Table of Contents
Numerous patents and references may be found that relate to studies of phosphate-rock defluorination processes. Most of these require sulfuric acid and heat, phosphoric acid and heat, or water vapor and heat for the defluorination reaction. One of the most thorough studies was made by K. D. Jacob and coworkers at the United States Department of Agriculture in the early 1930’s. The outgrowth of these investigations has been development of several commercial processes for manufacturing defluorinated phosphate rock. One of the processes, developed by Tennessee Valley Authority engineers, depends upon addition of enough silica to the charge to give a composition having a low melting point. In this process the phosphate rock and silica are fused in an oil-fired shaft furnace, and the melt is quenched and granulated by using high-velocity water jets. International Minerals & Chemicals Corp. developed a process in which P2O5, in the form of wet-process phosphoric acid, is mixed with finely ground phosphate rock before calcining. In a Coronet Phosphate Co. process described by Whitney and Hollingsworth, a large excess of silica is added to the charge to raise its fusion point above the defluorination temperature. Other processes in which phosphoric acid and heat are used to effect defluorination have been patented by Hollingsworth. The inclusion of aluminum phosphate in the calcining charge to promote and accelerate defluorination of phosphate rocks having a low silica content is described in a patent by Maust and Hollingsworth.
During the past several years considerable research has been done toward developing processes for removing fluorine compounds from industrial gases being vented to the atmosphere. Tennessee Valley Authority engineers developed a process for converting defluorination offgases into calcium fluoride by passing the gases through a tower packed with sized limestone. In a Coronet Phosphate Co. patently it is proposed that the hot fluorine-containing gaseous products of reaction be intimately contacted with a metallic compound capable of forming a fluoride therewith.
At this writing western producers of phosphatic materials are not recovering a salable fluoride from their plants; however, equipment for removing fluorine from the waste gases has been installed at most plant sites. Generally, an impure CaF2 product is being produced and stored in settling ponds until the economic situation with respect to fluorine compounds looks brighter. The Reynolds Metals Co. plant at Longview, Wash., is recovering fluorine from stack gases and converting it into synthetic cryolite suitable for use in alumina reduction cells.
Phosphate-Rock Beneficiation Research
Laboratory Scale
Western phosphate-rock deposits are sedimentary. They contain calcium phosphates in an amorphous state and many impurities. Some investigators have identified the phosphate-bearing material as calcium fluorophosphate, identical in crystal structure with fluorapatite, Ca10F2(PO4)6 Bureau of Mines petrographers prefer to use the term “collophane” in describing the principal constituent of western phosphate rock. Collophane is a particular species of the apatite group and is an intermediate member of the hydroxylapatite-fluorapatite series. It occurs as small nodules or oolites in the host rock, which in most instances is softer and more friable than the oolitic collophane. The host rock is composed of hydrous iron oxide, calcite, and minor metals in unidentified form, such as vanadium and chromium.
Various beneficiation techniques for separating low-melting-point impurities and vanadium from phosphate rock have been investigated. Low-melting-point impurities tend to retard the defluorination process at the temperatures involved. Vanadium may be toxic if the defluorinated product is used as an animal food supplement.
Two valuable products can be produced from some grades of western phosphate rock by controlled beneficiation practices – a sand product for use in production of phosphatic fertilizers and a slime product that contains most of the vanadium and chrome values. The potential value of the slime product may be increased by its vanadium and chrome content when used as a charge component in an elemental phosphorus furnace. These metals collect in the ferrophosphorus, a byproduct of elemental phosphorus smelting, and are recoverable from the ferrophosphorus.
Analyses of raw material and phosphorus products are given in this report as percent P2O5. Phosphate concentrations are recorded by many investigators as percent bone phosphate of lime (BPL or Ca3(PO4)2). For sake of comparison, percent P2O5 may be converted to percent BPL by multiplying by the factor 2.18.
Flotation Tests
The phosphate rock used in the initial defluorination tests was obtained from property of the San Francisco Chemical Co. near Montpelier, Idaho. The ore analyzed, in percent: P2O5, 31.1; SiO2, 6.6; Al2O3, 2.4; CaO, 46.0; V, 0.19; and F, 3.2. Minor constituents and CO2 known to be present in the rock are not included.
The flotation procedure used was one developed by the Mineral Dressing laboratory at Albany while making mineral-dressing studies of several samples of western phosphate rock in 1948. The phosphate rock was crushed to minus-65-mesh, pulped with water, and deslimed by decantation. The sand product was conditioned with sodium hydroxide and oleic acid, solids were allowed to settle, and the solution was decanted. The solids were repulped with water, and a silica concentrate was floated with lauryl amine hydrochloride, after which sodium silicate was added to depress the remaining silicates; the phosphate was floated, using oleic acid for the collector. The results of one of these beneficiation tests are shown in table 1.
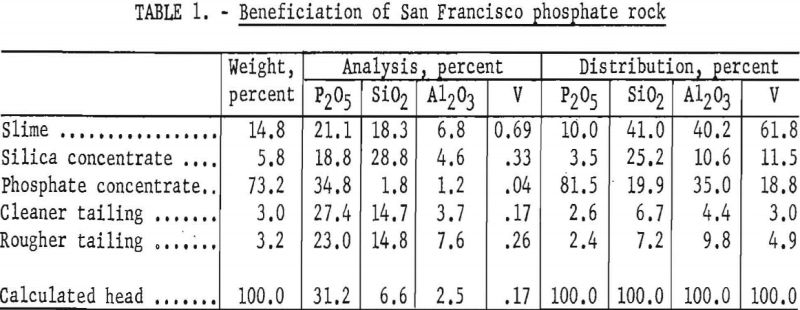
Desliming Tests
The grade of the flotation concentrates was not significantly higher than that of the deslimed phosphate rock. Consequently, the high-grade Simplot phosphate rock (32.9 percent P2O5), from near Fort Hall, Idaho, used in later defluorination tests, was only deslimed. The desliming procedure used in the initial laboratory tests was to pulp the sample with water at 20 percent solids, then permit the sands to settle 2 minutes before the slime was decanted. The pulping, settling, and decanting procedure was repeated three times.
Screening and elutriation tests have indicated the desirability of separating slime from the sand as soon as it is formed in the grinding operation. For instance, the minus-1,600-mesh (approximately 9 microns) fraction from stage crushing in rolls analyzed 0.60 percent V and 13.5 percent P2O5 and contained 32.3 percent of the V and only 1.7 percent of the P2O5 in 4.3 percent of the weight. The minus-1,600-mesh fraction, from a batch grind in a rod mill, analyzed 0.25 percent V and 20.6 percent P2O5 and contained 13.2 percent of the V and 3.6 percent of the P2O5 in 5.6 percent of the weight.
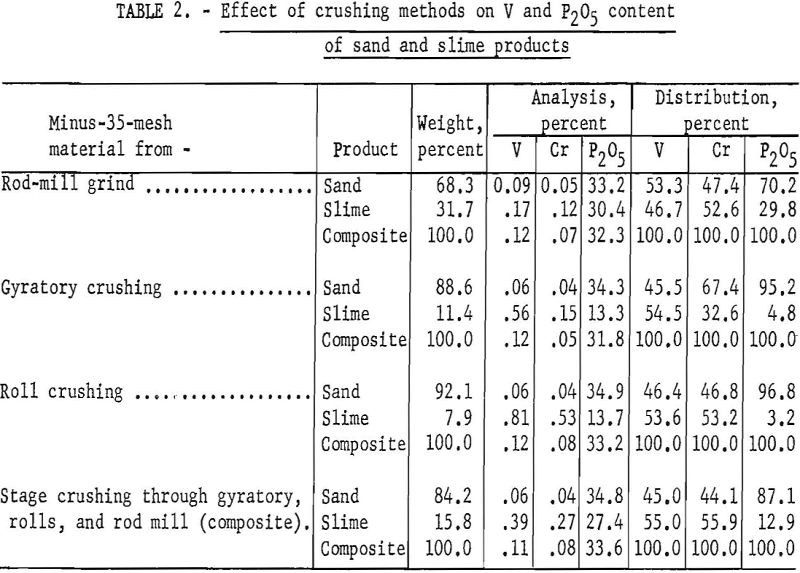
Results obtained from comparative desliming tests of Simplot phosphate rock further illustrate the advantage of removing the slime as soon as it is formed. Four samples were prepared for comparative desliming tests. The first sample was produced by grinding minus-½-inch phosphate rock to minus-35-mesh in a rod mill. The second sample was the minus-35-mesh fraction of phosphate rock crushed to minus-½-inch in a gyratory crusher. The third sample was the minus-35-mesh fraction from crushing the gyratory product to minus-6-mesh in rolls. The fourth sample was a composite of minus-35-mesh material from gyratory, rolls, and rod-mill crushing. All samples were deslimed as outlined above, and the sand and slime products were analyzed for V, Cr, and P2O5. Results of these tests are shown in table 2.
A beneficiation program started in March 1953 had two primary aims: First, development of an effective means for reducing the vanadium content of phosphate sand to a value as low as possible; and second, determination of the most economical grind and the best desliming method from the standpoint of effective phosphate-rock utilization.
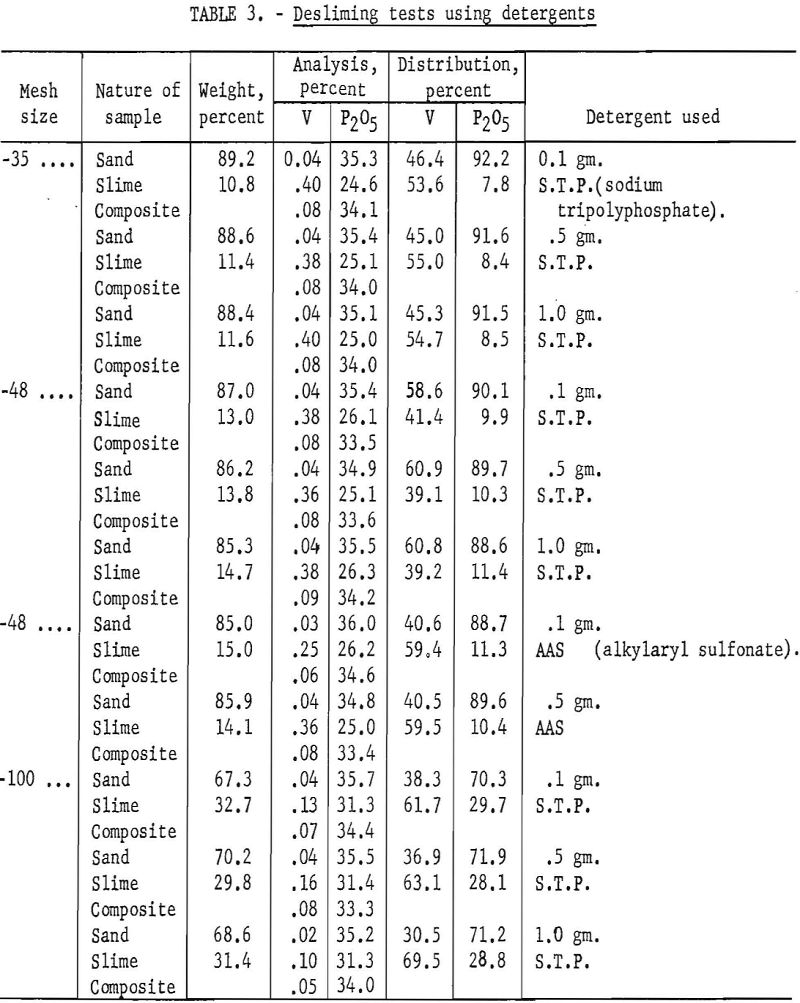
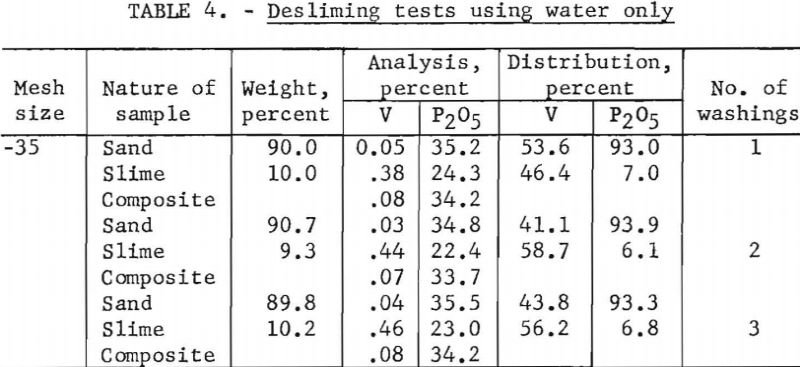
One phase of the program was a study to determine if detergents could be used to aid in removing residual slime from the sand grains. A detergent was added to the first desliming cycle, followed by three desliming cycles using water only. Sodium tripolyphosphate and an alkyl aryl sulfonate were tested in amounts equivalent to 0.4, 2, and 4 pounds per ton of phosphate rock ground to various mesh sizes. No significant differences in either P2O5 or V analysis were noted. Test data are shown in table 3. Following this study a short series of tests was made using water only to determine the minimum number of desliming cycles necessary for effective V removal. Indications are that more than two cycles do not remove a significant amount of V from the sand product. Results of these tests are shown in table 4.
Attention was now turned toward determining a beneficiation method that would produce both sand and slime products having economic value. A series of tests was made in which phosphate rock was ground to pass screens ranging in size from 10- to 150-mesh and deslimed by 2 water cycles. The main objective of the tests was to determine the fineness of grind necessary to produce a slime product usable as feed for an elemental phosphorus furnace.
Phosphate rock, coke, and silica rock comprise the usual phosphorus-smelting-furnace charge. Carbon, in the form of coke, is added to the furnace burden in an amount slightly in excess of the stoichiometric quantity required for reduction of all of the P2O5 and Fe2O3 to elemental phosphorus and metallic iron. A favorable SiO2:CaO ratio in the slag is obtained by the addition of silica rock. Smooth furnace operations require that this ratio be at least 0.7. For a complete discussion of the factors to be considered in the compounding of a furnace charge, the reader is referred to Waggaman or Curtis.
