Table of Contents
Demand for lead for transportation uses (1) has continued to grow as requirements for batteries have increased. The quantity of lead used in battery manufacturing has reached a record high, and the recycling and recovery of lead from lead battery scrap represented 56 percent of the total secondary lead recycled in 1972. The two methods that are most widely used in the secondary lead industry are direct conversion into metal using a reverberatory furnace or smelting the battery plates in a blast furnace. In one method, battery plates are fed to a reverberatory furnace and are converted into soft lead suitable for kettle refining. A more efficient method for treating battery scrap appears to be smelting in a blast furnace. This method is the most popular, for the direct product is antimonial lead. However, both methods produce copious quantities of SO2. Large quantities of SO2 are produced because of the large amounts of PbSO4 and H2SO4 found in the scrap battery. The principal reactions occurring during reduction are
PbSO4 + 2C → PbS + 2CO2…………………………………………………….(1)
PbS + 2PbO → 3Pb° + SO2…………………………………………………….(2)
PbS + 7PbSO4 → 4[PbSO4 · PbOj]+ 4SO2……………………………….(3)
3PbS + 2[PbSO4 · PbO] → 7Pb° + 5SO2…………………………………..(4)
No new methods have been developed for recovering lead from lead scrap since the advent of the lead blast furnace. Numerous reports and patents have been published that describe improvements in the efficiency of the lead blast furnace, but in all cases , SO2 is still an air pollutant.
Because of the SO2 emission during the smelting of the lead battery scrap, a new method that would not require the use of scrubbers, washers, and other pollution control apparatus is desirable. As part of its program in secondary metals research , the Bureau of Mines has developed such a method.
The research was divided into three phases. In the first phase, all work was performed using pure chemicals to test basic concepts and reactions to determine if the basic idea was feasible. The second phase used battery paste material to determine the optimum parameters for reducing and recovering lead. The last phase used complete battery plates for testing the complete recovery process.
Battery Composition
Two lead scrap batteries were drained and disassembled to determine the general composition. The metallic portion of the battery, which included the cells, terminals, cell bars, and sludge, averaged 79 percent of the total battery weight. The nonmetallic portion, which included the battery case and plate separators, averaged 21 percent. The average automotive battery uses 22 pounds of lead, of which 12 pounds is lead oxide and 10 pounds is a lead alloy that may contain 3 to 6 percent antimony depending upon the manufacture. Cells from five different scrap batteries were analyzed to get an idea of composition ranges. The paste material was separated from the metallic-grid material, and the general distribution for the five batteries is show in table 1. Wet analytical methods were used to analyze the paste material for metallic lead, total lead, and sulfate. X-ray analysis was used to identify the compounds in the paste material. The composition ranges of the components in the negative and positive paste are shown in table 2. The most important component is the sulfate because this is what causes the SO2 emission when the batteries are smelted in a blast furnace. The wide range of sulfate compositions is caused by differences in the state of charge of the batteries when discarded. The total cell reaction of positive and negative plates is
Pb° + PbO2 + 2H2SO4 → 2PbSO4 + 2H2O……………………………………………….(5)
When the battery is fully discharged, the reaction goes to completion.
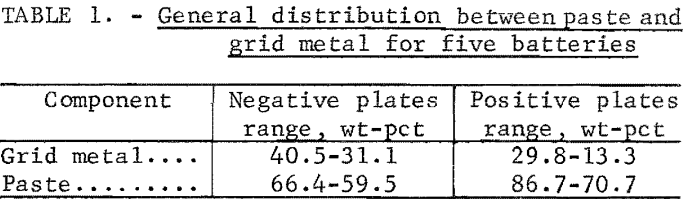
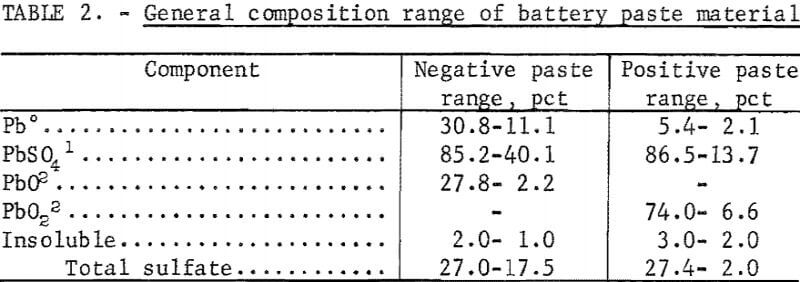
It is evident from the range of these results that it would be difficult to obtain a representative sample for a head composition. Therefore, all lead reductions and recoveries were based on the chemical compositions of the final products, and the lead recoveries were calculated on the basis of metallic lead recovered and the amount of total lead remaining in the reduced residue or spent flux. The reductions were calculated based on the amount of total metallic lead in the reduced sample.
