Table of Contents
One of the goals of the Bureau of Mines is to insure that an adequate supply of minerals is available to meet national, economic, and strategic needs. To help reach this goal, the Bureau is conducting research to advance minerals processing technology, which includes investigations for recovering metals from complex sulfide ores and concentrates.
Marketing complex sulfide concentrates that contain a variety of metals along with arsenic and antimony has always presented a problem. This situation has been compounded recently by increasing smelter costs. These factors have stimulated considerable interest in the development of hydrometallurgical procedures for recovery of metal values from sulfide concentrates.
Chlorination and acid-oxygen leaching procedures have been investigated for the recovery of metal values from sulfide concentrates. Favorable metal recovery values are obtained with the chlorination technique. However, the system results in the dissolution of metals such as iron and arsenic; and additional separation schemes are required to separate and remove these metals. The acid-oxygen leaching technique is more selective, but metal extraction is generally less than that obtained by chlorination. Also, some of the iron is extracted into solution and additional processing steps are required to remove it.
More recently, Haver and Wong developed a ferric chloride leaching technique for treating chalcopyrite and galena concentrates. In this system the metal sulfide is oxidized by ferric chloride to produce metal chloride and elemental sulfur:
CuFeS2 + 4FeCl3 → CuCl3 + 5FeCl2 + 2S………………………………………………………(1)
PbS + 2FeCl3 → PbCl2 + S + 2FeCl2……………………………………………………………..(2)
The system successfully treats classical concentrates such as chalcopyrite and galenas but as the concentrates become more complex in mineral composition, the ferric chloride system has several disadvantages. If a galena concentrate containing excessive amounts of iron, copper, and silver is treated the iron, copper, and silver are coextracted and must be separated. The simplest separation method to remove the silver is to use lead shot to precipitate the silver. The resulting silver-lead product must be fire refined to produce high-grade bullion. A diaphragm cell is required to recover the copper, and a turbo aerator sequence is required to reject the iron that dissolves during leaching and to regenerate the ferric chloride solution for recycle.
The objective of this investigation was to test a ferrous chloride-oxygen leach system as an alternative method for leaching a complex, refractory sulfide concentrate. The proposed ferrous chloride-oxygen leach sequence differs from ferric chloride leaching in that the combined use of ferrous chloride and oxygen effectively converts silver and base metals to chlorides, and simultaneously rejects iron as Fe2O3 and sulfur in the elemental form, all in a single step. The leach system operates without excess chloride ion so that copper-silver separation is achieved in the leach step.
Materials and Equipment
The composition of concentrate used in experiments described in this paper is shown in the following tabulation. This concentrate is the end product of a leaching sequence with a sodium hydroxide-sulfur mixture to remove the antimony from a tetrahedrite flotation concentrate. During leaching, a portion of the sulfide in the concentrate was converted to sulfate, which accounts for the high sulfate content of the concentrate. The oxygen content is residual hydroxide remaining after the leach step to remove the antimony from the tetrahedrite.
The ferrous chloride-oxygen leaching experiments were conducted in several different sizes of low-pressure glass or Teflon-lined reactors, with titanium, and Teflon agitators. The charge to the reactor ranged from 50 to 4,000 grams of concentrate, depending on the size of the reactor. Larger scale experiments were conducted in a 14-liter reactor constructed from an 18-inch length of 8-inch-diameter steel pipe flanged on both ends. A Teflon liner was employed to protect the pipe and flanges from the corrosive solutions, The bottom and top of the reactor were sealed by bolting ½-inch- thick titanium end closures to the pipe flanges. The stirrers, temperature well, and gas inlet were made of titanium, as were the ball valves for emptying the reactor. The apparatus is shown in figure 1. Oxygen was added at 40 psig and monitored by a totalizing mass flowmeter.