Data from the beneficiation test, which was aimed at slime utilization, are presented in table 5. These data indicate that grinding the phosphate rock to 35-, 48-, or 65-mesh will, in each instance, produce both sand and slime products that can be effectively utilized. However, since the primary aim of beneficiation is to produce the maximum amount of high-grade phosphatic material suitable for defluorination, the logical choice of grind is 35-mesh.
In table 6 analyses of the three previously mentioned suitable slime products from table 5 are compared with an analysis of a typical western phosphate shale suitable for furnace feed. Analyses indicate that, with little adjustment in charge composition, the materials could be used in an elemental phosphorus furnace. A comparison of the SiO2:CaO ratios shown in table 6 again indicates that the most suitable grind to use is minus-35-mesh.
Pilot-Plant Scale
One continuous beneficiation campaign was made to prepare phosphate sand for a pilot-plant fluorine-recovery test. The total amount of ore deslimed included approximately 6-½ tons of Simplot and 1 ton of San Francisco rock. The procedure used to produce the phosphate sand is outlined below.
The rock was dried, put through a gyratory crusher, and screened on a 35-mesh screen. The oversize then was crushed to approximately minus-10-mesh with crushing rolls. At this point all undersize and oversize were blended and fed to a small rake classifier in closed circuit with a small rod mill.
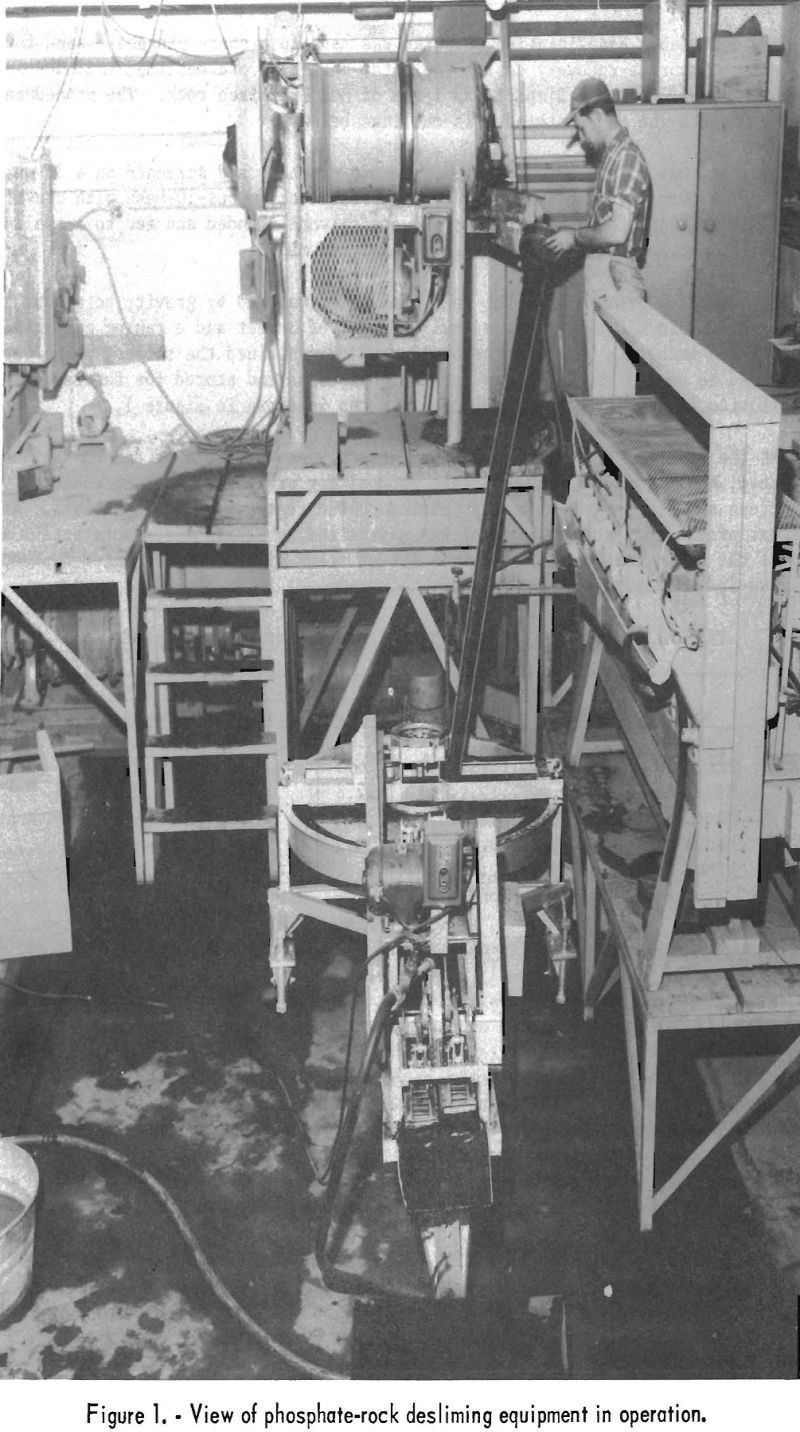
The overflow product from the rake classifier was fed by gravity to a bowl classifier. This classifier has a bowl diameter of 3 feet and a raking compartment 7 feet 4-¾ inches in length. The bowl overflow contained the slimes, which were discharged to waste; the clean sand product was dried and stored for future use. An overall view of the grinding and desliming setup is shown in figure 1.
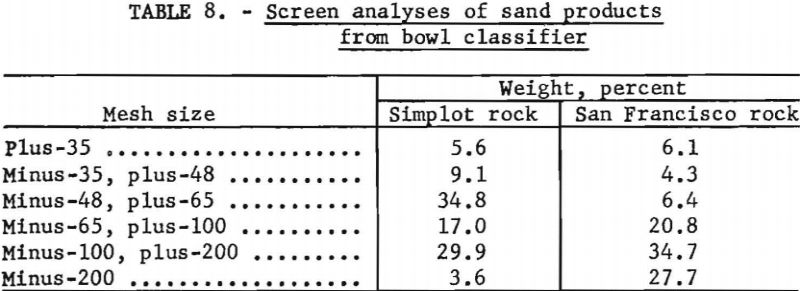
At the start of the campaign wet screen analyses were made of the bowl classifier feed, bowl overflow, and bowl rake product for process control purposes. Typical screen analyses made during this period of the test are shown in table 7. As the test progressed, process control was maintained by making a periodic screen analysis of the bowl classifier feed only. The grinding circuit was adjusted to keep the plus-35-mesh material to a minimum.
Enough high-grade Simplot rock was available so that both the grinding and desliming circuits could be properly adjusted to obtain good sand recovery and also to produce a material possessing desirable particle-size distribution. This was not true with the San Francisco ore; as a consequence, considerably more slimes were produced. The largest fraction (34.8 percent) of the sand produced from the Simplot rock was minus-48-, plus-65-mesh; the largest fraction (34.7 percent) for the San Francisco rock was minus-100-, plus-200-mesh. This difference in particle size is reflected also in the sand-recovery figures: 83.1 percent for the Simplot rock and 60.9 percent for the San Francisco rock.
Representative screen analyses of the dried sand from each lot of ore are given in table 8.
The deslimed Simplot phosphate-rock sample analyzed, in percent, as follows: P2O5, 35.0; CaO, 49.5, SiO2, 2.0; F, 3.6; and V, 0.06. The sample of San Francisco sand analyzed, in percent, as follows: P2O5, 33.6; CaO, 48.5; SiO2, 2.8; F, 3.5; and V, 0.06.
Defluorination Tests
Laboratory Scale
San Francisco Phosphate Rock
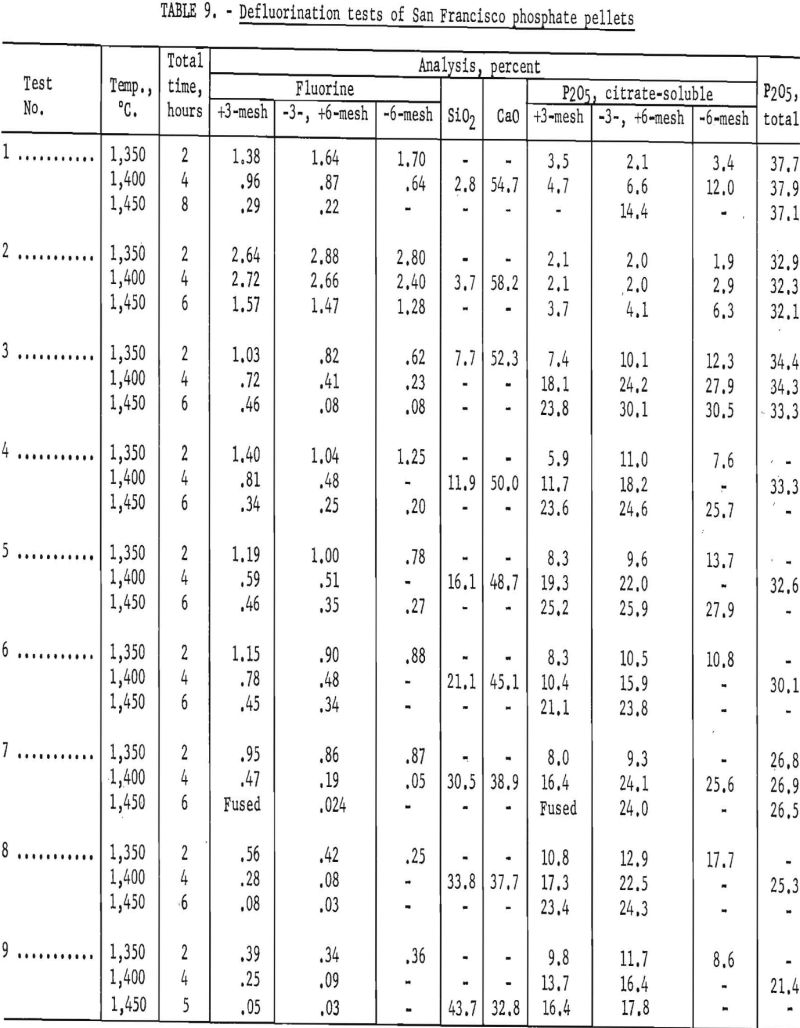
In a series of defluorination tests the effects of pellet size, silica content of pellets, temperature, and time at temperature were studied. The laboratory procedure generally used for the initial defluorination tests was briefly as follows: Shipping-grade phosphate rock ground to minus-48-mesh or finer was beneficiated by flotation or desliming as described previously and mixed with a predetermined amount of finely divided silica. The mixture was wetted and rolled into minus-½-inch, plus-10-mesh pellets in a rotating drum. The pellets were put into a crucible, then heated in an electric muffle furnace. Water vapor was introduced into the pellet charge through a quartz tube placed through a hole near the bottom of the crucible. After the pellets were fired at 1,350° C. for 2 hours, they were removed from the furnace, quenched in water, and sampled. The sample reject was returned to the furnace and fired for 2 hours at 1,400° C. Again the pellets were removed from the furnace, quenched, and sampled; then the procedure was repeated, using a firing temperature of 1,450° C. The pellet samples were sized on 3- and 6-mesh screens, and the sized samples were pulverized and analyzed. The results of some of these tests are shown in table 9.
In test 1 no silica was added to the beneficiated phosphate rock. In test 2 limestone was used in the charge. In test 7 the pellets fused at 1,450° C., and it is interesting to note that their P2O5 and CaO contents are similar to those of TVA’s fused tricalcium phosphate product. Pellets containing 33.8 percent SiO2 and over defluorinated satisfactorily without fusion.
Briefly summarized, this series of tests indicated that small pellets defluorinate more readily than large pellets; that a temperature near 1,450° C. is required; that excess lime retards defluorination; that pellets containing as little as 8 percent SiO2 will defluorinate; that SiO2 contents of 11 to 30 percent results in formation of low-fusion-point pellets which retards defluorination; and that the phosphorus in effectively defluorinated pellets is largely soluble in neutral ammonium citrate.
A few defluorination tests were made on raw San Francisco phosphate rock, with results that differed little from those on the beneficiated product. For example, 195 grams of minus-100-mesh phosphate rock was mixed with 4.7 grams of minus-100-mesh silica; the mixture was pelletized and defluorinated under the previously described conditions. The minus-3-, plus-6-mesh product analyzed, in percent: F, 0.07, SiO2, 8.6; CaO, 54.4; citrate-soluble P2O5, 31.4; and total P2O5, 34.7.
After it was determined that phosphate pellets could be defluorinated satisfactorily in the laboratory, a small pilot plant was designed and built. Since pelletizing facilitated laboratory defluorination tests, a shaft furnace was the logical choice for initial experimentation on pilot-plant scale. The pilot-plant tests are described in a later section.
Simplot Phosphate Rock
Through the courtesy of E. W. Hansen, former manager of the Simplot Fertilizer Co., Pocatello, Idaho, the Bureau of Mines was furnished with a carload of phosphate rock for pilot-plant defluorination tests. The carload comprised two lots, one being high-grade phosphate rock and the other phosphate shale. The high-grade rock analyzed, in percent: P2O5, 32.9; SiO2, 5.8; CaO, 46.9; F, 3.3; Fe2O3, 0.96; Al2O3, 1.2; C, 2.0; and V, 0.12. The shale analyzed, in percent: P2O5, 26.9; SiO2, 15.6; CaO, 39.5; F, 2.6; Fe2O3, 1.46; Al2O3, 2.3; C, 2.4; and V, 0.12. Only the high-grade rock was used for the tests described herein.
Several laboratory tests were made of the Simplot phosphate rock before using any of it in the pilot plant as the analysis differed slightly from that of the San Francisco rock previously tested. In the first test the minus-3-, plus-6 mesh pellets fused when treated for 2 hours in a water-vapor atmosphere at 1,450° C. Nevertheless, the pellets were well defluorinated. The test product analyzed 0.21 percent F, 9.8 percent SiO2 and 32.3 percent citrate-soluble P2O5. In later tests using less SiO2 in the charge, pellets were defluorinated in the laboratory without an appreciable amount of fusing. The most favorable procedure found for laboratory defluorination of this particular material was to grind the rock to minus-48-mesh, add little or no silica, form minus-3-, plus-6-mesh pellets by rolling in a drum, and heat-treat 4 hours at 1,450° C. in a water-vapor atmosphere. Upon removal of the pellets from the furnace they were immediately quenched in water. The products from such tests analyzed 0.1 to 0.3 percent F, 35 to 36 percent total P2O5, and up to 30 percent citrate-soluble P2O5.
