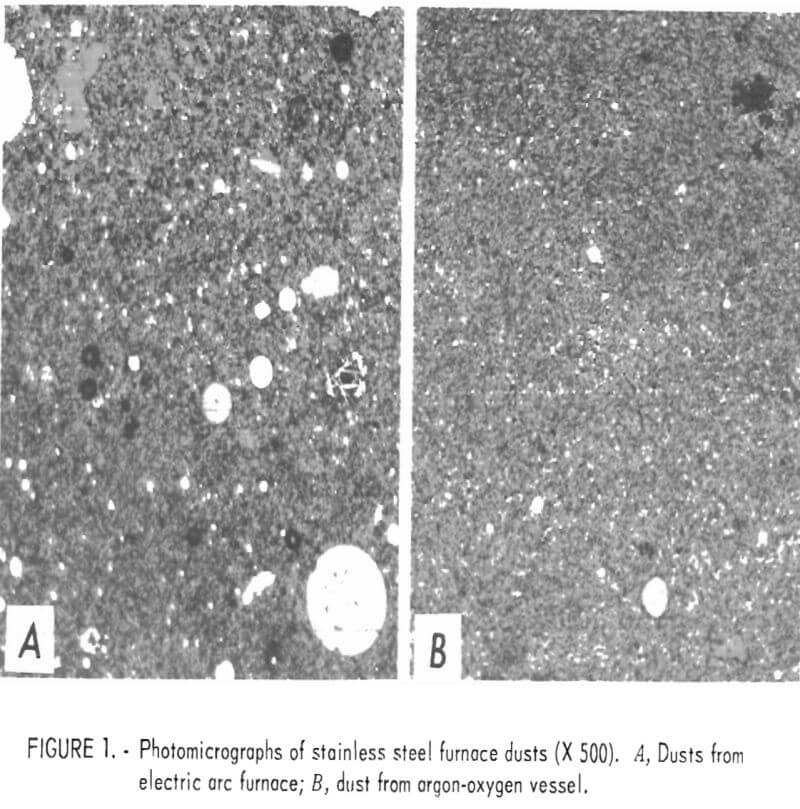
In general, electric arc furnace dusts, whether collected in scrubbers, baghouses, or electrostatic precipitators, are very finely divided mixtures of metal oxides and metal shot, with lesser amounts of slag, carbon, and other debris. About 90 percent of the dust is minus 400 mesh; much of it, including the metal shot, is submicrometer size. The bulk density of the dry dust is about 30 pounds per cubic foot. Figure 1 shows photomicrographs (x 500) of samples of baghouse dust as collected from an electric arc furnace (A) and an argon-oxygen vessel (B). In each, the round white spots represent metal shot, and the gray areas, metal oxides. These photmicrographs show the relatively large amount of metal present and the minute size of most of the metal shot. They further emphasize the large difference in particle size between the two furnace dusts.
Dust-collecting facilities are usually employed for a number of furnaces and in any given plant will collect dusts from more than one source and type
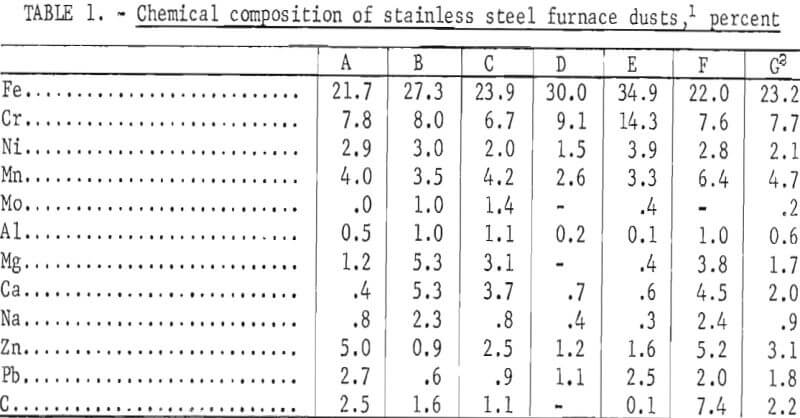
of production. As a result, the baghouse dust may represent a single alloy or a mixture of several. The chemical composition of five electric furnace dusts one argon-oxygen dust, and a composite of five of the dusts is given in table 1. Two of these dusts, A and F, are from plants producing only stainless steels; the others are from plants producing both stainless steels and other alloys steels. The analysis illustrates the wide variation in composition of wastes among plants.
Mill Scale
The definition of mill scale, as used in this report, includes not only the oxide scale and metal chips derived from hot rolling, forging, scarfing, and pneumatic chiselling, but also metal spills, pieces of refractories from furnace linings, and other debris from the mill floor. An “average” analysis of this scale would be meaningless, but an analysis of the scale used in these studies shows it to contain the following, in percent: 55.5 iron, 11.5 chromium, 4.9 nickel, 0.8 manganese, 0.8 molybdenum, and traces of zinc and lead.
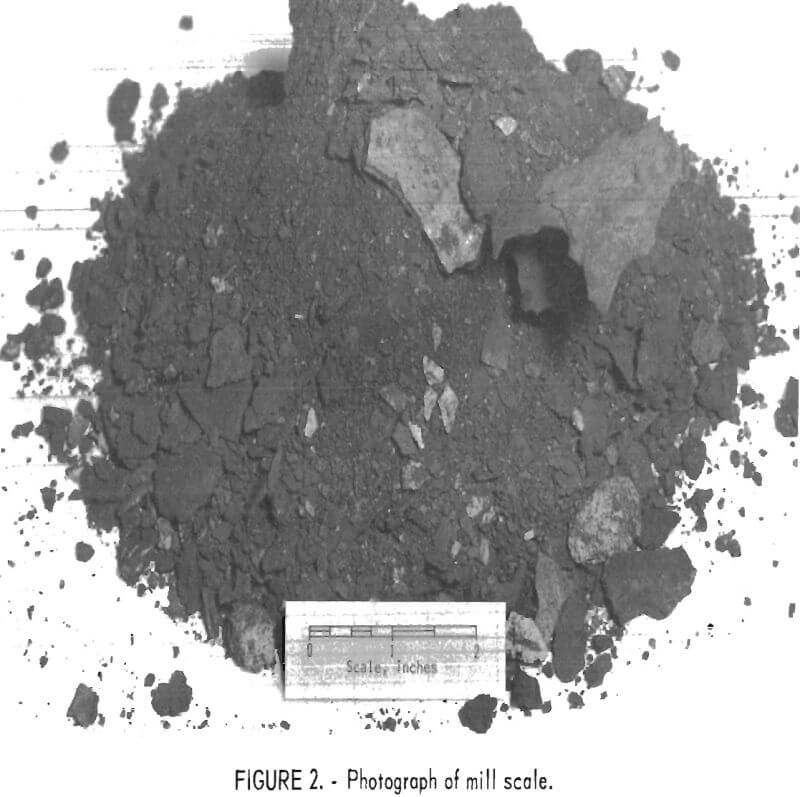
Figure 2 is a photograph of the as-received mill scale used in this work and illustrates the physical size and condition of the various constituents. With the exception of spilled metal, the scale crushes with no difficulty, except for a tendency to pack on rolls or in the jaws of crushers owing to its moist condition.
Centerless Grinding Swarf
This waste is produced through surface grinding of items such as shafts and rods. It is composed of fine metal chips and particles of oxide, carbide,
or other materials from the grinding wheels used in the operation. It is contaminated with water-soluble cutting oils and as collected from the plant, it is a lightweight, moist mass. Chemical analysis of a dried portion of the sample used in this study shows it to contain the following, in percent: 53.9 iron, 9.8 chromium, 2.3 nickel, 0.5 manganese, 0.1 molybdenum, and traces of zinc and lead.