First Phase using Pure Chemicals
The objective of the first phase was to determine if PbSO4 could be converted to PbO using Ca(OH)2 and if this reaction product could then be reduced to metallic lead.
Hydrometallurgical Conversion of PbSO4 to PbO
Ca(OH)2 + PbSO4 + H2O → PbO + CaSO4 • 2H2O……………………………………………(6)
where ΔH(25° C) = -5.5 kcal/mole
and ΔF(25° C) = -8.0 kcal/mole,
show that the reaction should go to completion at room temperature. No quantitative analytical method was available for determining the concentration of the compounds, that is, PbSO4 or PbO, while the Ca(OH)2 was being added. Thus , a semiquantitative X-ray diffraction procedure was used which reported relative amounts of products.
Tests were run using X-ray diffraction patterns to identify the different phases present during the reaction of PbSO4 and Ca(OH)2. Thirty grams of PbSO4 were slurried in 250 ml of water for these tests. A measured amount of freshly calcined lime (CaO) was slaked in a known volume of water to produce a slurry concentration of 0.025 g/ml. As the Ca(OH)2 was added, PbSO4 slurry pH measurements were made and samples were taken at intervals of 5 stoichiometric percent for X-ray analysis. The neutralization reaction apparently occurred in two steps, as follows:
4PbSO4 + 3Ca(OH)2 + 3H2O → 3PbO·PbSO4 + 3[CaSO4·2H2O]……………………………………(7)
3PbO·PbSO4 + Ca(OH)2 + H2O → 4PbO + CaSO4·2H2O……………………………………………….(8)
X-ray analysis identified only 3PbO·PbSO4 (white) and PbS04 (white) during the addition of 90 stoichiometric percent of the Ca(OH)2. At this concentration the slurry had become a thick paste. At 95 stoichiometric percent Ca(OH)2, some PbO (yellow) was detected and the slurry started to thin. A reaction time of approximately 5 to 10 minutes was required for the pH to stabilize when the final amount of Ca(OH)2 to reach stoichiometry was added. The slurry became very thin and turned the bright yellow color of PbO. A small amount of 3PbO·PbSO4 was still detected after all of the Ca(OH)2 had been added. An additional 10 stoichiometric percent of the Ca(OH)2 was needed before only PbO was detected in the slurry.
Three tests were run to determine the effect of temperature on the reaction rate for the conversion of PbSO4 to PbO using 110 stoichiometric percent Ca(OH)S. At 27° C it required 5 to 10 minutes for the pH to stabilize, and 2 to 5 minutes before the first appearance of the yellow PbO. At 40° and 80° C, the pH stabilized within 1 to 2 minutes and the yellow color of PbO appeared as soon as the addition of Ca(OH)2 was completed.
Two tests were run in which 20 grams of dry PbSO4 and 4.07 grams of Ca (OH)2 (10 percent excess) were mixed together and heated to 600° C. X-ray analysis showed that after 4 hours, one of the samples showed a trace (2 to 5 percent) of PbO. Therefore, a hydrometallurgical step was necessary for the conversion of PbSO4 to PbO using Ca(OH)2.
Reduction of Lead Reaction Products
Preliminary tests were run using 50-gram charges to study the effect of temperature on the reduction reaction. A 10- and 20-percent excess amount of carbon was mixed with the PbO-CaSO4 reaction products according to the following equations:
PbO + C → Pb° + CO………………………………………………………………………(9)
CaSO4 + 4C → CaS + 4CO……………………………………………………………….(10)
The mixture was placed in a furnace and heated to the desired temperature under a nitrogen atmosphere for a 30-minute reaction period. A semiquantitative X-ray diffraction procedure was used to identify the reaction products, and a chemical method was used for determining percent reduction. The results, presented in table 3, show that the major products were metallic Pb and CaS. The reduction of CaSO4 to CaS starts to occur between 700° and 800° C. The lead reduction reached a minimum between 700° and 800° C before increasing. Lead sulfide was also produced but decreased as the temperature increased.