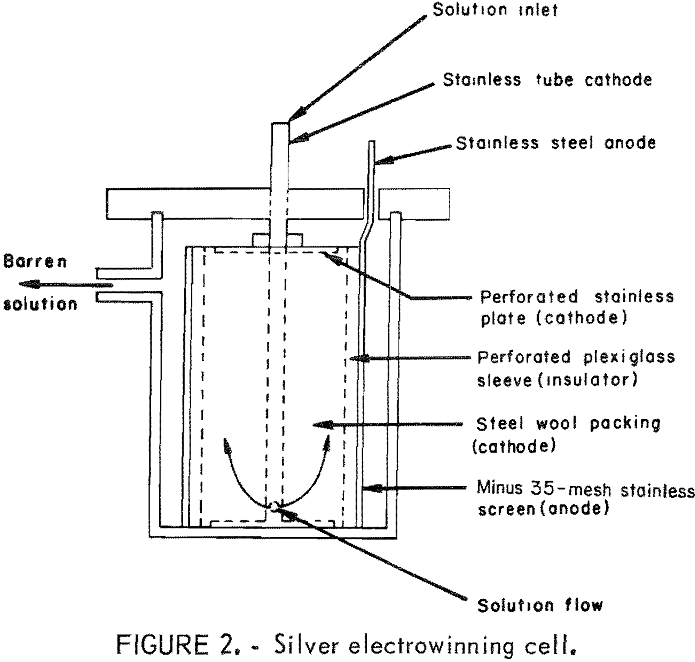
The cell consists of a 3-liter Pyrex beaker equipped with an overflow spout. The anode consists of a 35-mesh stainless steel screen, 4 inches in diameter and 7 inches high. The cathode consists of a stainless steel inlet tube with an attached circular bottom and top plates 3½ inches in diameter The top plate is perforated, and steel wool is wrapped around the inlet tube between the top and bottom plate. The anode and cathode are separated by a tubular, perforated, 3-7/8-inch-diameter plexiglass sleeve. Solution from a holding tank is pumped into the cell near the bottom of the tube and flows upward and outward through the insulating sleeve and anode. The solution exiting the cell container was returned to the holding tank and recirculated through the cell until the silver values in solution were depleted.

Metal extractions were determined by complete material balances. All solutions and residues were analyzed by either atomic absorption or standard chemical techniques.
Experimental Procedures
Experiments were conducted in the following manner: The concentrate and a predetermined amount of FeCl2·4H2O were slurried together with water in the reactor. Oxygen was added at a predetermined pressure until the system stopped consuming oxygen. The reaction of oxygen with the concentrate is exothermic, and it was necessary to control temperature in the 100° to 110° C range by cooling. At the end of the leaching period, the slurry was cooled to room temperature to allow trace amounts of lead chloride to precipitate. The slurry was then filtered and the filter cake was washed with a volume of water equal to the initial volume used in the reactor. The pregnant solution and wash solution were combined, and the copper was precipitated by adding iron metal in the form of shredded cans. The silver was extracted from the reaction filter cake by slurrying the solids with a cyanide solution and agitating the slurry for 1 hour. The silver was recovered from the cyanide leach solutions by electrolysis. A drawing of the electrowinning cell is shown in figure 2. This silver-recovery cell was developed by the Bureau of Mines to recover gold and silver values from activated carbon strip solution.
Results and Discussion
A conceptual flow diagram giving essential details of the ferrous chloride-oxygen leach process as applied to treatment of the silver-copper concentrate is shown in figure 3. Basically, the process consists of reacting the concentrate with oxygen and recycling ferrous chloride for 4 hours at 100° C and 40-psig pressure. Copper is extracted as soluble copper chloride; silver is converted to insoluble silver chloride. Copper is recovered from the leach solution as metal by cementing iron, and the resulting ferrous chloride is recycled directly for additional leaching. Silver is extracted from the original ferrous chloride leach residue by cyanidation and then recovered as metal by electrowinning. Copper and silver recoveries exceeding 98 pct each have been achieved.