Weighted Composite of Wastes
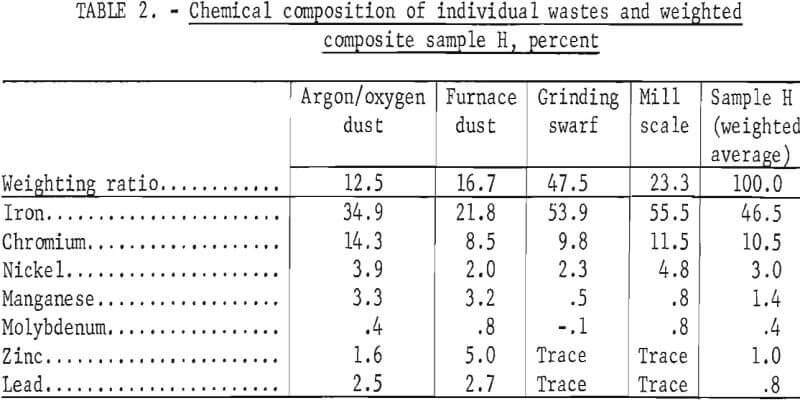
To demonstrate the feasibility of using the total waste generated in the production of stainless steel, four wastes from a single plant electric furnace dust, argon-oxygen dust, mill scale, and centerless grinding swarf-were combined in the ratio in which they were generated. The composition of each of the four-wastes and the composition of the weighted composite (sample H) are given in table 2.
Experimental Procedures
Preliminary studies were conducted to delineate the most promising areas for detailed research. These included physical separation of the metallized constituents, chemical leaching, and pyrometallurgy. The first two, physical separation and leaching, were found to be impractical. Metal shot in the dusts was much too finely divided to respond to gravity, magnetic, or other physical separation techniques, and their refractoriness made chemical dissolution of questionable value. In addition, nonmetals in the dusts would consume large quantities of the acid reagents, producing problems in waste disposal.
Results of the preliminary studies indicated that pyrometallurgy would be the most promising area for further research. Since plants producing stainless steel are based on pyrometallurgical processes, such techniques could be more readily incorporated into the plants. In addition, the products obtained through such processes would be recyclable directly by the producing plants without further treatment.
Pelletizing
Pelletizing or briquetting is a necessary prerequisite to the use of furnace dusts as a furnace feed; individual plant dusts, composites of the dusts, and the mill scale and grinding swarf were pelletized for further treatment. Although both disk and drum pelletizers were used, the drum type was found to produce the best pellet for both single and composite wastes.
In general, the several components for the desired pellet charge were mixed in a twin-shell blender, fed as-received to an 18-inch-diameter drum pelletizer, and moistened with a handheld water spray. (When mill scale was a constituent of the pellet, prior crushing was necessary to pass 4 mesh. All other wastes were used as-received.) Portland cement in amounts up to 5 percent was added to serve as a binder in essentially all of the work; however, two of the dusts investigated were found to pelletize satisfactorily without any binder. When necessary or desirable, reductants and modifiers such as coke breeze, lime, spar, or other material were blended into the pellet feed without deleterious effects. Water-soluble cutting oils in the grinding swarf had no adverse effect on the physical characteristics of the pellets containing this waste. Finished pellets were air dried for 24 hours, then oven dried at 250° to 300° C for at least 4 hours, to remove all traces of free moisture. These pellets were charged to the furnace, both cold and at full heat, without undue dusting or disintegration.
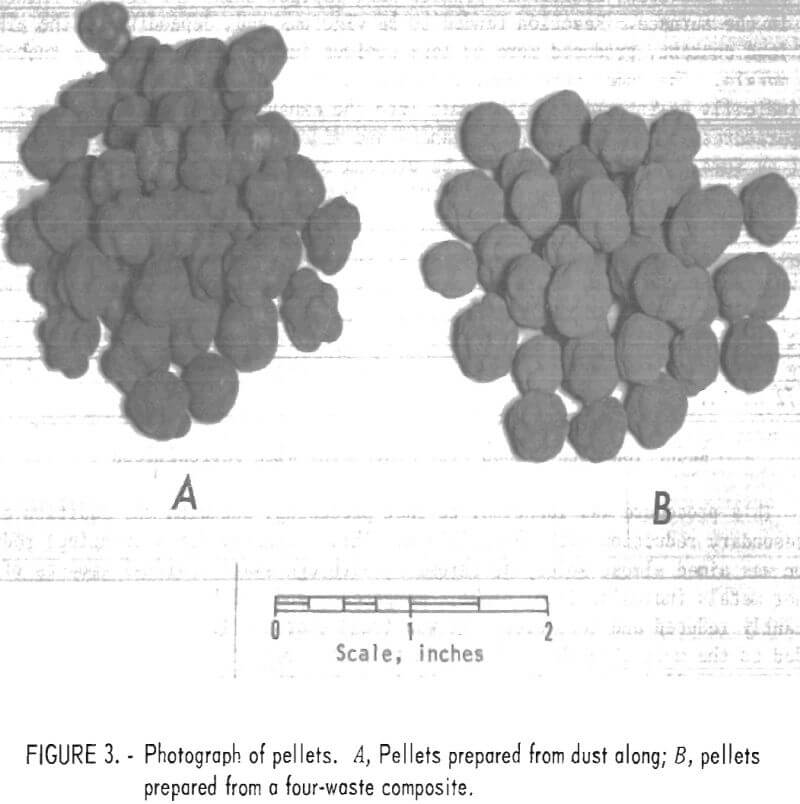
Although differences in their amenability to pelletization were noted among the several dusts used, no particularly difficult problems were encountered. The principal variants requiring control were amount of binder, rate of water addition, and retention time in the drum. Figure 3 shows the pellets prepared from dust along (A) and from the four-waste composite (B).
Smelting
All smelting was done in coreless induction furnaces ranging in size from 50- to 150-pound capacity using charges from 10 to 25 pounds. Because the charges were not electromagnetically susceptive, carbon, silicon carbide, or similarly susceptive crucibles were used to melt the charges. In most instances, the crucibles were machined from graphite stock.
Initial studies using reductants such as coke breeze, lignite, lignite char, and granular graphite showed that each was effective. Coke breeze sized to minus 35 mesh was used in most of the tests; however, in practice, final choice would depend on availability and cost. Carbon was found to be effective for reducing essentially all of the iron and nickel and about one-third of the chromium. Although ferrosilicon was found to be effective as a primary reductant, economics restricted its use to scavenging for the residual chromium, where small-staged additions were found to be the most efficient.
Operating temperatures ranged from 1,400° C to over 1,600° C; the higher temperatures were the most efficient. The reduction and sublimation of zinc and lead was very active at the lower temperatures and was complete before the higher temperatures were reached. Appreciable amounts of manganese were also evolved with the fume and recovered with the zinc and lead.
A problem that varied from dust to dust was that of slag control. In most instances, the slag tended to be very viscous and difficult to pour, however, additions of lime, spar, or other conventional reagents controlled the problem adequately. In some instances, the secondary reductant, ferrosilicon, served to fluidize the slag to a satisfactory degree.
A second problem in all tests utilizing graphite crucibles was the attack of the crucible by the molten metal. This was partly alleviated through the inclusion of a carbon reductant in the pellets, which was consumed before the crucible was appreciably attacked. Offsetting this undesirable feature was the fact that the reduced chromium was retained in the metal; with refractory crucibles, the chromium would oxidize and attack the furnace lining. Thus, the effect of crucible loss versus chromium salvage was not adverse to the overall process because the chromium was reduced to almost zero in the slag, and none was lost to the furnace lining.