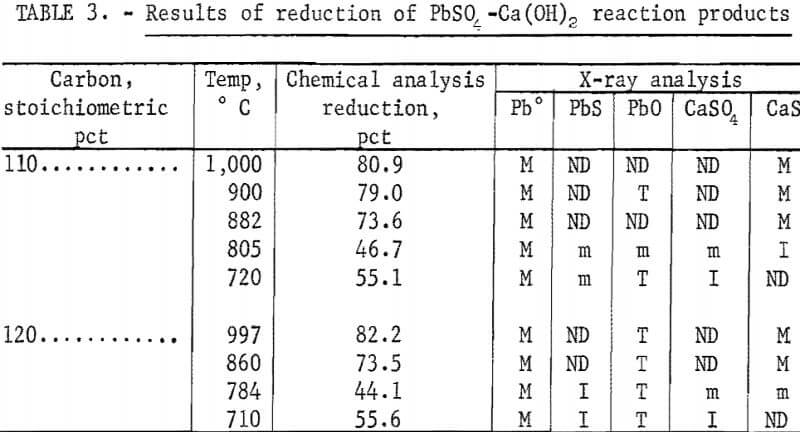
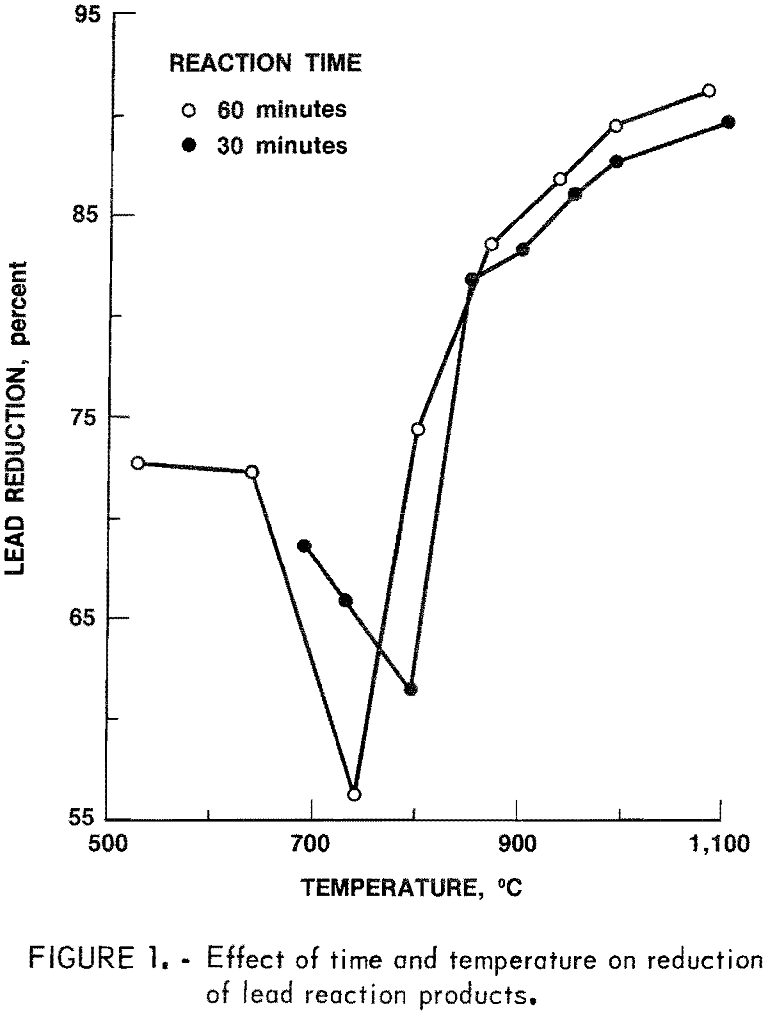
Tests run for 60- and 30-minute reduction times showed a difference in lead reduction of 2 to 4 percent at temperatures above 900° C, indicating that the reduction is rapid and can be achieved in 30 minutes or less at these higher temperatures. Figure 1 shows that the lead reduction decreased to a minimum at 750° C before increasing to a maximum at 1,075° C. Higher temperatures were not tried because of furnace limitations. No attempt was made to recover the reduced lead, although it was noticed that very little of the metallic lead was collecting in the bottom of the crucible.