The chemistry of the leach step is somewhat difficult to interpret because the original tetrahedrite mineralization was altered to an unidentified mixture of metal sulfides, which was caused by the pretreatment to recover antimony by leaching with a hot mixture of sodium hydroxide and sulfur. Because the feed material to the ferrous chloride-oxygen leach step is comprised of nonstoichiometric compounds, the following generalized equations serve only to give a qualitative description of the chemistry involved:
3FeCl2 + ¾ O2 → ½ Fe2O3 + 2FeCl3…………………………………………….(3)
6FeCl2 + 3/2 O2 + H2O → 2FeO(OH) + 4FeCl3………………………………………(4)
(CuFe2Ag)Sx + 2XFeCl3 → X(CuFe2Ag)Cl2 + 2XFeCl2 + XS……………………………………(5)
(CuFe2Ag)Sx + 4XFeCl3 + XO2 + 2XH2O → X(CuFe2Ag)Cl2 + 4XFeCl2 + 2XHCl + XH2SO4…………(6)
(CuFe2Ag)Sx + 2XHCl + X½ O2 → X(CuFe2Ag)Cl2 + XH2O + XS………………………..(7)
The initial reaction is the conversion of FeCl2 to FeCl3, which reacts with the metal sulfides. All of the reactions, 3 through 7, take place during

leaching. The major reactions are those in which ferric chloride is regenerated from ferrous chloride and reacts with the sulfide minerals to produce metal chlorides. The minor reactions are those, in which sulfate and hydrogen ions are formed. These reactions account for conversion of 6 pct of the sulfide contained in the concentrate to sulfate during leaching. The major difference between this sequence and ferric chloride leaching is the fact that iron is converted to oxides during leaching and silver remains insoluble in the leach solution.
Initial experiments to establish the leachability of the concentrate and to determine the effect of operating conditions were conducted on a 50-gram scale. The first parameter studied was the effect that the amount of ferrous chloride added to the reaction system has on copper and silver extraction.
The amount of ferrous chloride employed in the tests was ranged from 50 to 110 pct of the theoretical amount required to convert all of the cupric copper, lead, zinc, and silver in the concentrate to chloride salts. The results of this series of tests are shown in figure 4.
The data are interesting in that copper extraction increased almost linearly with increasing addition of ferrous chloride up to 97 pct and then leveled off. In contrast, silver extraction by subsequent cyanidation was

almost uneffected until 85 to 90 pct of the ferrous chloride had been added. When 100 pct of the theoretical amount of ferrous chloride had been added, silver extraction increased sharply to its maximum of about 98 pct. The data illustrate that the copper reaction takes place first, consuming nearly all of the reactants, and thus preventing reaction of the silver.
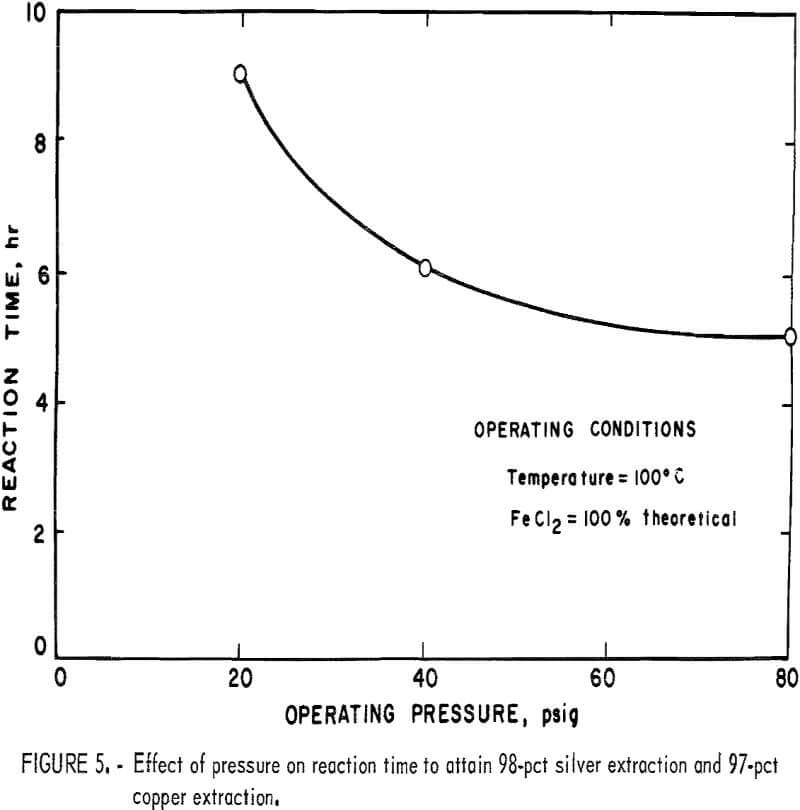
The operating pressure of the leach system is an important parameter because it affects the solubility of oxygen in the leach solution, which, in turn, affects the rate of reaction. Figure 5 shows the influence of oxygen pressure on the operating time required to reach 97 pct copper extraction and 98 pct silver conversion at 100° C when employing 100 pct of the theoretical amount of ferrous chloride required. As expected, the data show that time required to complete the leach reaction declines at a decreasing rate as the pressure is increased. Under these operating conditions, the reaction time becomes nearly constant at pressures above 80 psig.