The process was next tried in the pilot-plant shaft furnace with little success. Pellets that showed only slight fusion and defluorinated satisfactorily in the laboratory stuck together and caused serious mechanical difficulties in the shaft furnace. After poor results were obtained from the first two continuous pilot-plant tests, laboratory tests were initiated to determine what effect the P2O5 content of the pellets had on defluorination temperature and on fusibility of the pellets at the maximum temperature needed for nearly complete defluorination. Contents of P2O5 higher than that of the rock were obtained by adding both reagent-grade and fertilizer-grade phosphoric acid solutions, as well as phosphorus anhydride, to the pellet mixture. Both raw and deslimed rock was tested.
By using reagent-grade phosphoric acid in forming pellets, defluorinating temperatures as low as 1,350° C. were attained. Results of one test using phosphorus anhydride were very similar to those obtained from the use of reagent-grade phosphoric acid. Laboratory tests with fertilizer-grade phosphoric acid, 52 percent P2O5, indicated that the impurities in the acid cause the phosphate pellets to have a low fusion point, which apparently retards defluorination. Phosphoric acid used in subsequent laboratory and pilot-plant tests was either technical grade (75 percent H3PO4) or reagent grade (85 percent H3PO4).
The results of the tests shown In table 10 indicate that the use of reagent-grade phosphoric acid for making pellets will lower the defluorination temperature. When the equivalent of 15 grams, or more, of 100 percent phosphoric acid was used per 100 grams of raw phosphate rock, the pellets defluorinated at 1,350° C. X-ray analyses of the products indicated the major constituent to be beta-tricalcium phosphate. These analyses account for the material being low in citrate-soluble P2O5, as beta-tricalcium phosphate is only partly soluble in neutral ammonium citrate. Temperatures of 1,400° C. or higher are required to form the more soluble alpha-tricalcium phosphate.
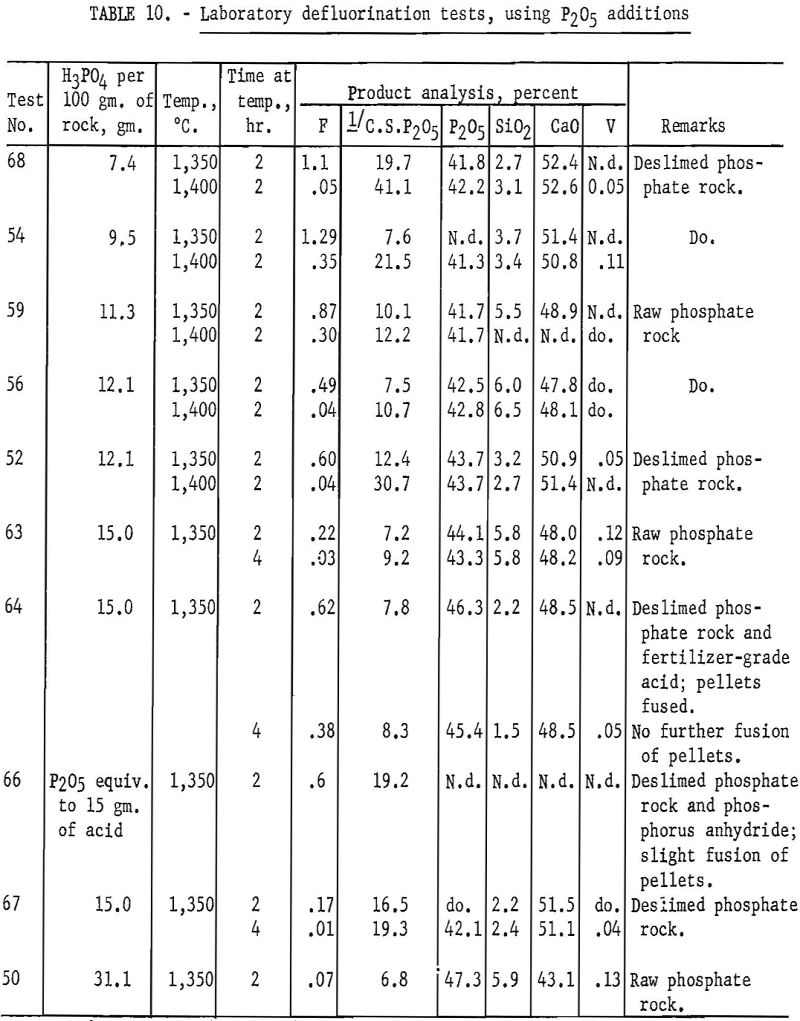
The most promising results were obtained from test 68, wherein the equivalent of 7.4 grams of 100-percent phosphoric acid was used per 100 grams of deslimed phosphate rock. The defluorinated pellets were low in fluorine and high in citrate-soluble P2O5. Defluorination temperature was 1,400° C.
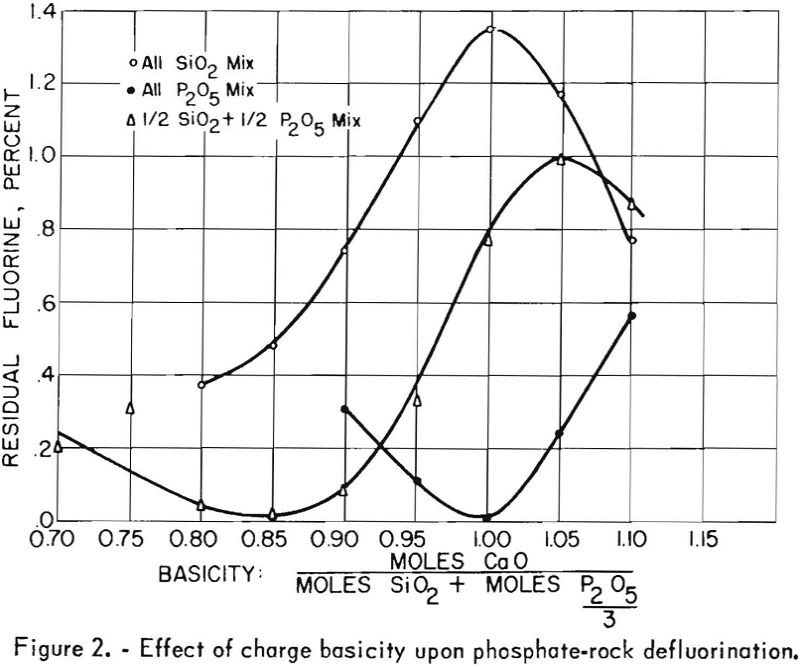
During this early experimental work on defluorination SiO2 and P2O5 additions varied widely; however, results of the tests indicated that the ratio of P2O5 and SiO2 to CaO is of vital importance in the defluorination reaction. Basicity factors, which were considered to be the ratio of moles CaO to (moles P2O5/3 + moles SiO2) calculated from the product analyses of previous tests, indicated that, at a basicity of approximately 1.10, both citrate solubility and fluorine volatility increased markedly.
A series of tests was made to check the validity of these apparent marked increases and also to investigate the effect of varying basicities over a fairly wide range. Three tests each were run at basicities from 0.70 to 1.10 in increments of 0.05. One test was made using pellets formed from raw phosphate rock, which has a calculated basicity of 1.17. Calculations for the above tests differed from the calculations made previously, in that the basicity is determined for the original charge instead of the defluorinated product.
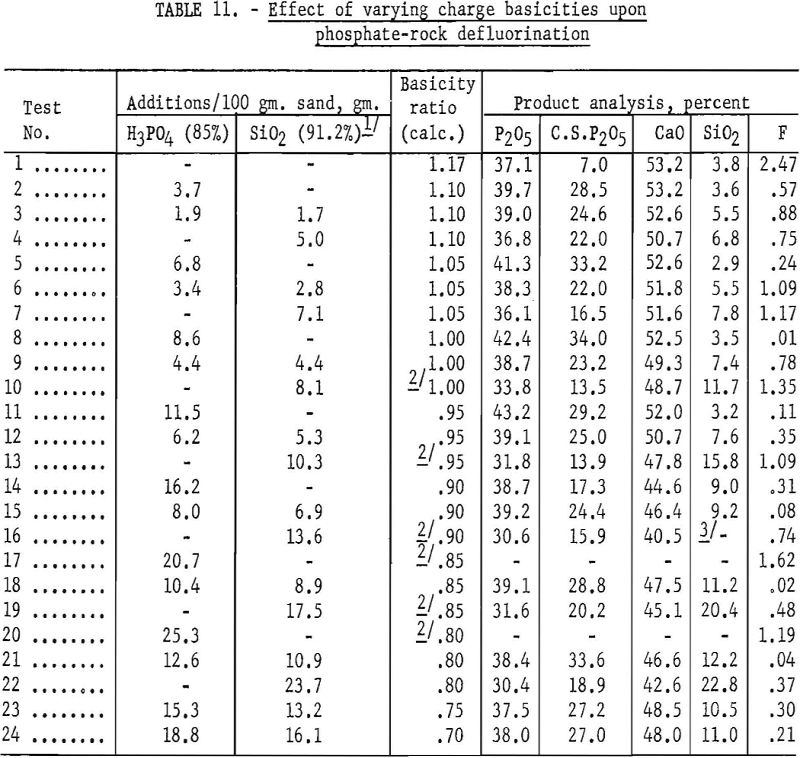
Two different samples of phosphate sand were used for the tests. For the first 7 tests the sand analyzed, in percent: P2O5 35.5; CaO, 51.0; and SiO2 1.6. The sand used in the remaining tests analyzed, in percent: P2O5, 35.0; CaO, 49.5; and SiO2, 2.0. Charges were prepared in which a definite basicity was obtained by admixing either reagent-grade phosphoric acid or silica alone or a mixture of the two acid constituents in equal molar quantities with the phosphate rock before pelletizing. The purpose in testing both phosphoric acid and silica as acidic constituents in the charge was to determine if silica, which is the cheaper material, could satisfactorily replace phosphoric acid in neutralizing the excess CaO in the rock. In a majority of the tests 200-gram charges were used, but some of the first charges made up with only SiO2 additions contained 250 grams. The pellets were handmade and generally plus-3-mesh in size. Water vapor was introduced into the crucibles at 1,350° C. and continued 2 hours at a controlled temperature of 1,450° C. At the end of this period the pellets were immediately quenched in cold water. Data for the test series are shown in table 11.
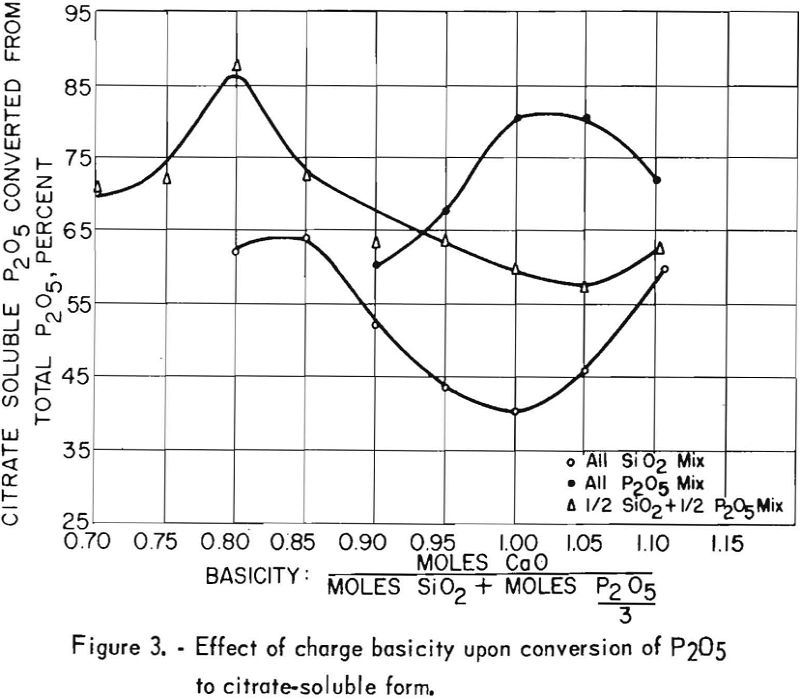
The test products were analyzed for CaO, SiO2, total P2O5 citrate-soluble and F. Satisfactory defluorination and high P2O5 availability were obtained by adding phosphoric acid alone to achieve a basicity factor of 1. The use of SiO2 additions without P2O5 did not produce satisfactory results at any basicity tried. The poor results obtained from this group of tests may be partly attributable to the fact that the charges at basicities of 1.00, 0.95, 0.90 and 0.85 fused. Equally good defluorination was obtained with ½ P2O5-½ Si02 additions at basicities of 0.90, 0.85, and 0.80. Tests in which the basicity was greater than 1 did not yield good defluorination results. The effect of phosphate-rock-charge basicity upon completeness of defluorination and conversion of P2O5 to the citrate-soluble form is shown in figures 2 and 3 respectively.
Other laboratory researchers have found it desirable to carry out calcination in the presence of a relatively large amount of silica. However, agreement on the part played by the silica in the reaction has not been unanimous. Maust claims that the action of the silica is mainly mechanical and not chemical. Petrographic
examination of products from tests 3 and 7, shown in table 11, seem to substantiate Maust’s observation, as chemical combination of the CaO and SiO2 could not be detected. Results of the examination showed that both samples were essentially alpha Ca3(PO4)2, and neither CaSiO3 nor Ca2SiO4 could be detected. Fluorapatite was also identified in both samples, with the sample from test 3 containing the larger amount.
Since no definite silica compounds can be detected in our products, it is reasoned that little neutralizing of the excess CaO is done by silica. Therefore, best defluorination results if all this excess lime is converted to tricalcium phosphate.