Second Phase Using Battery Material
The first phase showed that the PbSO4 could be converted to PbO using Ca(OH2). Battery paste was used in the initial tests of the second phase, and finally the complete battery plate consisting of paste and grid material was used.
Reaction of Ca(OH)2 with Battery Material
Equal parts (approximately 500 grams each) of positive and negative plates were ball-milled together and screened to separate the paste from the metallic-grid material. The paste was analyzed for sulfate, and a calculated amount of Ca(OH)2 slurry was added. A problem developed that had previously been noticed but had not been anticipated on a larger scale. When the Ca(OH)2 slurry was added rapidly, an exothermic reaction occurred, the mixture started to thicken, and suddenly all of the material in the reaction vessel set up in a hard, cementlike mass. To avoid this problem in subsequent tests, the lime was added gradually, with good agitation or mixing, over a 15- to 30-minute period depending upon the size of sample used. It was assumed that all of the PbSO4 had been converted to PbO although no final analysis was made because of the lack of an available analytical method for determining PbSO4 in a mixture of lime, gypsum, and battery material. No PbSO4 was detected using X-ray analysis.
Reduction of Reacted Battery Material
Two series of tests were run on 50 grams of reacted battery material at temperatures of 700° to 950° C using different amounts of carbon based upon the total amount of lead salts present in the sample. Carbon was added to the first series of tests on the basis that all the reducible lead would be in the form of PbO by the time the sample started to reduce at 550° C. Lead dioxide (PbO2) starts to decompose at 290°. A maximum of 82 percent of the
lead was reduced, and the maximum recoveries were approximately 60 percent.
Because the lead reductions and recoveries were low, additional carbon was added in the second series of tests to also reduce the CaSO4 to CaS. The results of these tests were identical to those of the first series of tests. Two things occurred during these tests. First, the reduced lead would not liquate and coalesce because of the extremely fine particle size. Second, some sulfur dioxide gas was produced at temperatures above 800° C. Morgan reported the following reaction will occur at high temperatures:
CaSO4 + Pb° + 3CO → PbS + CaO + 3CO2………………………………………………….(11)
Lead oxide in the paste material reacts with the PbS to form SO2 according to equation 2. Therefore, further high-temperature work was suspended.
Separation of Reduced Lead
The objective of this work was to find a flux that would not enter into the reaction and also could be recovered and recycled. A series of tests using 250 grams of reacted battery paste was run at 600° to 700° C to try to coalesce and recover the reduced metallic lead. Several methods were tried. Briquetting the reacted battery paste with powdered charcoal gave poor recoveries. Small beads of lead were formed when chunks of charcoal were used, but the lead still did not coalesce into a molten pool. Coalescing and separating the metallic lead was not satisfactory because of the extremely fine nature of the reduced lead particles. Several different low-temperature fluxes were tried. One such flux was composed of Na2CO3, K2CO3 , borax, and fluorspar. The flux was very fluid at 650° C, but when it was mixed with the reacted battery paste, the recovery of lead was low. The use of other fluxing agents (borax, Na2CO3 and silicate flux) aided somewhat in the coalescing of the lead but did not increase the separation sufficiently to justify their further use.
A flux mixture that was found to be satisfactory was a 50-50 mole-percent mixture of KCl-NaCl that has a melting point of 658° C. When mixed with the CaSO4 , the flux forms a very fluid ternary-eutectic mixture, which melts at 599° to 605° C. The eutectic mixture is composed of 19.5 mole-percent CaSO4, 40.25 mole-percent KgCl2 , and 40.25 mole-percent Na2Cl2. The KCl-NaCl flux acts to liquate or solubilize the CaSO4. The KCl-NaCl can be recovered for recycling by leaching with water.