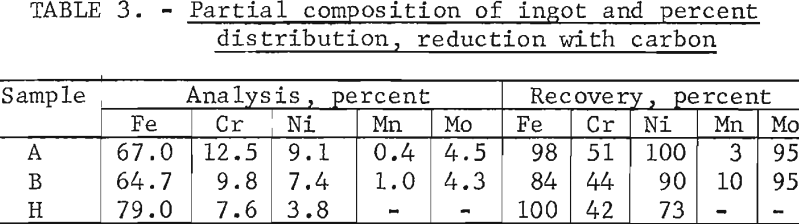
Reduction with Carbon
In a typical run using carbon as the only reductant, the pellets, containing 5 percent coke breeze, were charged to a cold furnace, then heated rapidly at a power input of 35 kilowatts. Melting began in about 20 minutes and, as the charge subsided, additional pellets were charged until the desired amount was in the furnace. Reaction tended to be vigorous and, depending on the zinc and lead content, produced more or less copious fumes of the oxides of these two metals. The fumes were readily collected in a baghouse and sampled periodically by inserting a fiber mat into the exhaust duct. At the higher temperatures, large amounts of manganese and minor amounts of chromium were lost as fume. The slags tended to be viscous and when necessary were fluidized with spar or other commercial media. Power input was changed as necessary to control the rate of reduction, which at times tended to become vigorous. Rate of reaction was judged by the degree of outgassing and turbulence in the melt. When the melt had become quiet and sufficiently fluid, the total melt (including both metal and slag) was poured into a preheateds cast-iron pig mold and allowed to freeze. Separation of solidified metal and slag was clean and complete. Total time from charge to pour ranged from 1 to 1-½ hours.
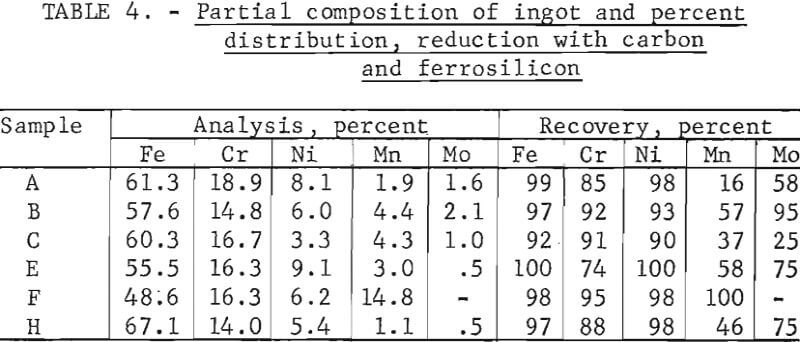
Reduction With Carbon Plus Scavenging With Ferrosilicon