This phenomenon is even more noticeable with the ½ P2O5- ½ SiO2 charges. The amount of phosphoric acid addition in test 9 was only about half the amount used in test 8. Defluorination was poor. As the amount of phosphoric acid addition (test 15) approached the amount used in test 8 defluorination was excellent. It should be pointed out that the amount of acid used in the charge of test 8 was nearly enough to yield a basicity ratio of 1 if no silica had been added. In subsequent tests at basicities of 0.85 and 0.80 the larger phosphoric acid additions increased defluorination. At basicities of 0.75 residual fluorine increased, and citrate-solubility values decreased.
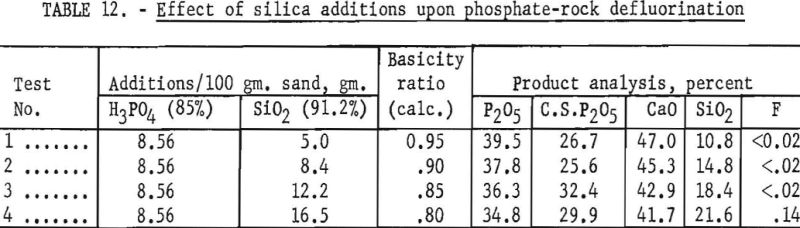
The defluorination reaction was investigated further by conducting several tests in which the P2O5 additions to the charge were kept constant and the SiO2 additions were varied. Enough phosphoric acid was added to the charge to yield a basicity ratio of 1 with P2O5. The quality of silica added varied from 5.0 to 16.5 grams per 100 grams of phosphate sand and was determined by the amount calculated to give basicity ratios of 0.95, 0.90, 0.85, and 0.80 in the defluorination charge. A residual fluorine content of less than 0.02 percent in each of the first 3 test products and 0.14 percent in the fourth test indicated that the amount of silica in the charge had little effect on defluorination, provided the charge contained enough P2O5 to neutralize the CaO. The percentages of total P2O5 converted to the citrate-soluble form were 67.7, 67.8, 89.4, and 86.0, respectively. Complete data for these tests are shown in table 12.
It has been commonly observed by various investigators of the phosphate-rock defluorination reaction that the amount of P2O5 converted to the citrate-soluble form is inversely related to the amount of fluorine removed from the rock. The curves shown in figures 2 and 3 illustrate this phenomenon quite well in most instances. One interesting exception may be noted for the ½ P2O5 – ½ SiO2 charges. The most complete defluorination for the tests in this series was apparently reached at a basicity very close to 0.85, but the most complete P2O5 conversion (87.6 percent) was reached with a basicity of 0.80. The high availability of phosphorus obtained at these basicities is corroborated by the data presented in table 12.
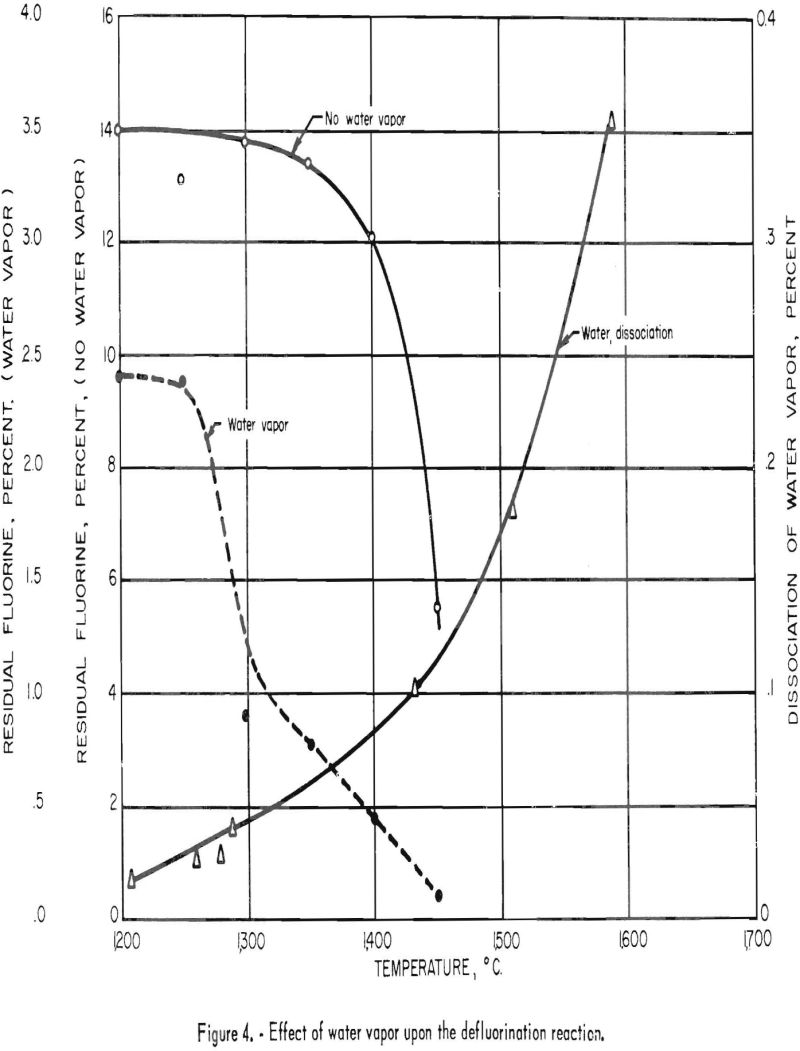
Additional information concerning the defluorination reaction was furnished by results of a series of tests made to determine the importance of water vapor to the reaction. At the same time the possibility of a definite relationship between the percentage of dissociation of water vapor and the degree of defluorination accomplished at different temperatures was studied. Values for the dissociation of water vapor were obtained from another source.
Previous defluorination experience indicated that a product essentially free of fluorine could be produced only at temperatures above 1,450° C. in a water-vapor atmosphere. The procedure used in this investigation consisted of heating pellets made from a mixture of quartz and fluorspar at various temperatures for periods of 1 hour each in a Globar furnace. A mixture containing 16 percent fluorine was chosen because the pellets would not fuse at the maximum test temperature. Fluorspar was chosen instead of phosphate rock because of the higher fluorine content. Two tests were made at each temperature setting: In the first test no water vapor was used; in the second test a measured amount of water vapor was introduced into the crucible. The amount of water converted to steam was kept as near 140 ml. as possible. Temperature settings for the test series were 1,200°, 1,250°, 1,300°, 1,350°, 1,400°, and 1,450° C. The products were analyzed for fluorine and the values plotted to illustrate the difference in amount of fluorine volatilized at various temperatures with and without water vapor. A curve showing the percentage of dissociation of water vapor in the range of temperatures studied is also included to show how the defluorination reaction is affected by this factor. These curves are shown in figure 4. Examination indicates that the amount of water vapor dissociated at any given temperature has little effect upon the removal of fluorine from the phosphate pellets.
Pilot-Plant Scale
Shaft-Furnace Tests
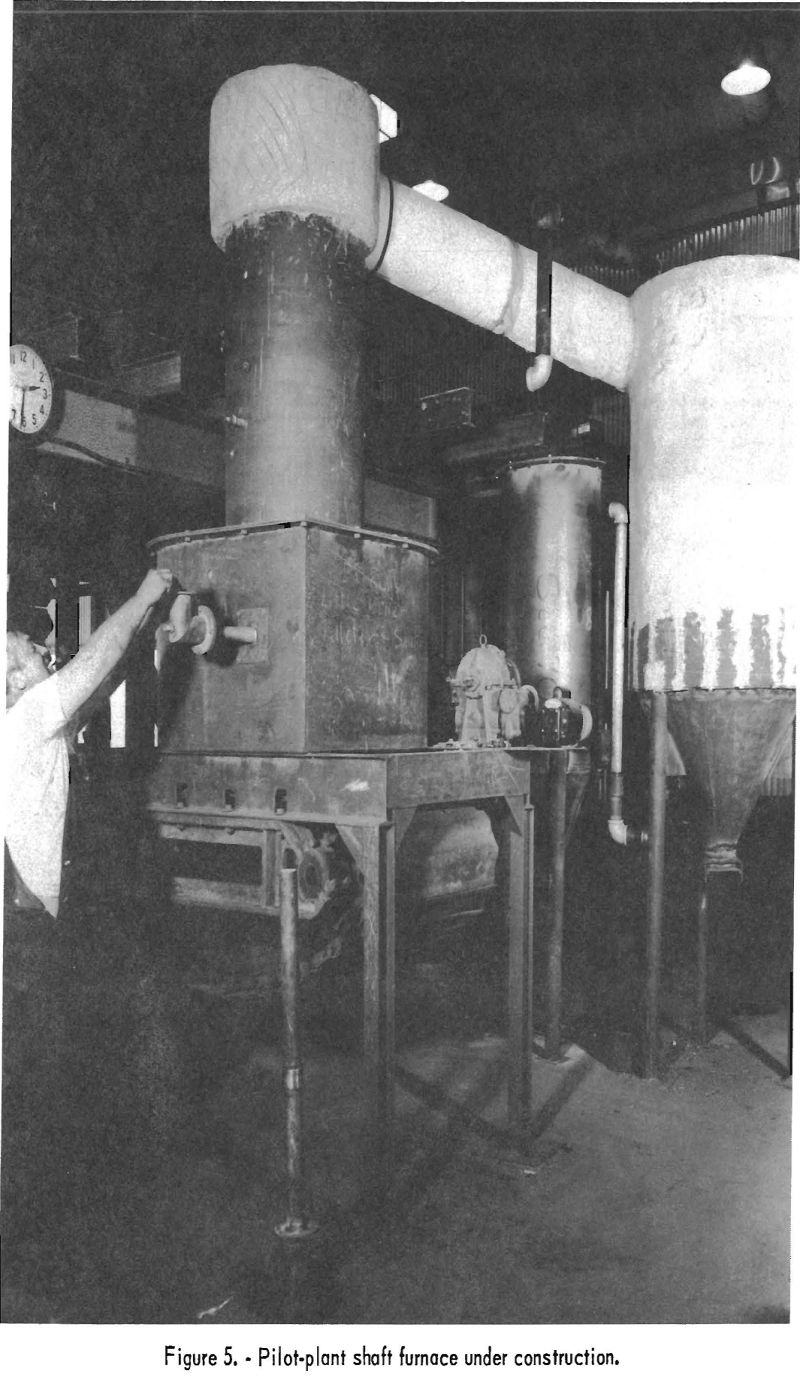
A shaft-type furnace, with necessary auxiliary equipment, was designed and built, as it was thought to be the logical choice for demonstrating that phosphate rock could be defluorinated on a pilot-plant scale, using the process developed in the laboratory. Figure 5 shows the pilot plant under construction. The piece of equipment in the foreground is the shaft furnace. Figure 6 shows, from right to left, the elevator, the pelletizer, and the conveyor, which were constructed to feed sized pellets continuously to the shaft furnace.
Between January 1952 and October 1952 several tests of 8 hours duration and 3 continuous tests ranging from 50 to 90 hours duration were made in this furnace.
The shaft had a diameter of 1 foot inside the firebrick lining and a height of 7 feet. The shaft was originally designed to have a cylindrical shape; following completion of the second continuous test its shape was changed to that of a truncated cone. This design change was made in the hope that the mechanical trouble encountered with fusion and expansion of the pelletized charge in the cylindrical shaft could be minimized. However, the major mechanical difficulty experienced in all of these tests was hanging up of the charge in the furnace shaft.
The furnace was fired with propane-gas burners installed tangentially to the shaft. The primary air for the burners was heated by passing the furnace exhaust gases through a heat exchanger. Water vapor required for the defluorination reaction, over that furnished by the combustion of the propane gas, was metered into the primary air line. The highest bed temperature attainable with operable furnace conditions was 1,400° C. These relatively high bed temperatures could be maintained only when the bed depth was limited to a maximum of 45 inches.
The cooled offgases were drawn by an exhaust fan through an absorption tower containing sized limestone in all except the third continuous test. These preliminary fluorine-recovery tests were patterned after work done by TVA engineers.
The products from the final test in the series retained 1.4 to 2.8 percent F; the percentage of P2O5 converted to the available (citrate-soluble) form ranged from 9 to 38. These results were typical of those obtained from other tests in the series.
Although there had been continual improvement in mechanical operation during the testing period, it was concluded that a shaft furnace is unsatisfactory for defluorinating phosphate rock by a calcination process.