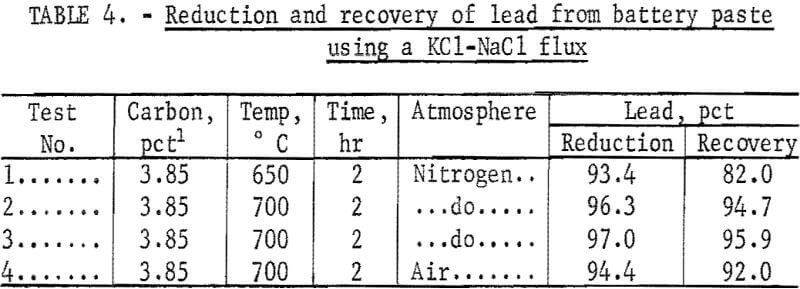
Preliminary reduction tests were made with battery paste that had been reacted with Ca(OH)2. Eighty grams of reacted material was mixed with an excess of carbon and KCl-NaCl flux, and an atmosphere of nitrogen was used to prevent oxidation. The results in table 4 show a high reduction and recovery of lead. Air was used in the last test and shows that the flux will protect the lead from oxidation. The reduction and recovery of lead was based upon the analyses of the final products because there was no way of obtaining an accurate analysis of the battery plate material.
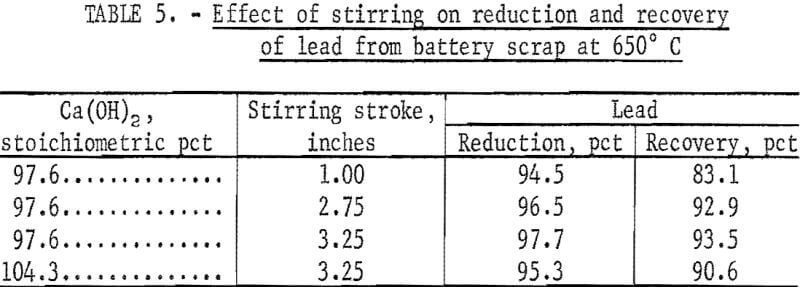
It was found that as the sample size was increased to 150 grams the lead reductions were decreased. Examination of the slag after the tests showed varying amounts of carbon. It appeared that the carbon collected in a layer between the metallic lead and slag and also near the surface of the slag. Rereduction or resmelting of the crushed slag for periods as short as 15 minutes without any additional carbon increased the lead reduction from between 75 and 80 percent to greater than 90 percent. Carbon has a lower density than the slag and, therefore , must be stirred into the molten slag. Stirring tests using a 600-gram charge were run in which the average depth of the melt was 4 inches. The molten lead was approximately 1.5 inches deep, and the slag was 2.5 inches deep. An up-and-down stirring action was used, and the stirring stroke or distance of travel was varied. The results in table 5 show that as the stroke distance was increased, the reductions and recoveries were increased. These tests show that the carbon must be pushed down into the molten slag to get good reductions. Additional tests showed that circular stirring produces a low lead reduction and recovery.
Reduction of Battery Plate Material
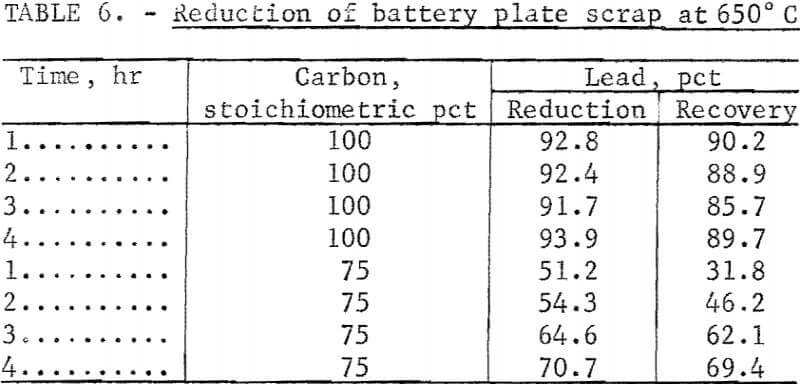
Complete battery plates (grids plus paste) were used to determine the effect of reduction time and the amount of carbon reductant needed. The paste was freed but not separated from the grid material by ball milling prior to the addition of the Ca(OH)2 slurry. A KCl-NaCl flux and varying amounts of carbon were added to the dried reacted material, and 200 grams of the resulting mixtures were used for the reduction test. The results in table 6 show there was little difference in the lead reduction at any time period when an adequate amount of carbon was present. The amount of carbon had a large effect. The percent of the stoichiometric carbon used was based upon equation 9. It was assumed that there would be an excess amount of carbon because carbon monoxide is produced which should also reduce some of the PbO.