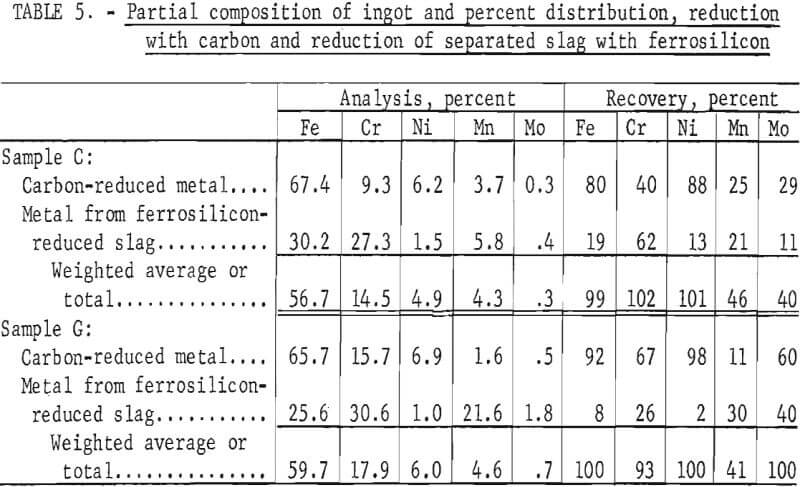
This procedure was identical to that preceding, but with the addition of a secondary reduction with ferrosilicon. This secondary (or scavenging) reduction was aimed almost solely at chromium, although small residual amounts of other metals including iron, nickel, manganese, and molybdenum were concommitantly reduced and recovered. It was found that the ferrosilicon could be added to the melt when the carbon reduction was complete but prior to tapping. However, equally good results were obtained when the slag was treated after separation from the metal. Because of the small (25-pound) size of the laboratory tests, it was not practical to separate the liquid slag completely; therefore, the solidified slag was returned to the empty furnace, remelted, and then scavenged with ferrosilicon. In the laboratory tests, ferrosilicon (25 Fe : 75 Si) was added in stages, primarily to determine the quantity that was required for essentially complete removal of chromium. In practice, it might be added in a single stage and, if so, less time would be required for complete reaction than was required in the reported test work.
Test Results
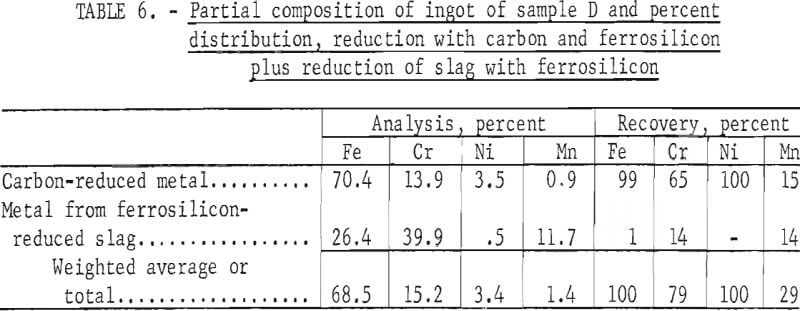
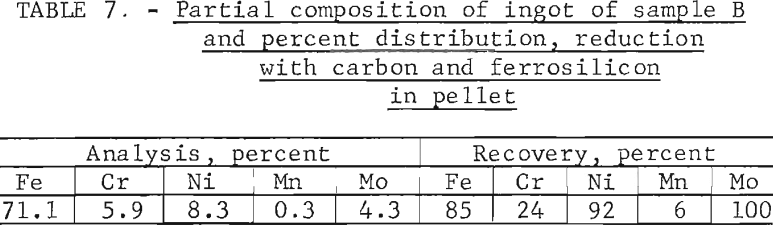
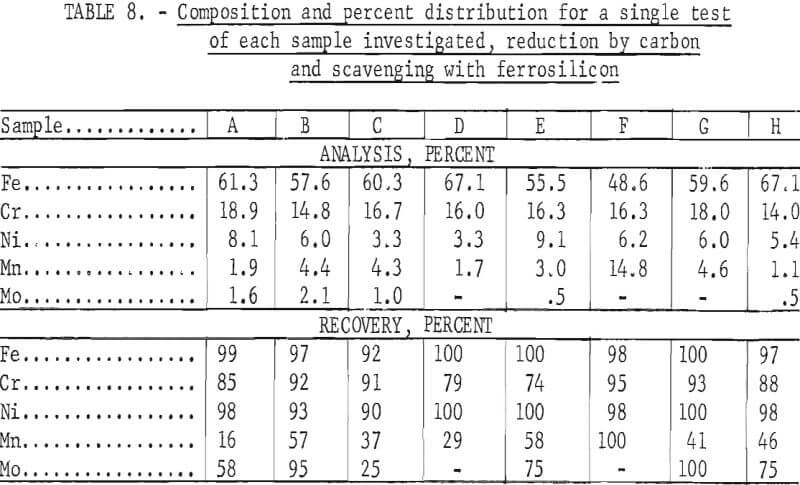
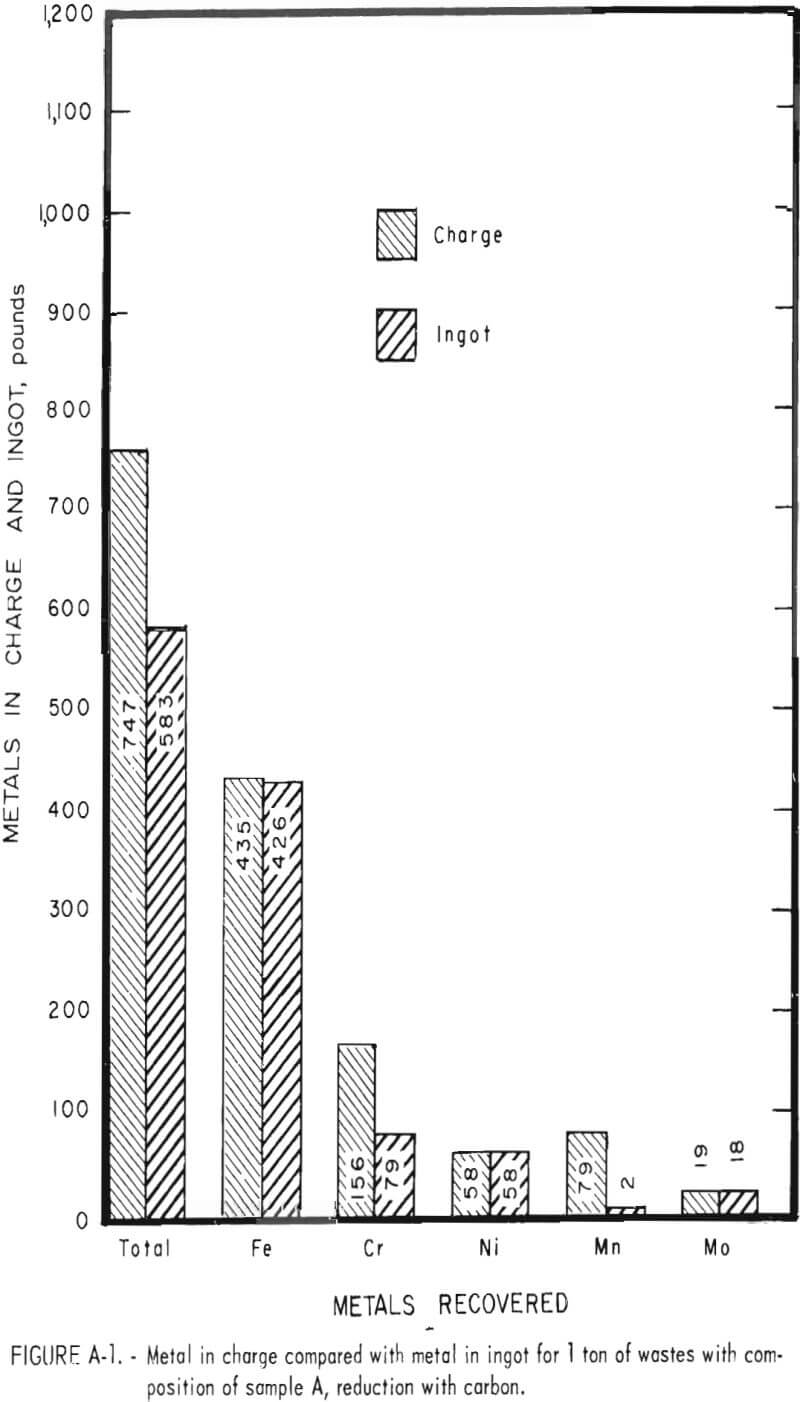
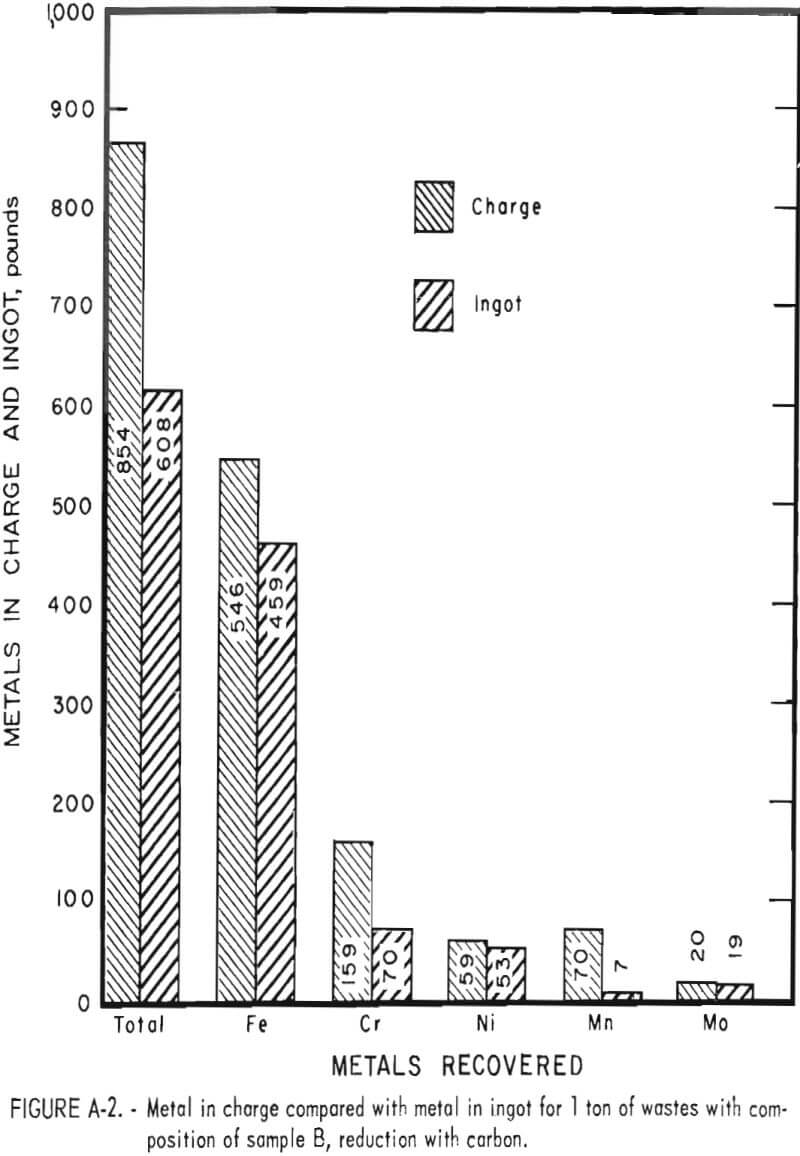
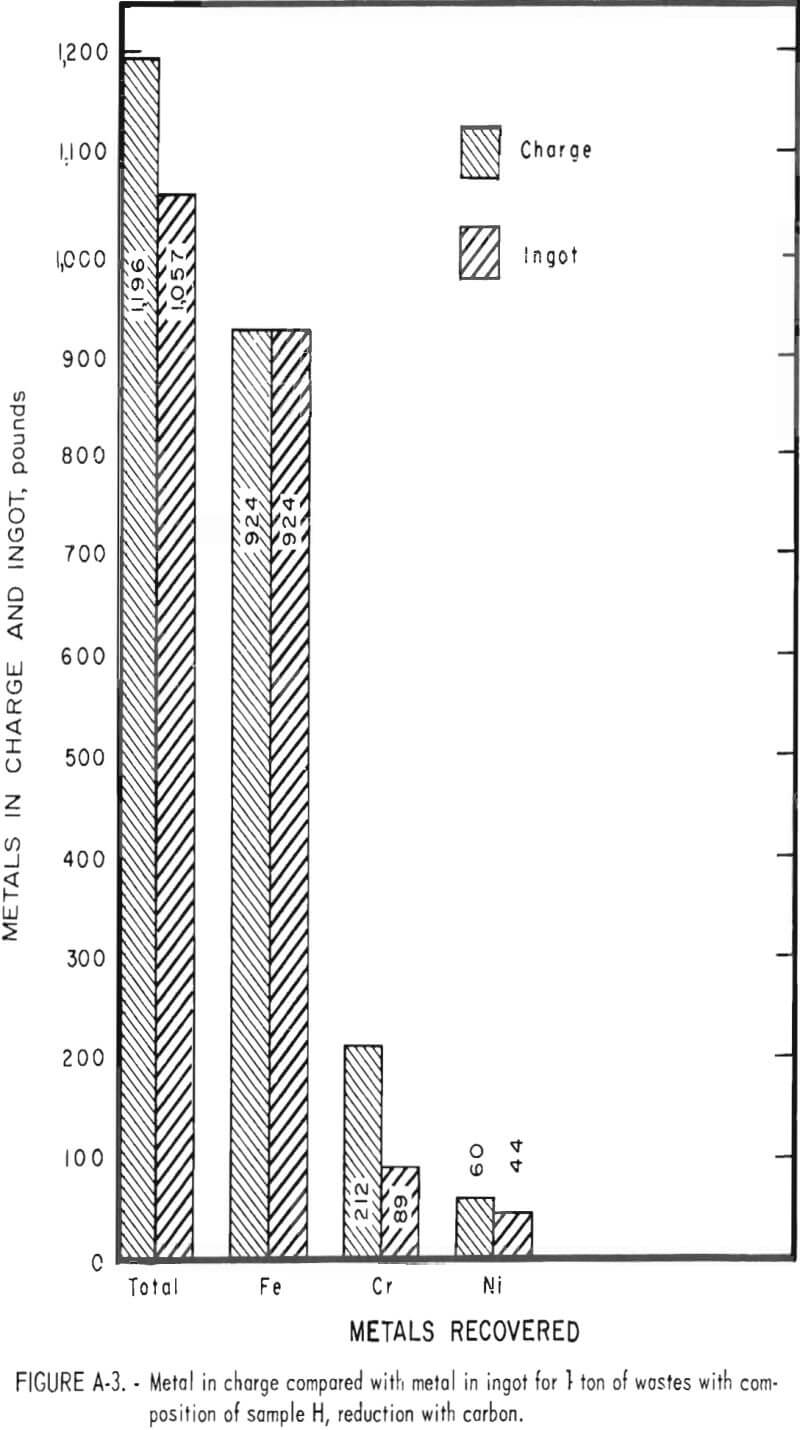
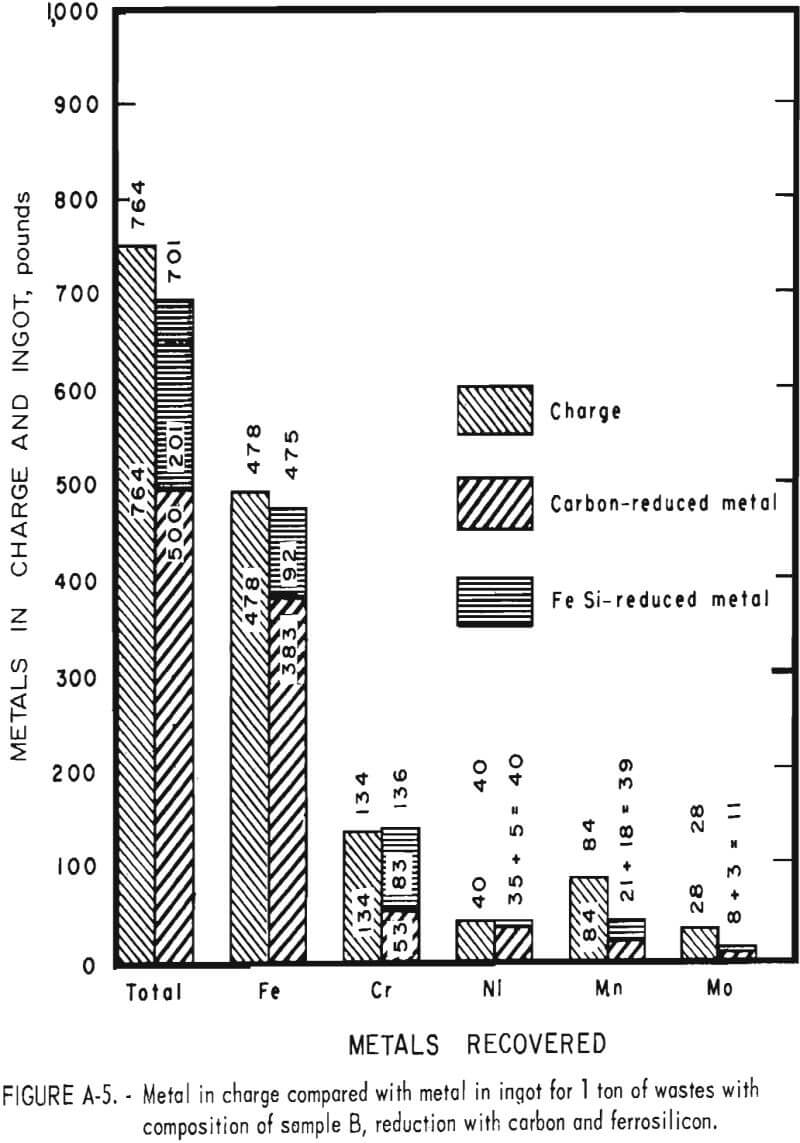