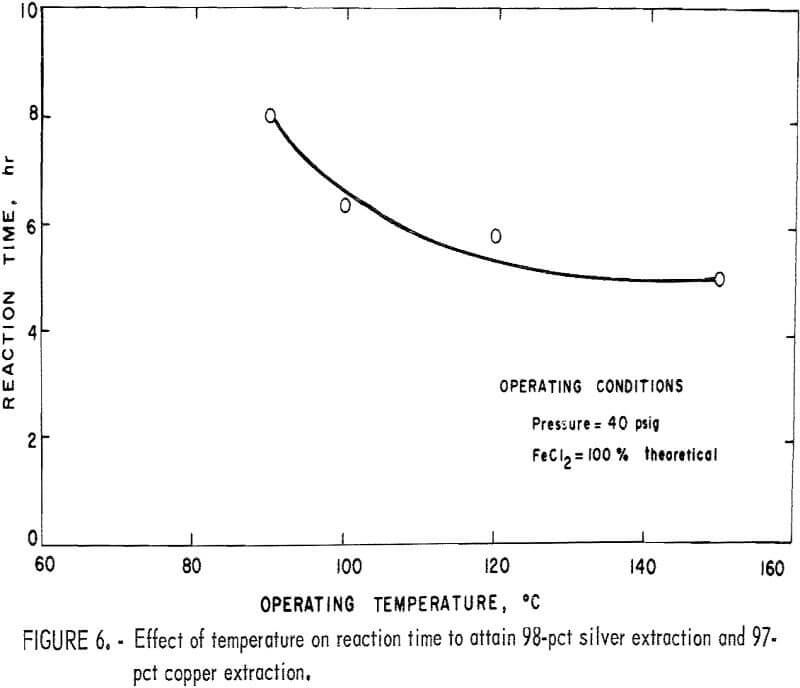
The rate of reaction is also affected by temperature. The effect of temperature on the time required to reach a constant extraction of 97 pct copper
and 98 pct silver at 40 psig when using 100 pct of the theoretical amount of ferrous chloride required is shown in figure 6. These effects are similar to those obtained by changes in operating pressure in that the time required to complete the leach reaction declines at a decreasing rate until it becomes nearly constant after 4 hours of leaching.
Also investigated was the effect of hydrogen ion concentration on the leach system because it was felt that this variable could be important in terms of what chemical reaction was predominant during leaching. The pH of the ferrous chloride solution generated during cementation of the copper is 1.74, and the use of this solution as a leachant resulted in a final pH of 1.8 to 2.0. The pH of the ferrous chloride leach solution was lowered to 0.32 stagewise in a series of experiments, and it was found that the copper and silver extraction was independent of hydrogen ion concentration within the range studied.