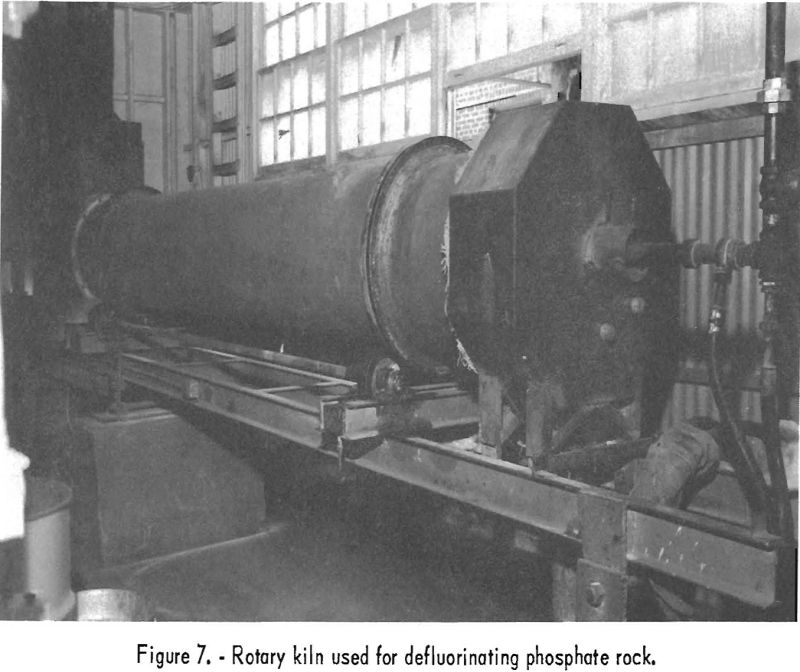
Rotary Kiln Tests
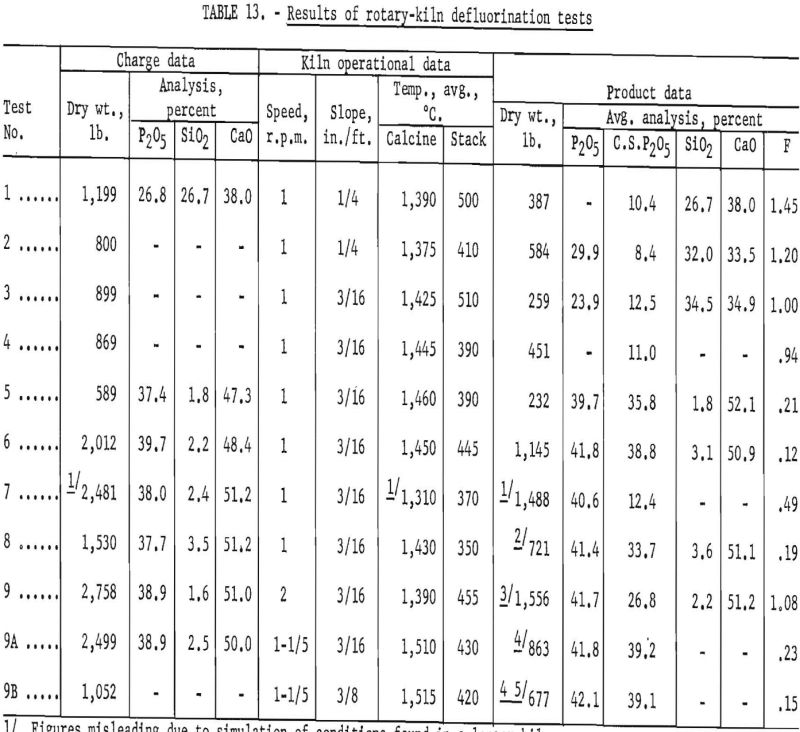
After considerable mechanical difficulty and poor defluorination results in two tests in the shaft furnace, the small rotary kiln shown in figure 7 was remodeled for operation at high temperatures. The kiln is 15 feet long, with an inside diameter of 1 foot. For the first 8 tests the lining consisted of 1 course of firebrick arches with 1 inch of silica insulating brick next to the steel shell. For test 9 the firebrick were replaced by 70-percent alumina brick. Different slopes and speeds were tried, but the optimum operating conditions were obtained with a slope of 3/16 inch per foot and a speed of 1 revolution per minute. Propane gas was used as a fuel to maintain a kiln temperature of 1,310° to 1,515° C., as measured by a Leeds & Northrup optical pyrometer sighted on the kiln load. Exhaust-gas temperature, determined with a chromel-alumel thermocouple at the base of the stack, varied from 350° to 500° C. Optimum calcining temperatures ranged from 1,450° to 1,525° C. The latter value was reached toward the end of test 9 and then only for a short period. The temperatures recorded in table 14 are averages for each of the tests. The average temperature of 1,310° C. shown for test 7 is misleading, as the first pass of the charge through the kiln was at a relatively low temperature to simulate conditions that might be found in a large commercial kiln.
Charge material for tests 1 and 4 was in the form of pellets, while material for tests 2 and 3 was finely ground (approximately minus-48-mesh). From test 5 onward the phosphate rock was ground to minus-35-mesh and deslimed according to the procedure outlined in the section on laboratory-scale desliming tests. The composition of each charge is appropriately noted. Phosphoric acid used in preparing defluorination charges was either reagent grade (85 percent H3PO4) or technical grade (75 percent).
The first six rotary-kiln runs (hereafter referred to as RKR’s) were of an exploratory nature and are described only briefly. Tests 7, 8, and 9 were more satisfactory, and detailed operating data are presented.
In the first test pellets that previously had been passed through the shaft furnace were used. Above 1,400° C. these pellets began to fuse, thereby limiting operating temperature to a maximum of 1,395° C. Due to the low fusion point of these pellets, defluorination was not complete.
In the second test finely ground phosphate rock and quartz were used for the charge material. Fifty-two pounds of high-grade Simplot phosphate rock and 23 pounds of quartz were ground in the rod mill for 45 minutes. The ground product was then charged to the rotary-kiln feeder. In this test temperatures as high as 1,440° C. were maintained. When the temperature rose to 1,450° C. large nodules formed. The results of this test differed little from those of the first test.
Before starting RKR 3, the kiln lining at the discharge end was built up to form a retaining ring having an inside diameter at the discharge of about 9 inches. The slope of the kiln was also decreased from ¼ inch to 3/16 inch per foot. This 16-hour test was made on the same charge composition as the second test. Due to the above mechanical changes in the kiln there was a decided improvement in defluorination results. The product from the second shift analyzed 0.49 percent F.
The fourth test was made to determine whether the same charge, in pellet form, would defluorinate as well as finely ground rock under similar operating conditions. It was observed that the pellets could be heated to a slightly higher temperature without fusing than could the finely ground charge. The average temperature for this test was about 20° C. higher than for the previous test. Nevertheless, the defluorination results were poor compared to the previous test, that is the fluorine content of the product from the second shift of the fourth test was virtually double what it was from the second shift of the third test.
The charge for RKR 5 was prepared by treating 86 pounds of sand containing about 7.5 percent moisture with 7.0 pounds of reagent-grade phosphoric acid. After the acid treatment the product was dried and charged to the kiln feeder. The average operating temperature exceeded 1,450° C. Little fusing of the charge was experienced. Occasional rodding was necessary as the charge tended to hang up in the kiln, especially at the feed end. Encouraging defluorination results were obtained in this test. The fluorine content dropped to as low as 0.06 percent, while the citrate- soluble P2O5 was as high as 37.4 percent. The total P2O5 content was 39.7 percent.
RKR 6 was operated continuously for 48 hours. Deslimed phosphate rock was mixed with phosphoric acid and dried before being charged to the kiln feeder. The optimum ratio for good defluorination appeared to be 7 parts of 85 percent phosphoric acid to 76.5 parts of dry sand. When the kiln was operated at 1,465° C. a 63-pound sample of product analyzed, in percent: F, 0.06; citrate-soluble P2O5, 40.9; total P2O5, 41.7; CaO, 51.5; and SiO2, 3.0.
In RKR 7 the optimum ratio described above was maintained. This quantity of acid used is the stoichiometric amount needed to neutralize the excess basic components in the deslimed phosphate sand, so that all of the CaO in the final defluorinated product might be combined as either 3 CaO·P2O5 or CaO·SiO2. The object of this test was to determine whether sticking of charge material to the kiln’s lining could be reduced by simulating conditions of a longer kiln, or by feeding a pelletized charge to the kiln. In the first part of the test the dried, phosphoric acid-treated phosphate rock was charged to the kiln and the kiln operated at 1,000° C. for 28 hours. The product from this period was recycled through the kiln at near 1,450° C. to simulate conditions in a longer kiln; 349 pounds of defluorinated material was produced. This product analyzed 0.20 percent F and 40.7 percent citrate-soluble P2O5. Approximately 50 percent more propane gas was required to produce this defluorinated product than when the material was passed through the kiln once.
In the second part of this test the phosphoric acid-treated phosphate rock was pelletized before being charged to the kiln. Some unusual operating difficulties were encountered during this part of the test since the power was off 2 hours during the last day of the run, some charge at the point of incipient fusion froze to the kiln lining. The kiln product from this part of the run was high in fluorine and varied from 0.25 to 0.77 percent; the citrate-soluble P2O5 varied from 36.5 to 12.4 percent. The results of the test indicated that it was neither advisable to pass the material through the small rotary kiln twice nor to pelletize the charge before defluorination.
In RKR 8, 25 percent of the excess basic component of the deslimed phosphate rock was neutralized with SiO2 and 75 percent with technical-grade phosphoric acid. A total of 1.6 pounds of silica and 6.9 pounds of phosphoric acid was added to every 75 pounds of deslimed phosphate rock.
Operationally, the test was satisfactory, for temperatures as high as 1,480° C. were reached without appreciable fusion. During 5 shifts of operation, 721 pounds of product averaging 0.20 percent fluorine was recovered. The citrate-soluble P2O5 analyses varied from 26.8 to 36.9 percent. Heat-energy requirements were calculated to be 32 million B.t.u. per ton of product. Results of the tests indicated that at least part of the acidic addition could be made satisfactorily with SiO2.
Two separate lots of high-grade Simplot phosphate rock were prepared for test RKR 9 by stage crushing to minus-35-mesh and desliming. Lot 1 analyzed, in percent: P2O5, 35.5; CaO, 51.0; SiO2, 1.56; and F, 3.92. Lot 2 analyzed, in percent: P2O5, 35.5; CaO, 50.0; SiO2 2.52; and F, 3.90. Technical-grade phosphoric acid was used in this test. Calculations from the analysis of lot 1 indicated an acid:ore ratio of 12:101 for neutralization of the excess CaO in the charge. Although analytical results of lot 2 were not available before the acid was mixed with the sand, the same ratio was maintained without serious discrepancies noted in either operating procedure or product analysis.
To make the process more economical, a higher discharge rate was sought by appropriate alteration of the slope and speed of rotation. In nearly all previous tests these conditions were fixed at 3/16 inch per foot and 1 r.p.m., respectively. The speed of rotation was set for about 2 r.p.m. for the first 28 hours of operation, with the slope remaining fixed. A total of 2,320 pounds of acid-treated sand with an average moisture content of 3.10 percent was charged during this period and 1,245 pounds of defluorinated phosphate rock recovered; 152 pounds of material contaminated from previous calcining test in the kiln was discarded. The average calcining temperature was 1,390° C. for this initial phase of the test. Heat-energy requirements were calculated to be 26 million B.t.u. per ton of product.
Grab samples taken during operations indicated that about 50 percent of the fluorine was being eliminated. It is known that temperatures around 1,400° C. are not sufficient for good defluorination, so an attempt was made to raise the temperature. Failing in this attempt, the speed of rotation was changed back to the original setting of 1 r.p.m., with the slope remaining unchanged. These operating conditions were maintained for the next 45 hours. The kiln charge consisted of 737 pounds of recycled high-fluorine material from the swing shift and the graveyard shift of the first period and 1,080 pounds of raw feed from lot 2; the total amount of product discharged was 1,485 pounds. The average operating temperature for the period was 1,510° C., and a maximum of 1,600° C. was reached briefly. Above 1,540° C. frequent rodding of the sidewalls became necessary, and formation of large nodules caused operational trouble. This portion of the test was the most successful with respect to defluorination. The defluorinated product varied from 0.28 down to 0.08 percent F, and the citrate-soluble P O varied from 36.9 to 41.2 percent. Heat-energy requirements were increased to 34 million B.t.u. per ton of product for this test period.
Dust loss was negligible when raw feed, containing 2.7 percent moisture, was used. When the recycled material was charged, approximately 50 percent of the feed was blown into the dustbox and had to be returned to the hopper. Spillage accounted for part of this amount, because constant leakage around the feed-hopper gate caused the kiln to overflow.
The last part of the test was conducted with the same rotational speed, but the slope was increased to 3/8 inch per foot. The average temperature during this 21- hour period was 1,515° C., and the operation was very smooth. The charge consisted of 598 pounds of material remaining from the first period which required recycling; the total amount discharged was 960 pounds, including 160 pounds from kiln cleanout. The latter portion was discarded. Solubility of P2O5 in neutral ammonium citrate varied from 38.4 percent to 39.6 percent, and the residual fluorine content averaged 0.15 percent. The heat-energy requirement was 28 million B.t.u. per ton of product, and dust loss was high.
A material balance using the P2O5 content as a base was made to determine the unaccountable loss. The balance for the initial test period showed a loss of approximately 300 pounds, which may be mainly attributed to the normal load carried by the kiln during operations. Likewise, the final period of the test yielded much more product than was accounted for in the kiln cleanout. Load conditions were fairly static during the second period, and a more accurate balance was made. Calculations indicated the weight losses for this period were 11.7 percent. This figure agrees well with the average 10-percent weight loss due to calcining noted in laboratory defluorination tests. It is assumed that losses over 10 percent were in the form of dust carried out by the stack gases.
In anticipation of higher F content in nodules formed the product from each shift was sized on a 9-mesh screen. The undersize was kept in individual lots until analyzed, but the nodules were mixed. After chemical analyses for F and citrate-soluble P2O5 were received on both the fines and oversize, three composite lots were made. As shown by subsequent analysis the fluorine content of the nodules was not enough higher than that of the fines to warrant their removal in future tests. One lot totaling 1,087 pounds had the following analysis, in percent: F, 0.17; citrate-soluble P2O5, 42.3; CaO, 51.0; and SiO2, 2.50. The second lot analyzed, in percent: F, 0.24; citrate-soluble P2O5, 39.6; and total P2O5, 42.3 (the total weight of this lot was 450 pounds). The third lot, totaling 710 pounds, contained all of the plus-9-mesh material. This lot analyzed, in percent: F, 0.25; citrate-soluble P2O5, 35.4; and total P2O5, 41.6.
All three lots of defluorinated material were ground to minus-100-mesh in a Hardinge conical ball mill with closed classifying circuit. Typical data pertaining to the rotary-kiln tests are summarized in table 13. Another kiln test is described in the section covering fluorine-recovery tests.
Fluorine-Recovery Tests
Laboratory Scale
Preliminary Experiments
In October 1952, after demonstrating that fluorine could be successfully removed from western phosphate rock, the problem of recovering this fluorine as a valuable byproduct was attacked. A literature survey indicated that either synthetic cryolite (Na3 AlF6) or acid-grade fluorspar (CaF2) would be desirable end products. The latter compound was chosen for initial experimentation, as it is a starting material for the production of hydrofluoric acid and many other useful compounds. However, a majority of the research to date has been centered on the production of artificial cryolite.