Examination of the spent flux or slag after the reduction revealed that some carbon was still present when 100 stoichiometric percent carbon was used. When only 75 stoichiometric percent of carbon was used, the reduction and recovery of lead were decreased by 30 to 40 percent. Therefore, 100 stoichiometric percent of carbon should be the minimum amount required.
Small-Scale Tests
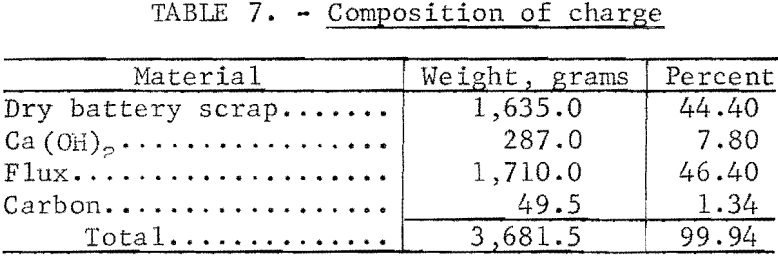
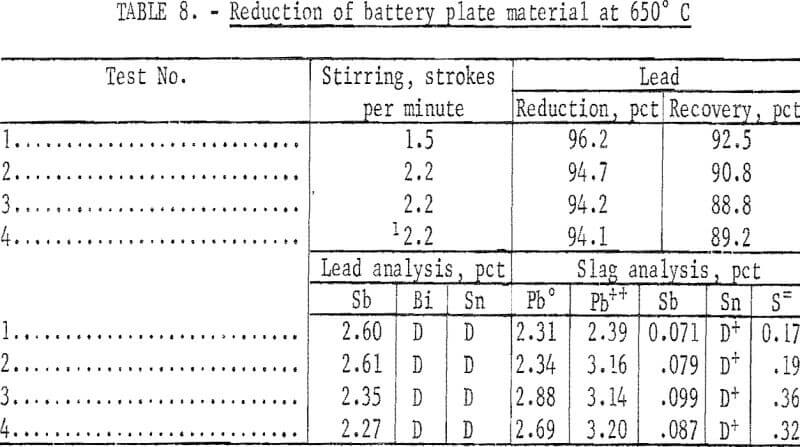
Small-scale tests were run utilizing the best conditions as determined by previous tests. Mixed battery plates were ball-milled for 1 hour in a ceramic mill to free the paste material from the grids as a fine powder. A stoichiometric amount of Ca(OH)2 slurry was added based on the sulfate analysis. The mixture was ball-milled another 30 minutes to insure good mixing and a complete reaction. After the reaction was complete , the material was filtered and dried at 120° C. Carbon and a 50-50 mole—percent KCl—NaCl flux were added and mixed. The composition of a complete charge is given in table 7. For the small-scale tests , 700-gram batches of the charge were used for the reduction tests run at 650° C, and results are given in table 8. A flow diagram is shown in figure 2.