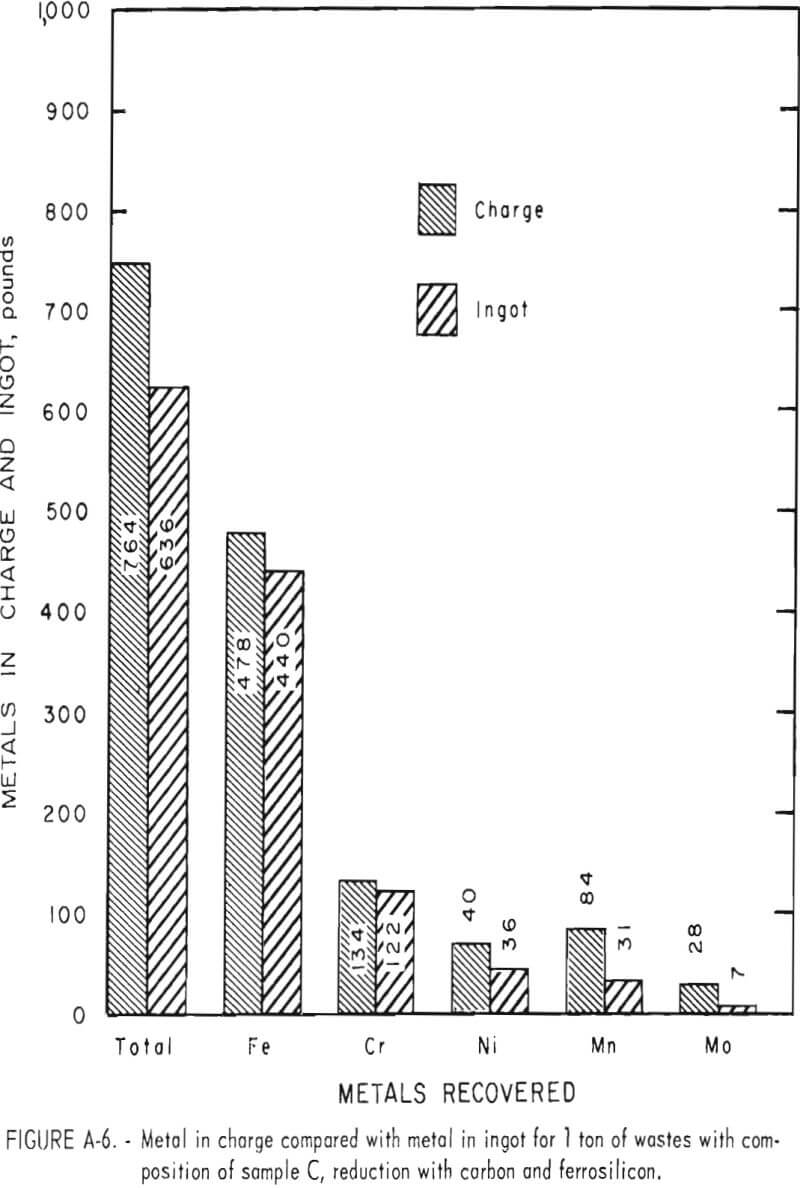
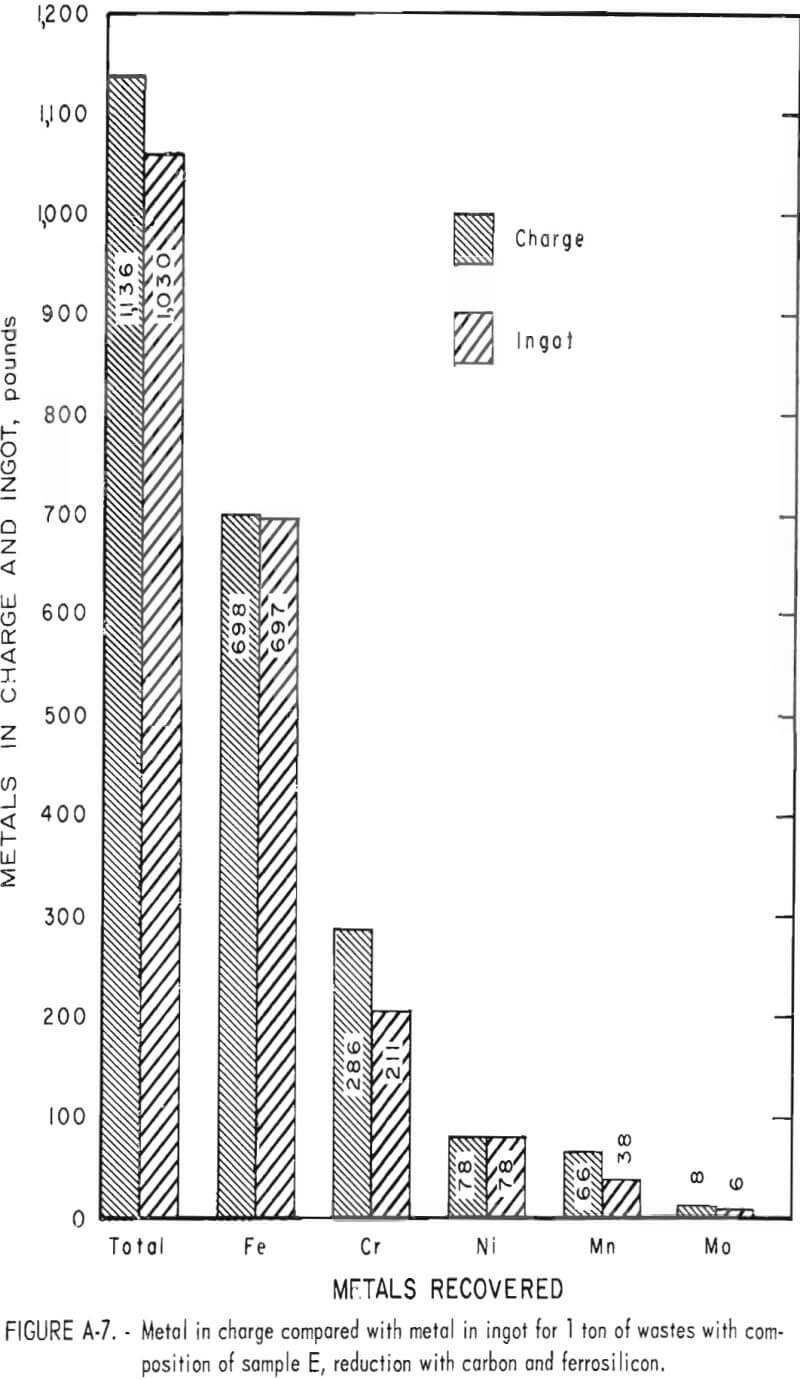
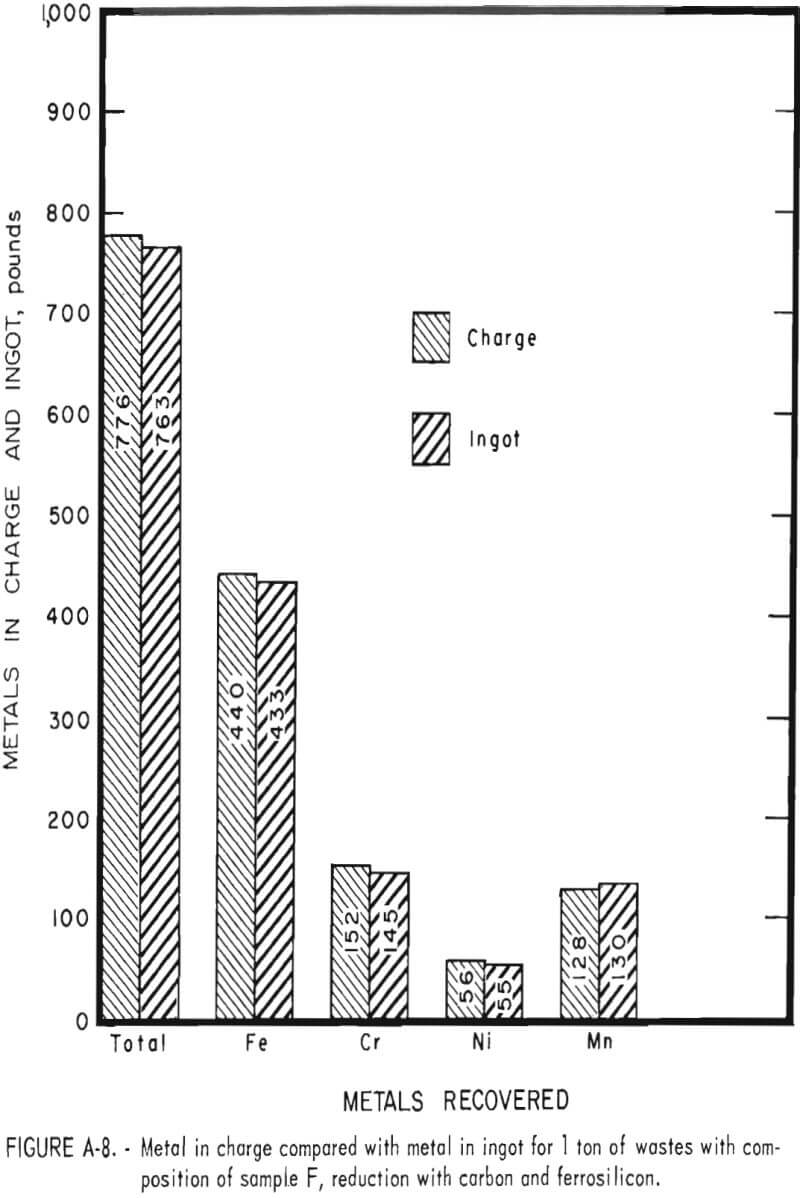
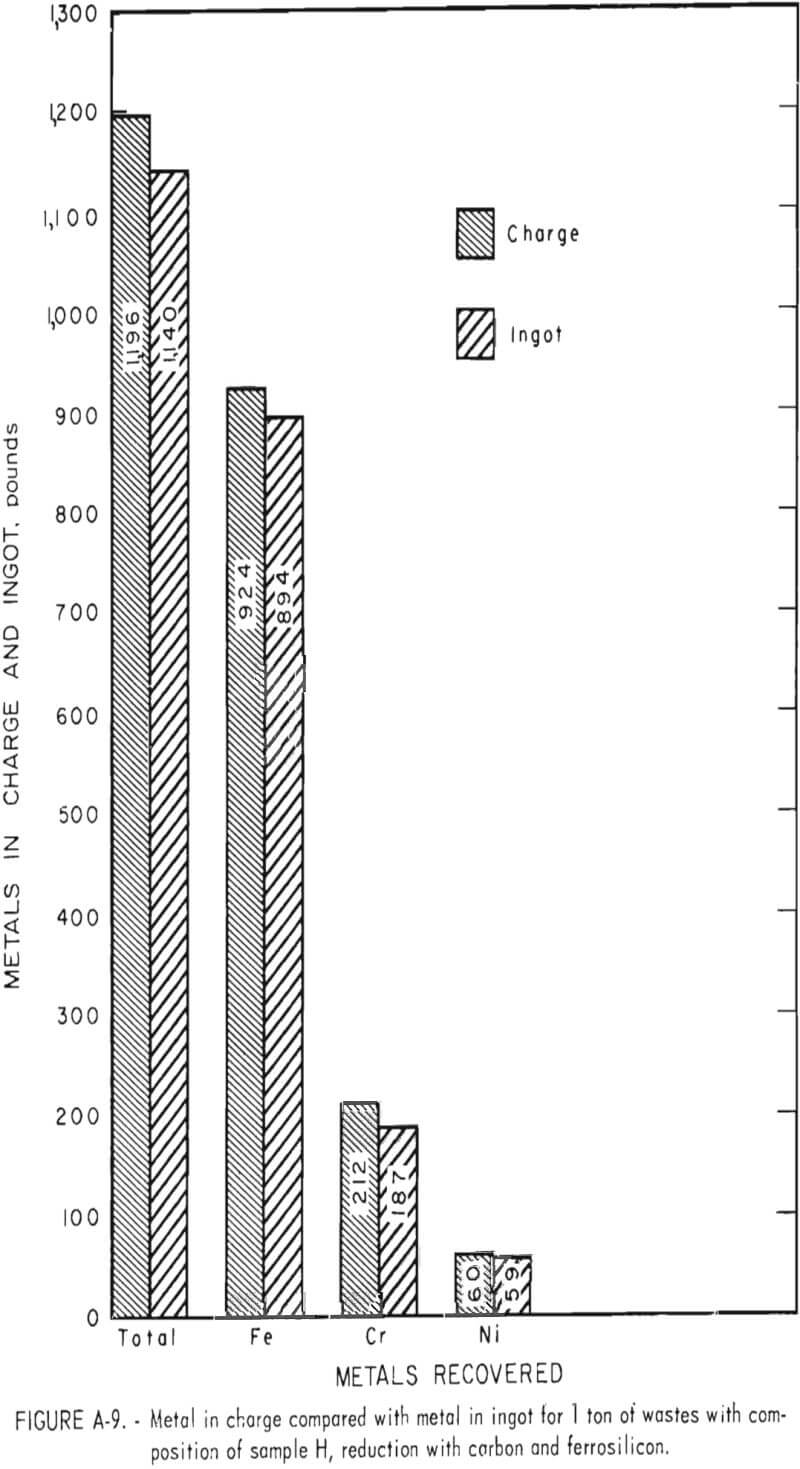
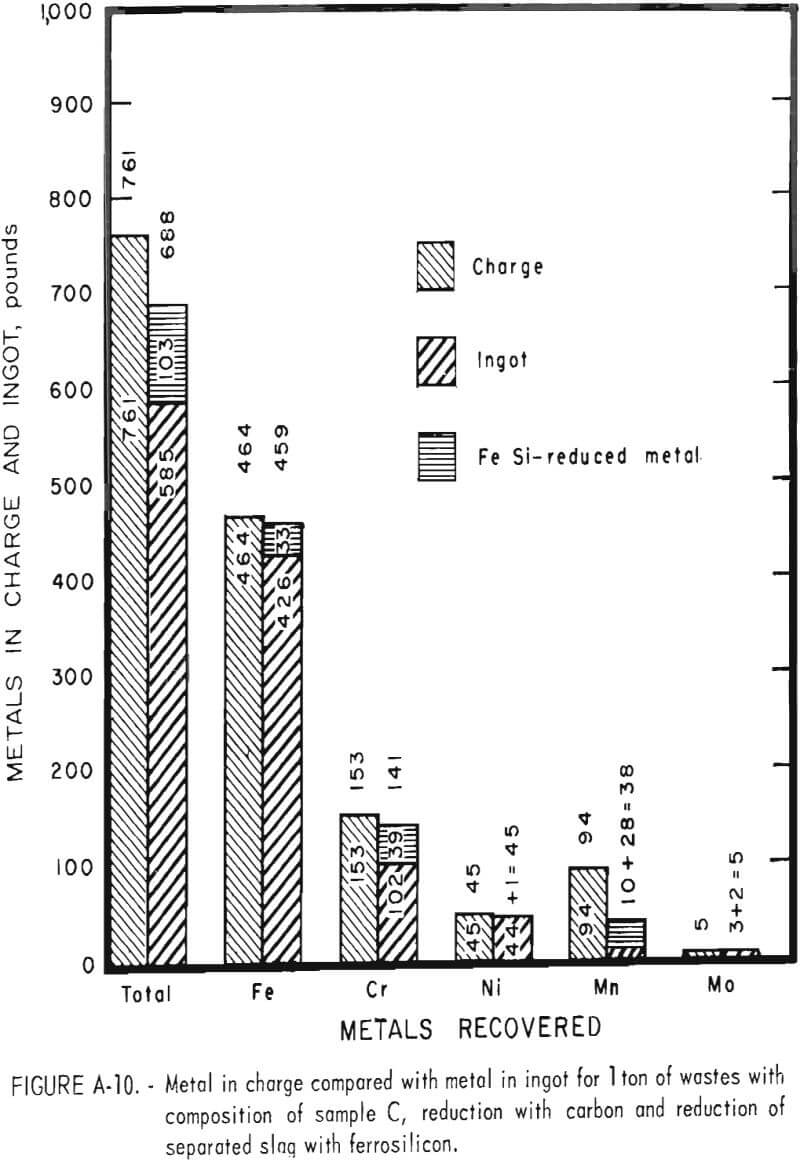
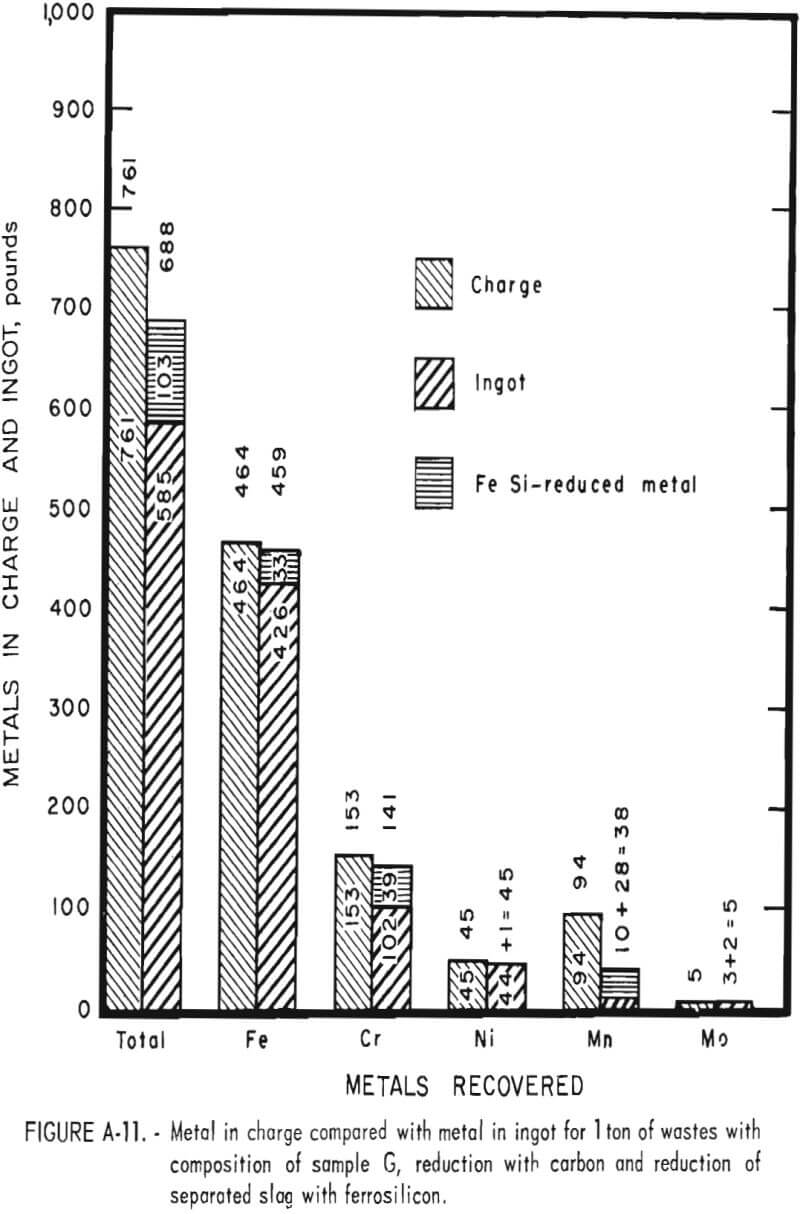
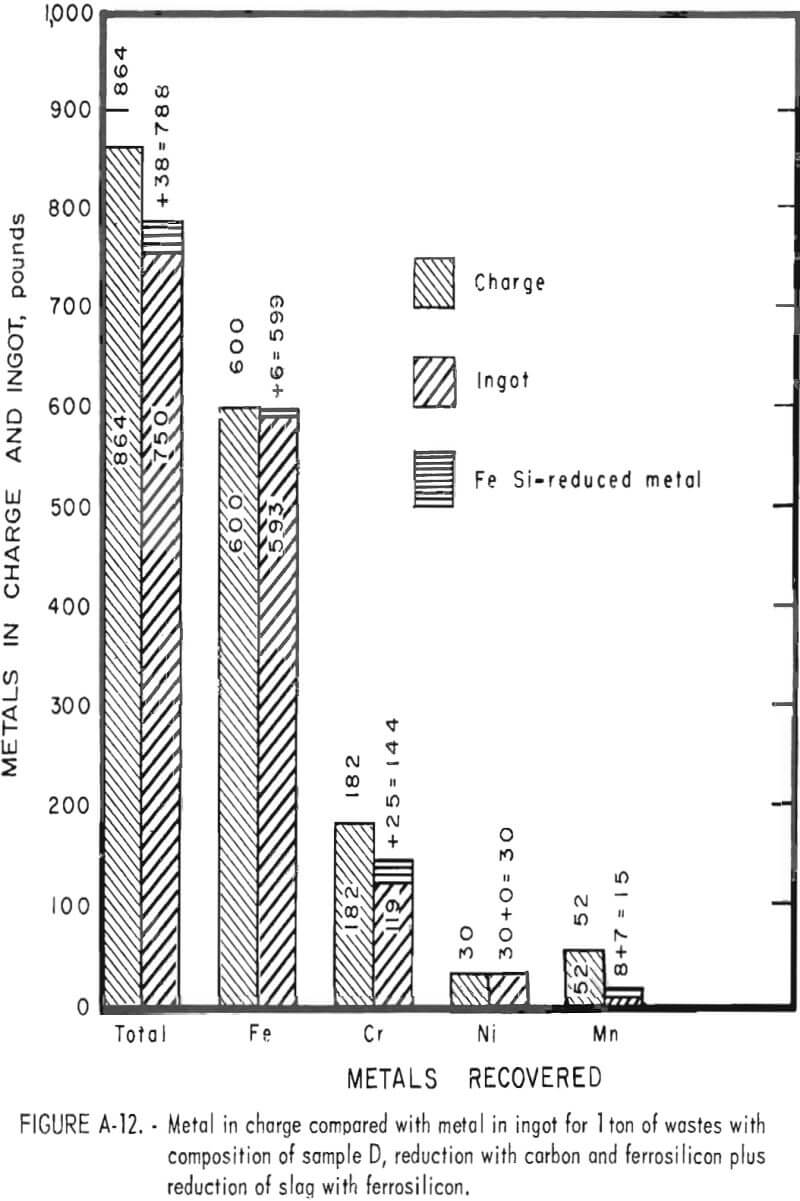
Tests conducted in accordance with the aforementioned procedures were made on the various dusts, mixtures of dusts, and mixtures of plant wastes. Typical analyses of the ingots produced and the percentage of recovery of each metal from the various tests are shown in tables 3 through 7. Figures A-1 through A-13 of the appendix present additional data in the form of bar charts that compare the amount of metal contained in the wastes (charge) with the amount recovered (ingot). This comparison is based on a calculated charge of 1 ton of waste and presents the amount (in pounds) of each metal of interest versus the amount in the 1-ton charge. Figures A-1, A-2, and A-3 show the results of carbon reduction of samples A, B, and H, respectively. Figures A-4 through A-9 show the results of reduction with carbon followed by stage reduction with ferrosilicon of samples A, B, C, E, F, and H. The total addition of ferrosilicon was 6 percent. Figures A-10 and A-11 show results obtained for samples C and G, respectively, by reducing with carbon, separating the slag, then stage-reducing the slag with ferrosilicon. Again, the total ferrosilicon used was 6 percent. Figure A-12 is a variation