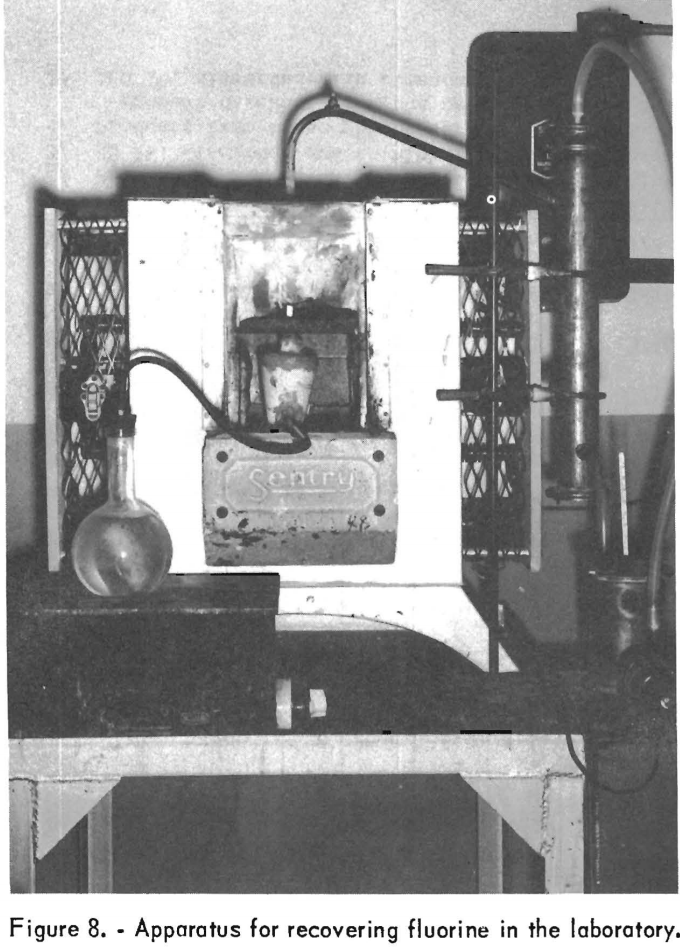
Essentially the same setup was used for the fluorine-recovery tests as for the phosphate-rock-defluorination tests. However, to recover the fluorine compounds, lids were added to the fire-assay crucibles and a quartz tube of the same diameter (3/16 inch nominal) as the steam-inlet tube led the evolved gases outside the muffle furnace. All points of connection were sealed with a refractory cement. A view of the defluorination apparatus in place in the Globar furnace is shown in figure 8. The furnace door and fume hood were removed before the picture was taken, to show the furnace interior. The concurrent-type scrubbing tower at the right of the furnace is described later.

The first nine tests were based upon the chemical reaction 2 HF + CaCl2 = CaF2 + 2 HCl, and a packed tower was used for scrubbing the defluorination gases. A length of copper tubing slightly larger in diameter than the quartz tube was used in the initial tests to convey the evolved gases to the scrubbing tower. The gases entered the bottom of the tower and were exhausted from the top by means of a small vacuum pump on the outlet side of the tower. The pump was installed in this position to minimize corrosion of its parts. Contamination of the solution and products by copper prompted replacement, in succeeding tests, of the copper tubing by nickel at the furnace exit and by polyethylene at a point beyond the furnace where heat would not be too great. It was found that nickel was also a contaminant but not as serious as copper. Factors investigated in the above tests were: Effect of tower height, rate of gas flow, size of packing material, and concentration of solution on the completeness of fluorine removal from the gas stream. The choice of packing material was also investigated.
For the first 3 tests and test 9, a pyrex tube 1-½ inches in diameter and 24 inches in height was used. A pyrex column 1-5/8 inches in diameter and 48 inches in height was tried in test 4 to allow more time for the reaction to take place. This same tower was used in the next four tests. The type and size of packing material used included minus-3-, plus 10-mesh fluorspar, minus-10-, plus-35-mesh ferronickel (analyzing 22 percent Ni), plus-3-mesh aluminum shot, and random-size nickel chunks. Gases were scrubbed with CaCl2 solutions in all tests except 7 and 8, wherein water was the scrubbing medium. Calcium chloride concentrations used in the scrubbing solutions were 5, 10, and 20 percent.
Actual gas-flow rates were not measured, as a study was being made only of the behavior of the rising gas bubbles and the relative amount of restriction offered to the flow of gas by the various particle sizes of packing used. It was found that, unless the rate of gas flow was quite high, the inlet tube soon became plugged.
These tests were only exploratory. Only a small amount of product was obtained from each run. None of the packing materials tested appeared to yield promising results; in fact, at this time it was decided that use of any packing in a scrubbing tower would be unsatisfactory for two reasons: First, the small amount of product obtained from defluorinating a charge of phosphate pellets in the laboratory, even with 100-percent recovery (15 gm. CaF2), would be difficult to recover; and second, production on a larger scale would present operating difficulties due to accumulation of the product in the packing, which would, in turn, plug the tower. Production of CaF2 was recessed until suitable apparatus could be devised.
In the interim, a German method for making artificial cryolite was used as a pattern for the next few tests. In this process, aluminum hydrate is added to an agitated hydrofluoric acid solution at such a rate that the temperature is maintained between 85° and 95° C. When the alumina is completely dissolved, caustic soda is added slowly and the mixture is kept hot and agitated until the cryolite precipitates. Our tests were conducted by bubbling the effluent defluorination gased through a sodium aluminate solution to determine whether cryolite would be formed according to the following chemical equation: NaAlO2 + 2 NaOH + 6 HF = Na3AlF6 + 4 H2O. The solution was kept at temperatures between 80° and 95° C. and constantly agitated. This heating and agitating was continued for several hours after defluorination of the pellets was completed. Operationally, the tests were fairly successful, but no product was obtained. It is believed that this method is not feasible due to the low concentration of HF in the defluorination stack gases. No further tests were made.
A concurrent-type scrubbing tower, similar to one used successfully for disposal of fluorine on a relatively large scale by personnel of the Manhattan Project, was constructed to replace the packed pyrex tower for the remainder of the tests. The tower is 2 feet in height, has an I.D. of 2 inches, and is flanged on both ends. A sprayhead is bolted onto the upper flange, and a blank cleanout plate is fastened to the lower flange. The gases evolved from defluorination of phosphate rock are drawn into the tower just below the spray plate through a 3/8-inch-I.D. nickel pipe. A settling tank, a small centrifugal pump, and closed circuit piping constitute the accessory equipment. All components are of stainless steel except the 7/16-inch-I.D. polyethylene tubing, which is used to return the liquid from the pump to the spray-head. To date, no packing material has been used.
In operation, the tower solution passes through the spray plate, absorbs the effluent HF, and is discharged to a tank, where the insoluble fluorine compounds formed by reaction of the absorbed HF with the solution settle. The overflow solution is continuously pumped to the top of the tower. The downward flow of solution produces an aspirator effect on the gases, and no additional pumping is required. The inert gases are vented to the atmosphere.
The first test in this apparatus was exploratory, to check performance of equipment and determine what data should be recorded in future tests. Water was used for the tower solution, and the temperature at the end of the run was 60° C. Corrosion was quite severe, as the product contained 16.4 percent Fe. The top side of the spray plate was attacked the most severely. This test confirmed results found in the literature, which stated that stainless steel resists dilute aqueous hydrofluoric acid at atmospheric temperatures but is subject to an accelerated corrosion rate by hot acid solutions. A total of 2.56 grams of iron was also present in the tower solution, which is added evidence that stainless steel is not entirely suitable as a construction material for use with water as the scrubbing solution.
After satisfactory equipment performance was obtained, tests 16 and 17 were made, using a 10-percent calcium chloride solution. Operationally, the tests were successful, and approximately 5 grams of material was obtained from each run. Chemical analyses of the product from test 16 were, in percent: Ca, 41.1; F, 42.5; and Cl, 16.4. The filtrate from this test analyzed, in grams per liter: Ni, 2.18; F, 0.24; Ca, 0.90; and Cl, 9.90. Similar analyses were reported for test 17. Evidence of corrosion was not as apparent as in the test using water. The final temperature of the solution was 40° C.
In tests 18, 19, 20, and 21 reagent-grade sodium aluminate (assay, 75 percent NaAlO2) was used in the tower solution. The product recovered from the first test amounted to 25.5 grams but analyzed only 1.58 percent fluorine. The bulk of the precipitate may have been aluminum trihydrate. A literature reference states that solutions of the pure sodium aluminate product are not stable but hydrolyze readily, with precipitation of alumina trihydrate. Solutions can be stabilized by using excess NaOH. The sodium aluminate used for these tests had 10 to 15 percent NaOH added, but it was apparently insufficient for our purpose. Soon after the second test was begun, a white precipitate began to form, A small amount of NaOH was added to the settling tank, and the solution cleared immediately. The amount of product recovered was 6.5 grams, and it analyzed 6.2 percent fluorine. Addition of the excess NaOH apparently kept most of the alumina in solution, as the amount of fluorine recovered equaled 0.4 gram in both tests. Excess caustic was added to the tower solution before the next two tests were begun. One test was terminated early, owing to operational trouble, but the other test yielded 13.1 grams of product, which contained 0.9 gram fluorine. Improved recovery of fluorine is indicated. However, this figure is misleading, as three different batches of pellets were defluorinated before the tower solution was changed. The filtrate and defluorinated pellets analyzed 0.05 gram per liter and 0.04 percent fluorine, respectively. The percentage of fluorine recovered was 6.5. Test 22 was made with a 10-percent calcium chloride solution in the tower to see if the choice of tower solution affected fluorine recovery. Four and four-tenths grams of material analyzing 45.3 percent fluorine was recovered. The filtrate contained 0.6 gram fluorine per liter, and the pellets retained 0.07 percent fluorine. The percentage of fluorine recovered was raised to 43.3. Severe corrosion of the spray plate or scrubbing tower was not apparent in any of these tests, but an average of 1.2 grams per liter of nickel was present in the scrubbing solution.
In the next six tests excess NaOH was added to reagent-grade sodium aluminate to keep all reactants in solution. A material balance was made on each of the six tests to determine the distribution of fluorine. The small amount of precipitate obtained from each test yielded a maximum of 0.04 gram fluorine. The tower solution contained 1.91 to 3.64 grams; and the residual fluorine in the pellets amounted to 0.01 gram. Less than 50 percent of the fluorine contained in the original pellets could be accounted for in the first two tests, so research was concentrated in the later tests on improving recovery of fluorine as well as precipitating cryolite.
The belief is that the defluorination gases contain the fluorine mainly in the form of HF. Since water vapor is introduced into the furnace, it is conceivable that the reaction 2 HF + H2O = OF2 + 2H2 could take place to some extent. If the compound OF2 is formed, a literature reference states that it may be decomposed by caustic solution, provided contact time exceeds 1 minute. This is not possible in our small equipment. Also, investigators found that the use of reducing agents in the caustic solution increased the capacity of the unit for fluorine absorption. Concentrations of sodium sulfide ranging from 0.02 to 0.04 molar with respect to the Na2S were tried, with indications of improved recovery. The accountable fluorine in 1 of these tests was 82.6 percent, as compared with 43.0 percent, which was obtained in an earlier test without addition of a reducing agent. Another literature reference suggested acidifying the final liquor to a pH between 4 and 6, allowing the precipitate to settle for 3 to 4 hours before the supernatant liquor, was decanted and the cryolite slurry filtered. This was done with the tower solutions from the last two tests. The resulting precipitate from 1 of these tests analyzed 17 percent fluorine and contained 83 percent of the accountable fluorine.
Another possibility investigated was the production of sodium fluoride by the proper choice of solutions in the scrubbing operation. The sodium fluoride could either be recovered for its own value or used to produce other fluorides, such as cryolite. One of the most promising reactions investigated used a sodium bicarbonate as the scrubbing medium. Six laboratory-scale fluorine-recovery tests were made to obtain data on fluorine pickup in a sodium bicarbonate solution. These tests showed that 50 to 70 percent of the volatilized fluorine was recovered, depending upon how tightly the system was sealed. The resulting sodium fluoride solutions were stored in polyethylene bottles until enough was on hand for initiating the laboratory-scale cryolite-production tests described in the following section.
These preliminary laboratory tests demonstrated that the fluorine-recovery process would work, but the small amount of fluorine (approximately 3 percent) contained in phosphate rock makes extensive laboratory testing impractical. Consequently, the bulk of the testwork on fluorine recovery was done on a pilot-plant scale. Test data and results of a typical pilot-plant campaign are detailed in a later section of the report.
Production of Synthetic Cryolite
Until June 1953 most of the fluorine-recovery testwork was exploratory, and a definite goal had not been set. Indications were, however, that either artificial fluorspar or artificial cryolite could be produced from the defluorination gases. After various methods of possible cryolite manufacture had been evaluated, the following one was selected for more thorough investigation. The offgases from a kiln or other furnace producing defluorinated phosphate rock are scrubbed with a sodium bicarbonate solution. The sodium fluoride thus formed is reacted with sodium aluminate and carbon dioxide to precipitate cryolite. Sodium bicarbonate is regenerated for use in scrubbing more gas. One attractive feature of the process is that part of the required CO2 can be obtained from the defluorination stack gases after the fluorine is removed.
The general procedure used for producing cryolite in the laboratory may be summarized as follows: After the sodium fluoride concentration of the scrubbing-tower solution has been determined by the analytical methods described in the appendix, it is heated to 85° to 90° C. in a stainless steel vessel. Sodium aluminate is dissolved in water and added slowly to the hot primary solution, which was being agitated. After addition is completed, carbon dioxide is bubbled into the solution at atmospheric pressure and at a rate of approximately 2 liters per minute. The carbonation period varies from ½ hour to 6 hours. After precipitation is believed to be completed, the product is separated by filtration, calcined at 600° C., weighed, and analyzed for fluorine content. The carbonation vessel used was made of stainless steel, except for the use of a carbon-steel vessel in test 7 to determine the amount of iron pickup to be expected from this type of apparatus.
Table 14 shows the operating data for 18 cryolite-preparation tests. Products from tests 1, 3, and 10 were discarded. Tests 2 and 4 were conducted with synthetic sodium fluoride solutions, while the remaining tests were made with solutions collected from previous fluorine-recovery tests. The last five tests of the series were made using solutions produced from defluorination of synthetic fluorspar-quartz mixtures. These fluorspar mixtures, compounded to contain approximately 16 percent fluorine, were used so that more highly concentrated scrubber solutions could be obtained than from defluorination of a single charge of phosphate pellets.