Based on data obtained in tests using a small reactor, the treatment sequence was scaled up to a reactor 14 liters in size. A charge of 4,000 grams of concentrate, 977 grams of ferrous ion as FeCl2·4H2O, and 8 liters of water were added to the reactor. Pressure in the reactor was held at 40 psig with oxygen. The temperature quickly increased to 100° C, and the temperature
was controlled between 100° and 115° C by cooling. The reaction was complete in 4 hours. The oxygen consumption was 300 grams or 150 lb/ton of concentrate. The reaction time of 4 hours is 2 hours shorter than the time required for similar experiments in a 50-gram-capacity reactor. The shorter time is attributed to the more violent agitation achieved in the 14-liter reactor. Silver and copper extractions of 99.4 and 98.6, respectively, were obtained when the pulp was treated by the sequence described in figure 3. Silver and copper extractions were also higher in the larger reactor when compared with those in the 50-gram reactor. The reason for this increase, again, is due to the increased agitation achieved in the larger reactor, which gives better contact between the mineral particles and the oxygen. This experiment was repeated three more times, and silver and copper extractions ranged between 99.1 to 99.7 and 98.2 to 98.6, respectively. The data obtained in these experiments showed that 65 pct of the sulfide portion of concentrate reacted with the ferric chloride, and, of this, 94 pct of the sulfur was oxidized to elemental sulfur with the remainder being converted to sulfate ion. The sulfate originally contained in the concentrate remained insoluble during the ferrous chloride-oxygen treatment. More than 99.9 pct of the iron, antimony, and arsenic were converted to insoluble oxides and reported to the tailings.
Cyanidation and Electrowinning
Silver was recovered from the leach residue by cyanidation followed by electrowinning. Experiments conducted at a pulp density of 25 pct, initial cyanide concentration of 33 g/l, and ambient temperature to determine required reaction times indicated that a constant silver extraction of 99.7 pct could be obtained with only 1 hour of cyanidation.
Silver was recovered from pregnant cyanide solutions by electrolysis in a cell operating at a fixed voltage of 1.8 and an amperage of 2.15 to 4.30. The silver content of the solution was reduced from 14.5 g/l to 0.1 g/l during electrolysis. The power requirements were 0.0224 kwhr/oz of silver recovered, corresponding to a current efficiency of 77.2 pct. After electrolysis, silver was recovered from the steel wool cathode by adding HCl to dissolve the iron and fire refining the residue. The resulting ferrous chloride solution can be used in the ferrous chloride-oxygen leaching step. The barren cyanide electrolyte would be recycled to the cyanidation step. The solid tailings from the cyanidation step would have to be washed for appropriate removal of cyanide prior to disposal or subjected to an oxidation step to destroy the entrained cyanide content.
Copper Recovery
Copper was recovered from leach solutions by cementation with iron as shown by the following equation:
CuCl2 + Fe° → Cu° + FeCl2………………………………………………………..(8)
A typical copper solution produced by ferrous chloride-oxygen leaching contained 122 g/l copper, 8 to 10 g/l zinc, 15 to 20 parts per million (ppm) lead, 20 to 30 ppm silver, 20 to 30 ppm iron, and 10.2 g/l sulfate ion. The cementation reaction, was performed by adding shredded iron cans to the copper solution and agitating vigorously for 20 min. The solution was filtered, and the cement copper was washed with water. Cement copper containing 88 pct copper, 3.1 pct iron, and 0.7 pct chloride ion was produced using 120 pct of the theoretical amount of iron required. The source of chloride ion is cuprous chloride. The resulting barren solution contained 0.009 g/l copper and 125 g/l ferrous ion. This solution in plant practice would be recycled to the reactor to treat a fresh batch of concentrate. With time, the concentration of zinc and sulfate ion would build up in the solution. Ion exchange or sulfide precipitation could be used to remove zinc from the recycle solutions, and sulfate ion could be precipitated by adding calcium chloride.
A 500-lb/day miniplant incorporating the leach and recovery sequence depicted in figure 2 is being operated at the Reno Metallurgy Research Center to quantify reagent requirements and determine optimum operating conditions for extracting metal values from this complex concentrate.
Conclusions
Silver and copper can be effectively recovered from a pretreated complex concentrate by a ferrous chloride-oxygen leach, followed by cyanidation and electrowinning. Silver extractions of 99.7 pct and copper extractions of 98 pct were obtained in 4 hours of leaching, employing 150 pounds of oxygen per ton of concentrate at a pressure of 40 psig and a temperature of 100° to 110° C. Greater than 99.9 pct of the iron, sulfur, arsenic, and antimony content of the original concentrate reports to the tailings as insoluble products.