of the stage-reduction procedure. In this test, sample D was reduced with carbon followed by a single-stage reduction with 3 percent ferrosilicon before a slag removal, and an additional 3-percent-ferrosilicon reduction of the slag after it was removed from the melt. Figure A-13 shows the effect of a single-stage carbon reduction of sample B with 3 percent ferrosilicon added to the pellet during pelletization. In all tests, the ingots were high in carbon, which consistently ranged from 4.5 to 4.8 percent. Silicon in the metal was related to the excess of ferrosilicon used and varied widely, ranging from less than 1 percent to nearly 5 percent.
Summary
Table 8 provides a summary of data from the individual dusts, the composite of five dusts, and the composite of four plant wastes. These data were obtained via the reduction with carbon plus a scavenging reduction with ferrosilicon, and illustrate technical efficacy of the process for recovering the metals of major interest iron, chromium, and nickel. Both recovery and composition of the alloy were high for these metals. For the minor metals-manganese and molybdenum results tended to be unpredictable and erratic. Much managanese sublimed to the fume, as did an appreciable quantity of the molybdenum; however, these fume values were also erratic and were not controllable or reproducible.
Discussion and Conclusions
Evaluation of the data presented in tables 3 through 7, figures A-1 through A-13, and observations made during the experimentation led to the following conclusions:
- Electric furnace dusts from the production of stainless steel are amenable to reduction and yield a recyclable stainless steel.
- Maximum recovery of the metals, especially chromium, requires reduction with carbon followed by a scavenging reduction with ferrosilicon.
- With induction furnace melting, a carbon crucible (or liner) is necessary because the stainless steel furnace dusts are not electromagnetically susceptive. However, the carbon serves a dual purpose; because it does not react with the reduced chromium, it permits essentially complete removal of chromium from the slags.
- All electric furnace dusts investigated were amenable to pelletization; however, requirements for binder vary from dust to dust and each must be evaluated individually. Portland cement in amounts varying between 2 and 6 percent yielded acceptable pellets for all wastes considered in this work. Air drying for at least 24 hours plus oven drying at 250° C for at least 4 hours produced pellets that charged to either hot or cold furnaces without undue dusting or disintegration.
- Mill scale or centerless grinding swarf may be incorporated into the pellets with furnace dusts and smelted without change in technique. Cutting oils in the swarf do not have a deleterious effect on the pellets or on the subsequent smelting process.
- Slags produced during the smelting of furnace dusts and other stainless steel production wastes are generally viscous and require the use of modifiers such as spar, lime, or other conventional modifiers to fluidize them to an acceptable degree. Ferrosilicon, in additon to reducing the metals, also fluidizes the slag. Requirements vary from dust to dust, and each must be considered alone.
- Although an economic evaluation of the process was not made due to the small scale of the tests (25-pound charges), there is reason to believe that the proposed process is economically feasible and attractive. Both capital and operating costs for induction furnace smelting are well known, and the proposed process conforms to conventional practice. In addition, the value of the product is high. One ingot from sample A, analyzed and evaluated by a major producer of stainless steels, was priced at $385 per ton based on 1973 prices for nickel and chromium. All alloys recovered were similar in composition; thus, the dollar value would be similar for all wastes. Pelletizing is also straightforward and costs are well documented. The fume, a rich zinc lead product, should be of economic interest either as a directly salable product or as a product for further refining. Slags are hard and friable and because they are free of toxic metals , they should find ready sale as aggregates. Additional research is in progress to obtain operating and cost data to establish the economics of the proposed recycling process.
![]()
![]()