The amount of sodium aluminate used in the first 9 tests was 20 percent in excess of the stoichiometric proportion required for combination with all of the sodium fluoride. The next three tests were made, using information contained in a patent.
It was stated that the amount of sodium aluminate used should be equal to or slightly less than the stoichiometric quantity required to combine with all of the sodium fluoride. The combining proportions of sodium aluminate used in these tests were 93, 97, and 100 percent, respectively.
Originally, it was planned to use 100 to 110 percent stoichiometric NaAlO2 for all of the remaining tests, as earlier test results indicated that at least 100 percent was required for optimum yields of cryolite. The low yields obtained in tests 15 and 18 were due to errors in preliminary calculations which indicated use of only about half the required amount of NaAlO2 in the reaction.
Results of chemical and spectrographic analyses of the cryolite produced in these tests are shown in table 15. Methods used for determining constituent elements are described appropriately in the appendix.
Appreciable quantities of nickel, picked up from the laboratory fluorine-recovery equipment, were present in the test solutions in an unidentified form. If excess NaHCO3 was added to the solution at near boiling temperatures, a majority of the nickel precipitated as an amorphous compound which has not been identified.
A spectrographic analysis of the product obtained by evaporating one of the test solutions to dryness showed the following major elements to be present, in the following percentage ranges: Ni and Na, over 10; Si, 1 to 5; and Ca, 0.1 to 1.0. The high percentage of Na is explained by the X-ray diffraction report, which showed the sample to be mostly NaF. Chemical analysis of the sample indicated that it contained 14 percent fluorine.
Spectrographic analysis of the cryolite samples showed that in all samples tested the nickel content was low, except in test 15. The solution used in this test had not been treated for removal of nickel.
Contamination of either the sodium fluoride solutions or the cryolite by nickel will not be an immediate problem in pilot-plant tests, as steel and other standard construction materials are used for all equipment except the scrubber. The scrubber is fabricated from Haveg 43, a molded, graphite-filled phenolic resin. Later, if iron is found to be a serious contaminant in pilot-plant tests, more corrosion-resistant material, such as Monel, may be used.
Fluorine, sodium, and aluminum analyses of the samples do not match the theoretical values too closely. An explanation of this apparent discrepancy in analyses may be found in Simons. This source describes the work of several investigators who believe that the compositions of natural and synthetic cryolites differ somewhat from the commonly accepted composition represented by the formula Na3AlF6.
Fe2O3 content of the cryolite products is satisfactory in all tests reported except test 7. The high Fe2O3 content of this test sample was due to use of a carbon-steel carbonization vessel to obtain comparative data on contamination of cryolite by Fe.
SiO2 values for the products are inconsistent and generally higher than desired. No explanation can be given for products from tests 7, 14, and 18, which contained silica in excess of 1 percent. It is conceivable that the use of fire-clay crucibles and quartz tubing contributed some silica to the products.
Typical specifications of aluminum-grade synthetic cryolite from both American and European producers are included in the appendix for comparative purposes.
Pilot-Plant Scale
A pilot-plant-scale fluorine-recovery campaign, RKR 18, was completed in December 1954. The campaign was of 3 weeks duration and was divided into 3 parts. This was necessary, as the fluorine-recovery process, being new and untried on any but a laboratory scale, required changes to be made in equipment or operating procedures as the test progressed. The production of a low-fluorine phosphate product, although of secondary importance, was not neglected.
Figure 9 shows the fluorine-recovery equipment used for the test.
The kiln offgases, after passing through the cyclone dust collector, were scrubbed by a sodium bicarbonate solution, which was recycled through a fume scrubber. This scrubber, which is resistant to HF, is made of Haveg 43, a phenol-formaldehyde resin combined with graphite as a filler. The scrubber nozzle is made of Teflon. The effluent from the scrubber discharged into a 230-gallon-capacity reservoir provided with a lid, wherein a concentration of sodium fluoride was gradually built up. Carbon dioxide and other products of combustion or reaction were then exhausted from the tank by means of a blower. Sliding dampers were placed in each duct to control the draft. Standard materials of construction, such as steel or brass, were used for piping, ducts, tank, and valves.
Before entering the scrubber, the kiln stack gases were cooled in two stages: In the first stage, the gases were cooled from an average temperature of 540° to approximately 170° C. by a curtain of water flowing down the upper portion of the kiln stack; in the second stage, part of the scrubber recycle solution was bypassed and sprayed into the duct between the cyclone and the scrubber, further cooling the gases to a temperature (max. 130° C.) that would not harm the scrubber. A coil of ½-inch copper tubing in the tank helped to maintain the temperature of the scrubber solution between 50° and 60° C.
Data recorded from laboratory defluorination tests, as well as results obtained from previous pilot-plant tests, were used as a basis for the defluorination phase of the test. Technical-grade phosphoric acid was added to the deslimed phosphate rock in an amount necessary to convert the excess CaO in the raw feed to Ca3(PO4). The so-called “basicity factor” under these conditions would be 1.0.
The kiln feed contained approximately 5 percent moisture and analyzed, in percent, as follows: P2O5, 38.2; CaO, 47.0, SiO2, 2.4; and F, 3.6. Kiln operating conditions, such as slope, rotational speed, and calcining temperature, were selected from previous successful rotary-kiln runs. A rotary cooler was used in the first two parts of the test in place of a quenching pan to eliminate the necessity of drying the product.
During the first week the kiln was operated continuously for 36 hours. The fluorine-recovery equipment was put into operation 12 hours after the test was started and was operated semicontinuously for the remainder of this first part of the test. One hundred and fifty pounds of sodium bicarbonate was initially added to approximately 200 gallons of water in the scrubber collecting tank. Samples were withdrawn from the tank at regular intervals as the test progressed and tested for bicarbonate by titrating with nitric acid, using methyl orange as the indicator. The titration results determined the rate of bicarbonate addition. The strength of the bicarbonate solution was kept between 8 and 10 percent. The above samples were also analyzed for fluorine to determine the rate and extent of buildup of sodium fluoride concentration in the scrubbing solution. The highest concentration of fluoride obtained during the short run was 5.1 grams per liter.
Analyses of grab samples of the kiln product taken during operations indicated that about 50 percent of the fluorine was being eliminated from the charge. These poor defluorination results prompted the conduction of laboratory tests to determine if the correct proportions of rock and phosphoric acid had been used in the charge preparation. Results of these tests showed that the charge mixture could be satisfactorily defluorinated. A materials balance made for fluorine indicated that between 40 and 50 percent of the total fluorine was recovered. A balance made for the period of time that the scrubber was actually in operation would yield higher recovery figures. Before operations were begun the second week, the scrubber reservoir was drained; part of the solution was siphoned into steel barrels for use in future experiments. A sample of the tank sludge analyzed, in percent, as follows: P2O5, 2.3; SiO2, 0.32; and F, 0.58.
It was originally intended that only natural draft be used on the kiln and fluorine-recovery system; however, the presence of sulfur in the phosphate feed in an amount equal to about 0.60 percent caused the formation of sulfurous compounds, which were creating a safety hazard to operating personnel. This situation was remedied by installing a blower to positively exhaust all gases from the system. Trouble was experienced with adjustment of the dampers to obtain just the right amount of draft for efficient kiln operation. It was discovered later that some of the draft-regulation trouble could be attributed to accumulation of material in the crossover pipe leading from the cyclone to the scrubber. Numerous other mechanical and operational difficulties, similar to those just mentioned, were encountered throughout the campaign. These difficulties at least partly contributed to the poor defluorination and recovery values indicated above.
During the second phase of the test, the pilot plant was operated continuously 128 hours. Again, the kiln offgases were vented directly to the atmosphere during the kiln-warmup period. No major changes were made in operation of the fluorine-recovery equipment.
An inspection of the equipment at the end of the first week’s run showed that material was being deposited in the crossover pipe between the cyclone and the scrubbing tower in the vicinity of the solution-bypass nozzle. During this portion of the test considerable trouble was again experienced with deposition in the pipe; in fact, the pipe section had to be removed toward the end of the period and thoroughly cleaned before the test was continued.
Approximately 20 pounds of material analyzing 25.1 percent F and 6 percent P2O5 was recovered. An X-ray analysis of the material revealed that the major constituents were NaF and 3 CaO·P2O5, with some quartz and other crystalline material that was not identified. It was also noted that a considerable amount of material having a similar appearance was deposited inside the scrubber, particularly, around the Teflon nozzle.
Pressures on both the solution return pipe and the solution bypass pipe were varied throughout the run to determine optimum operating conditions for removing fluorine from the kiln offgases. It was determined that a pressure of 60 p.s.i.g. on the solution return pipe caused excessive loss of scrubber solution, as the violent agitation of the solution at this pressure formed froth which was carried up the stack. Loss of solution was negligible when pressure was reduced to 30 p.s.i.g., but it was not determined if the corresponding quantity of solution was adequate to attain maximum recovery of fluorine. Flow through the bypass line was regulated to keep the temperature of gases entering the scrubber below 95° C.
Analyses of the products taken as a composite for each shift showed that considerably better defluorination was achieved during this part of the test, although the fluorine content of the defluorinated products ranged from 0.23 to 3.16 percent. It is difficult to explain this latter value, as temperature and other operating conditions were apparently very similar to those that produced the low-fluorine material. The degree of defluorination achieved in this part of the test is indicated by the product analyses in table 16. Operating data are also included in the table for general information.
Loss of the pump packing near the end of this part of the test forced removal of the scrubber from the defluorination circuit for periods of 1 hour or more at a time.
Despite all the attendant mechanical troubles mentioned, fluorine recovery for this period rose to nearly 60 percent, and the concentration of F in the scrubber solution at the end of the period was determined to be 26 grams per liter, or 58.8 grams per liter if present as NaF. Since the maximum concentration of sodium fluoride in water at the temperature of the scrubber solution during normal operations (60° C. average) is given by Seidell as 44.7 grams per 1,000 grams of saturated solution, the excess fluorine may exist as HF or more possibly as the acid salt NaF·HF. More recently, X-ray examinations have been made of crystals that formed when the solution was heated to near 90° C. in the laboratory. The crystals were identified as Na3FSO4, which might be also written as Na2SO4·NaF.
After all solid material was allowed to settle, the clear solution was siphoned into barrels for use in further recovery experiments. Seventy-six pounds, dry weight, of material was recovered from the scrubber tank. It analyzed, in percent, as follows: F, 9.3; P2O5, 16.4; Ca, 9.25; SiO2, 1.48; and Fe, 1.4.
The pilot plant was operated continuously only 22 hours during the third and final portion of the campaign. The principal data gathered from the fluorine-recovery unit during this period concerned efficiency of the scrubber in removing fluorine from the gases. The apparatus set up for sampling the stack gases consisted of a stainless-steel sampling tube, a Fisher-Milligan gas-washing bottle, a wet test gasmeter, a dry trap, and a vacuum pump, in the order named. Samples of gas were taken at both the entrance and exit of the scrubber for purposes of comparison. Analyses indicated that 20 to 40 percent of the fluorine entering the scrubber was passing through unreacted.
These analytical results agree fairly well with the calculated value for the overall recovery of fluorine, which in this portion of the test approached 60 percent. It must be pointed out, however, that the kiln gases were not routed through the scrubber during the first 10 hours of the period while the kiln was heating up. The scrubber solution from this last part of the test reached a concentration of approximately 5.0 grams per liter. The tank sludge was not analyzed.
In this final test period attention was also directed toward improving the defluorinated product. The kiln slope was lowered to the original setting (3/16 inch per foot), and the quenching pan was reinstalled so that additional water vapor might be drawn into the kiln, thereby aiding defluorination. Although the run was of too short duration for obtaining reliable data, the reduction of fluorine in the product from 1.8 to 0.4 percent indicates that the quenching pan may have furnished the additional water vapor required. If a rotary cooler is used in future tests to cool the product, an auxiliary source of steam probably will have to be provided.
Although the data obtained from this initial pilot-plant test are not too complete, needed improvements in operating technique were definitely indicated. Some changes in the equipment are also contemplated before further fluorine-recovery tests are conducted.
Use Tests of Defluorinated Phosphate Rock
Several agricultural colleges and the United States Department of Agriculture conducted cooperative tests to determine the value of defluorinated western phosphate rock as either a mineral supplement for animal feed or as a fertilizer. Brief descriptions of test methods used and 4 results achieved from crop response tests and 3 animal-feeding experiments are included to show the commercial possibilities of defluorinated western phosphate rock.
Use As a Fertilizer
Two field tests were made on acid soil, and 1 field test was made on calcareous soil; 1 acid test soil was west of the Cascades and the other was near the Washington-Idaho border. The test plot containing calcareous soil was west of the Cascades in southern Oregon. Greenhouse tests were made using 2 calcareous-soil samples and 1 acid-soil sample by K. D. Jacob and his staff at the United States Department of Agriculture research station, Beltsville, Md.
Dr. H. M. Reisenauer, Washington State College, reported on tests conducted on acid clay soil near Cusick, Wash., in Pend Orielle County. In addition to defluorinated phosphate rock produced at the Albany Station, several commercial fertilizer materials were tested. The phosphate materials with 40 percent N and 40 percent K2O were applied in bands 1 inch to the side and 1 inch below the seed at planting. Rainfall in the area was quite favorable for wheat production. Yields on the fertilized plots varied from 36.2 to 42.0 bushels of wheat per acre. The test plot produced 37.2 bushels of wheat per acre when defluorinated phosphate rock was applied in an amount equivalent to 40 pounds of P2O5 per acre, while the control plot which was not fertilized produced 32 bushels per acre. Commercial superphosphate applied at the same rate produced a yield of 39.1 bushels per acre.
Other field tests using phosphate carriers were made at the Washington Experimental Station, Puyallup, Wash., by Dr. Walter P. Mortensen. Experimental techniques employed and other pertinent data contained in this report are detailed below.