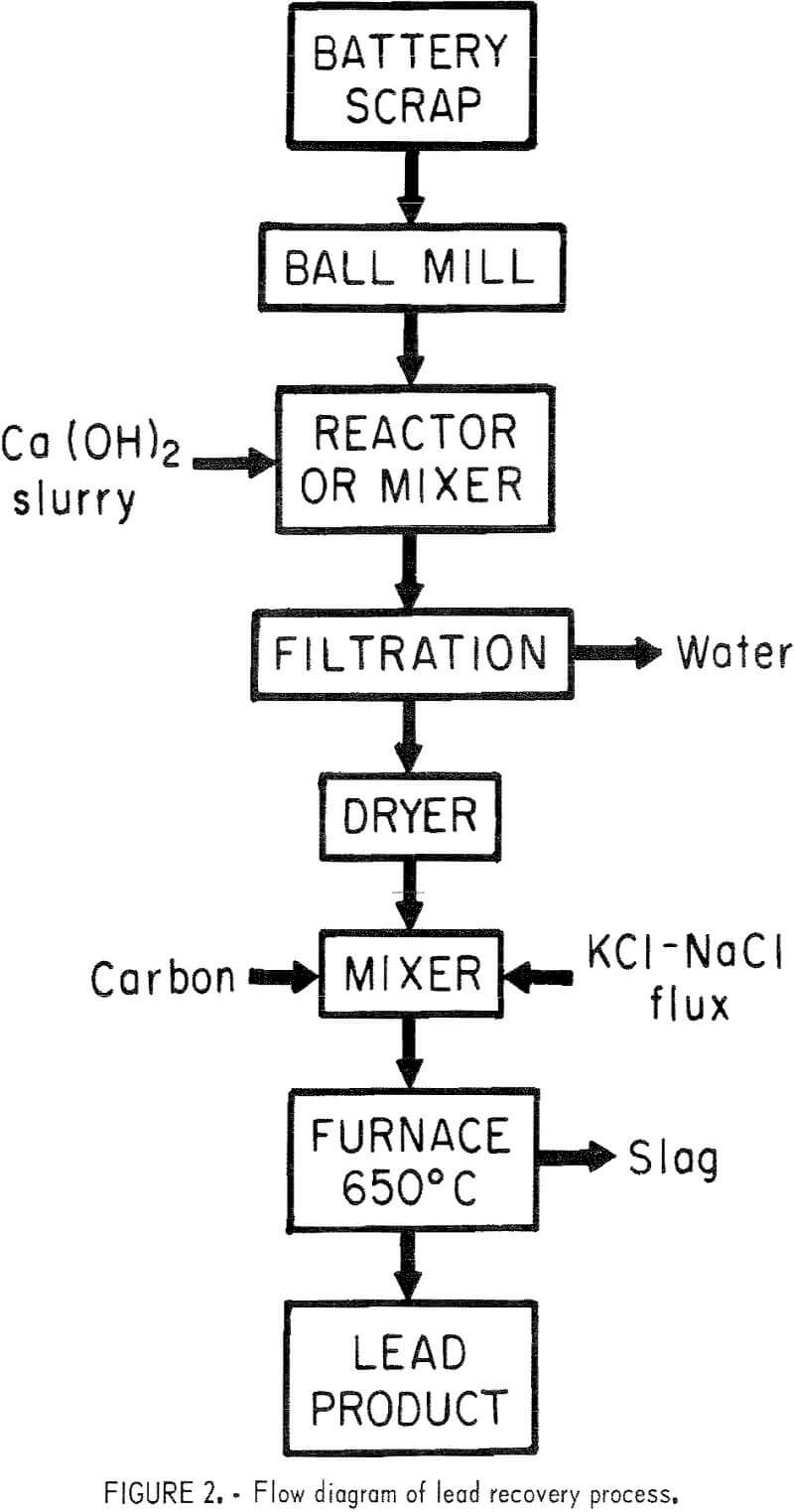
A simulated four-stage countercurrent water leach was used to recover the KCl-NaCl salts from the spent slag at room temperature. The saturated leach solutions were evaporated to reclaim and recycle the KCl-NaCl flux. This was a straightforward simple unit operation, and no difficulties were encountered.
Conclusion
An effective method was developed for recovering lead from lead battery scrap without producing SO2 by first using a hydrometallurgical step to convert the SO2-producing compounds to CaSO4·2H2O. A pyrometallurgical step was used to reduce 97 percent of the PbO to lead. The CaSO4 was solubilized at 600° C using a KCl-NaCl flux. The major steps are as follows:
- Ball mill the battery plates to free and grind the paste to a fine powder.
- Add the stoichiometric amount of Ca(OH)2 slurry to the paste, at room temperature, based on the sulfate content.
- Filter and dry the reacted material, at 120° to 200° C. This temperature is not important because the water of hydration of the CaSO4·2H2O will be lost when the sample is heated for reduction.
- Add 100 percent of the stoichiometric requirement for carbon (see equation 9) and a calculated amount of KCl-NaCl flux to give an eutectic mixture of 19.5 mole-percent CaSO4, 40.25 mole-percent K2Cl2 , and 40.25 mole- percent Na2Cl2.
- Reduce the battery scrap-lime-flux mixture at 650° C using some type of up-and-down stirring motion to insure that the carbon is stirred down into the fluid mixture.
- Recover the KCl-NaCl from the spent slag by water leaching and evaporation.