The plots were established on a Sultan silt loam, which has a pH of about 5.8. The field-experiment results were obtained in replicated tests, usually six replicates, which employed randomized block designs. A uniform rate of 100 pounds P2O5 per acre was used in all the experiments except those involving potatoes, in which 150 pounds was used. Rates of nitrogen and potassium addition were constant and were supplied by ammonium nitrate and potassium chloride. Supplemental water was applied through sprinkler irrigation to all crops as needed throughout the growing season. All fertilizers were applied in bands at distances from the seed previously determined to be optimum for the crops concerned. Yield data were obtained by harvesting the crops when mature.
Average yields obtained from using two similar lots of defluorinated phosphate rock were, in tons per acre, as follows: Pole beans, 9.03; cucumbers, 7.35; sweet corn, 7.18; and potatoes, 18.39. Similarly, the control plot (no phosphate) produced as follows: 3.45; 1.55; 4.80; and 13.94. Yields obtained from the use of various commercial phosphate fertilizers were not significantly higher in most instances.
The response of barley to phosphatic fertilizers was studied by Dr. A. R. Halvorson at the Oregon State College Experiment Station near Klamath Falls, Oreg. The field trials were conducted on a well-decomposed muck soil having a pH averaging 7.7 to 8.0, with a fairly high salt content. Various types of phosphate fertilizers and trace elements such as copper, manganese and zinc were used. The crop response appeared to be greatly affected by the trace elements; however, the results of the tests indicated that the defluorinated phosphate rock did not give as great a crop response as water-soluble phosphate materials.
In addition to the field trials at Pacific Northwest experimental farms, greenhouse tests were made by R. Starostka, M. Norland, and J. MacBride under the direction of K. D. Jacob of the United States Department of Agriculture Experiment Station, Beltsville, Md. Two samples of defluorinated phosphate rock were compared with fused tricalcium phosphate and triple superphosphate as sources of phosphorus for alfafa on three soils.
The former 3 products, ground to pass a 200-mesh sieve, and the triple super-phosphate, ground to pass a 28-mesh sieve, were applied at the rates of 50 and 150 pounds of P2O5 per acre. Two of the soils, Fort Collins and Millville, were calcareous soils from the western United States and the Chester was an acid soil from the eastern United States.
The composition of the phosphate fertilizer materials tested is shown in table 17. The N and K2O, applied to all soils at the rates of 200 and 400 pounds per acre, respectively, were supplied by NH4NO3 and KCl. Trace elements, including B, Mn, Cu, Zn, and Mo, were applied in solution.
The Fort Collins and Millville soils, although moderately high in extractable phosphorus, were selected because they are representative soils of the western United States, where the defluorinated phosphate would most likely be produced. The Chester silt loam of the eastern United States was selected so that the performance of the phosphatic materials could be tested under acid conditions.
Application of phosphorus increased the yield of alfalfa significantly for all cuttings on the Millville and Chester soils and for the sum of three cuttings on the Fort Collins soil. The defluorinated phosphate with the higher available phosphorus (3141) gave a greater yield than the other defluorinated phosphate (3142) for the third cutting on Fort Collins and Millville soils and for the sum of three cuttings on Millville soil. Defluorinated phosphate and fused tricalcium phosphate gave lower yields than triple superphosphate for the total harvest on Millville soil. All sources of phosphorus gave comparable yields on the Chester soil.
These United States Department of Agriculture researchers concluded from the results that the nutritive value of the defluorinated phosphates compares favorably with that of fused tricalcium phosphate. Furnace products, such as the defluorinated phosphates and fused tricalcium phosphate, generally show a lower nutritive value than triple superphosphate on calcareous or alkaline soils, though possibly equal to that of triple superphosphate on slightly acid or acid soils, especially when the furnace products are finely ground (minus-200-mesh or finer) and mixed throughout the plow layer of the soil.”
Use as a Mineral Supplement in Animal Rations
Where livestock is fed rations composed largely of grasses and various other forages, diet supplementation may be necessary to avoid vitamin and mineral deficiencies, especially calcium and phosphorus. Feeding grain as a roughage supplement often makes good such deficiencies, since grains are fairly rich in phosphorus. In recent years there has been a trend toward using relatively cheap carbohydrate (or “energy”) supplements, such as molasses, to replace the more expensive grains. Since these supplements are sometimes quite deficient in phosphorus, it is necessary to add phosphorus in some other manner.
Phosphate rock is cheap to produce and has a relatively high phosphorus content. It has the disadvantage of a high fluorine content (3 to 3.5 percent), and ingestion of materials of this nature would exert deleterious effects upon animals. Even 1 percent of it in the concentrate mixture would provide an intake approximately 10 times the permissible level set in 1950 by the association of American Feed Control officials. These regulations state that “Rock phosphate or other fluorine-bearing ingredients may be used only in such limited amounts in feeding stuffs so that they will not raise the fluorine of the total concentration of the (grain) ration above the following percentage for cattle, 0.009; for sheep, 0.010; for swine, 0.014; and for poultry, 0.035.” The official publication of this organization further defines the term “defluorinated.” It is to be used in connection with a phosphate product only if the product contains 1 part fluorine or less to 100 parts of phosphorus.
Nutrition tests using rock phosphate produced at Albany were conducted at the University of Wisconsin and at Oregon State College to determine: (1) If the fluorine contained in the phosphate material caused toxicity; (2) if the supplement had enough nutritional value; and, (3) if the defluorinated phosphate rock in itself was palatable enough to insure adequate intake of the phosphorus-containing supplement. Rock phosphate used for the Oregon State College feeding trials met the aforementioned specifications, as the fluorine content was 0.17 percent for 18.4 percent phosphorus. The phosphate used at the University of Wisconsin was slightly above specifications, as the fluorine content of the sample was 0.20 percent.
The tests at the College of Agriculture, University of Wisconsin, were under the direction of Dr. Paul Phillips. White rats were fed for a 6-week period from weaning onward. They were given 2.5 percent rock phosphate and fed in comparison to the performance of animals fed the stock ration. The experiment was set up in such a manner as to furnish about 50 to 63 p.p.m. of fluorine to each of the test rations. At the end of 6 weeks all animals had made approximately the same weight gain, which indicated no adverse effects of the phosphatic material as far as fluorine toxicity was concerned. The physical appearance of the teeth and analysis of the femur bones were criteria used in these tests; normal results were indicated, as far as the short-term experiments were concerned. However, Dr. Phillips concluded that material containing over 0.20 percent fluorine, when fed over long periods, would be expected to be somewhat toxic.
Two graduate students working under the direction of Dr. J. E. Oldfield, assistant professor in the animal husbandry department of Oregon State College, made investigations concerning the use of phosphate rock as a source of phosphorus for animals. Allen Anglemeier conducted preliminary tests, using sheep to determine palatability of defluorinated material, while William W. Ellis fed rats both raw and defluorinated phosphate rock to determine the nutritional value of the materials, as well as to observe tendencies toward fluorosis in the animals.
The palatability tests were conducted by feeding finely ground defluorinated phosphate rock, on a free-choice basis, to six groups of feeder lambs for a definite period of time. Diets for 2 of the groups included barley and oats; diets for 4 of the groups included ammoniated molasses without grain. Consumption of the rock phosphate varied widely and not in any definite relationship to the various dietary treatments. As the rations fed the lambs were not truly low in phosphorus and a study of the levels of blood phosphorus, after slaughter, indicated that none of the lambs suffered from phosphorus deficiency, the test results are considered inconclusive.
In the tests at Oregon State College, using rats to determine the biological availability of phosphates, a modified low-phosphate diet was used as the basal ration. Calculated amounts of bonemeal, defluorinated phosphate rock, and raw phosphate rock (3.6 percent F) were added to supply the desired P level. The method used in conducting the tests is described by Ellis as follows:
Weanling Wistar-strain rats in individual cages were accustomed to the experimental regimen for 2 or 3 days before actual administration of the test diets. During this period a mixture of standard Oregon State College formula rat pellets and the complete synthetic basal ration of Jones and Foster was fed, free choice; tap water was provided as desired during the entire course of the experiment.
The rats were divided into four groups with respect to the best equal distribution of sex and lot weight possible. Since the total number of animals was not available at one time, concurrent experiments were undertaken with the four lots. These consisted of: Controls (basal ration – low P); basal ration plus bonemeal; basal ration plus defluorinated phosphate rock; basal ration plus raw phosphate rock. Later a second small group of animals at a different level of raw rock phosphate was started.
Initially, the experiments were planned so that the same level of P would be supplied to each of the three groups other than those on the basal ration. Differences in the results of analytical procedures for P upon which the initial calculations were based caused slight deviation from this original purpose. However, in all cases (except possibly the raw group I) an amount of P adequate for maximal growth was supplied, providing, of course, that it could be satisfactorily utilized by the rat.
Individual rations were allotted daily, and an estimate of total food consumption was calculated. Individual weights were taken at weekly intervals and any abnormal symptoms noted on occurrence. After 5 weeks on the experimental diets, representative individuals of each of the groups were photographed for tooth-color and body-size comparison; the rats were then sacrificed.
Results of this growth test indicated that the defluorinated phosphate-rock sample (0.17 percent F) submitted by the Bureau was equally as good as, or slightly better than, bonemeal with respect to available phosphorus, as measured by the criteria of weight gain, serum inorganic phosphate level, bone weight, and bone ash. Accumulation of fluorine in the bones of rats receiving the defluorinated material was approximately 1.5 times that in the bonemeal group but was within published normal ranges. Raw phosphate rock was shown to be definitely unsuitable as a phosphorus supplement in livestock feeds.
To complete the study on availability of phosphorus, Ellis carried out chemical assays to compare availability of the phosphorus in the Bureau’s product with bonemeal. These assays were patterned after work by Hill, Reynolds, Hendricks, and Jacob. These investigators had found that the amount of phosphorus extracted from a sample with 0.4 percent HCl is a rough estimate of the expected feed value, or degree of phosphorus utilization by the organism. A sample of defluorinated phosphate rock analyzed for HCl extractable phosphate and compared with bonemeal showed that the Bureau product contained about 91 percent available phosphorus as compared with 97.3 percent availability for bonemeal.
Discussion of Test Results and Conclusions
Beneficiation Tests
The series of desliming tests carried on in connection with the project yielded data on the distribution of vanadium and P2O5 in both the sand and slime fractions, the number of washes necessary for removing a majority of the slime from the sand grains, and the effectiveness of adding detergents to the wash water to aid in removing slime. The following conclusions were reached from the desliming tests:
- Vanadium concentrates in the slime fraction.
- The most economical grind from the standpoint of economic utilization of both the sand and slime fractions of the phosphate rock is indicated to be minus-35-mesh. The sand fraction is high enough in P2O5 and low enough in impurities to be defluorinated in a rotary kiln. The P2O5 content of the slimes is high enough to permit them to be used for feed to elemental phosphorus furnaces.
- Disregarding the potential value of the slimes, grinding to minus-20-mesh will produce a sand product low enough in V for production of defluorinated phosphate rock for use in animal foods deficient in phosphorus.
- Washing the phosphate ore twice with water is effective in separating slimes from the sand product.
- The use of detergents to aid in removal of residual slime from the sand grains is ineffective.
Defluorination Tests
The following conclusions concerning defluorination of western phosphate rock were reached after satisfactory results had been achieved from the rotary-kiln tests.
- It is necessary to beneficiate the phosphate rock by desliming to eliminate vanadium and low-fusion-point impurities.
- Furnace-grade phosphoric acid is desirable for use in this process.
- Quenching the hot, calcined product in water improves the solutility of the phosphorus in neutral ammonium citrate, as it favors retention of alpha-tricalcium phosphate, the highly soluble form produced at temperatures above 1,400° C.
- A minimum temperature of 1,450° C. and an adequate water-vapor atmosphere inside the kiln are essential for good defluorination.
- A shaft-type furnace is not suited to the operation, primarily because of mechanical difficulties encountered by fusion of the charge at defluorination temperatures.
- The defluorination charge should contain enough acid constituents (P2O5 and SiO2) so that all of the CaO in the final product is neutralized.
- The SiO2 in the charge apparently does not combine with excess CaO to form either mono- or di-calcium silicate in the defluorinated product.
- Excellent defluorination was obtained by using only phosphoric acid to neutralize the excess CaO in the phosphate sand. The basicity factor for this condition is 1. Equally good defluorination was attained and the citrate solubility of the product was improved when equal molar proportions of P2O5 and Si02 were used at a basicity of.0.85 to 0.80 in laboratory tests.
Fluorine-Recovery Tests
The following conclusions were reached from the results obtained from the laboratory- and pilot-plant-scale fluorine recovery tests.
- Either artificial fluorspar or synthetic cryolite is a desirable byproduct, which can be produced from phosphate-rock-defluorination stack gases.
- The most satisfactory scrubbing tower to use is of the concurrent type, without any packing.
- Fluorine-recovery tests on a laboratory batch scale, do not give a true indication of results to be expected in a commercial operation owing to the relatively small amount of fluorine present in a charge of phosphate pellets.
- Sixty percent or more of the fluorine evolved from the pilot-plant kiln during the defluorination reaction can be collected by scrubbing the offgases with a dilute sodium bicarbonate solution.
- Synthetic cryolite pure enough to meet specifications required by the aluminum industry can be produced from laboratory solutions.
- Further tests will have to be made before any definite conclusion can be reached as to the correct proportions of combining ingredients needed to obtain optimum yields of cryolite. Other process factors that should be studied include improvement in method and technique of carbonating the cryolite solution and growth of the crystals to a size that can be handled conveniently without dust hazard.
Use Tests
The series of cooperative use tests of defluorinated phosphate rock yielded the following conclusions.
- Application of the material to acid soils generally give crop responses practically equivalent to those obtained from commercial water-soluble phosphatic materials.
- Although the crop response to defluorinated phosphate rock on calcareous soils was not equivalent to that of water-soluble phosphate fertilizers, its value was not conclusively proved or disproved.
- The nutritive value of defluorinated phosphate rock compares very favorably with that of fused tricalcium phosphate.
- Defluorinated phosphate rock is suitable for use as a supplement to animal diets. Nutritionwise, the material is comparable with bonemeal. Residual fluorine in the phosphate product will not cause serious toxicity if fed for a moderate length of time; however, the tests, being of relatively short duration, did not indicate the effect of long-term feeding of the supplement upon tooth and bone structure.
