Table of Contents
We initiated a program was concerned with the utilization and disposal of steel scrap, as the pollution and unsightliness caused by the accumulation of steel scrap in junkyards. Maybe we could use heat to Separate Copper from Steel Scrap? In the past decade the problem has become increasingly serious; greater amounts of steel scrap are being generated while the percentage of scrap recycled by the steel industry has been decreasing because of technological changes, such as the shift to basic oxygen furnaces and the use of beneficiated ores to produce blast-furnace hot metal. It has been estimated that in 1965 alone these factors resulted in the accumulation of some 6 million tons of scrap.
Our program is divided into resource studies and metallurgical research. Resource studies are concerned primarily with factors relating to the accumulation and distribution of scrap and economic factors affecting the disposal or utilization of scrap. Metallurgical research is concerned with determining optimum means for the disposal or utilization of scrap either by the development of new technology or by modification of existing technology. Particular emphasis is being placed on automobile scrap because it represents a major portion of the scrap that is accumulating in junkyards, and because it contains valuable metal resources now being wasted.
The presence of residual elements either in or associated with ferrous alloy scrap is an important factor bearing on scrap utilization. Copper is one of the most persistent of these residual elements, and the amount of copper in scrap is generally used as a yardstick by scrap buyers to establish quality in terms of tramp elements. Copper in commercial steels is required to be under 0.5 percent in rebar steels, 0.3 percent in normal steels, 0.10 percent in high-quality steels, and 0.05 percent for deep-drawing steels. In general, copper contents above 0.05 percent impair the fabricability of steel, especially where it is subjected to deep-drawing operations. Although the presence of copper does not adversely affect many types of steel and in fact may be beneficial, concern has been expressed because of increased amounts of copper in all types of steel over the past 20 years. This increase is due partly to the recycling of ferrous scrap and to the fact that only negligible amounts of copper are, or can be, removed by conventional pig iron or steel making practices.
The problem of removing tramp elements, including copper, is extremely complex, involving both economic and technological factors. In large urban areas contiguous to steel making facilities where fragmented automobile scrap is being recycled into the steel industry, costs of transporting scrap to a shredder installation become a major factor; however, some installations draw from a radius of 200 to 300 miles. Processing of automobile scrap by fragmentation is usually followed by magnetic separation to remove nonferrous metals and other nonmagnetic material; in this operation tramp elements including copper are reduced to tolerable levels.
Another method of utilizing automobile scrap now being developed by the Bureau of Mines involves using scrap steel to convert nonmagnetic taconites to magnetite. Grinding, roasting, and magnetic separation reduce copper and other tramp elements to tolerable levels. Again, this process is limited to large urban centers where the cost of transporting automobile hulks and ore to a common location is not excessive.
Langenberg and Lindsay have prepared a survey, containing an extensive bibliography, on the problems of removing copper from steel. In general, none of the methods disclosed in this paper or in more recent literature appears to offer an economical means for removing copper from steel. Even with the use of the exceptions noted previously, in which scrap is either fragmented or utilized as a reductant to form magnetite and the copper removed by magnetic separation, some copper is recycled back into the steel industry.
Another approach to copper removal involves thermal treatment of automobile scrap either before or after fragmentation. In almost all cases, bulk copper such as generators and radiators is removed from the automobile bulk before it is processed; however, significant amounts of copper in the form of wire copper-plated surfaces, radios, under-seat heater cores, small motors, and other miscellaneous items, frequently remain with the scrap. If this contaminated scrap could be heated above the melting point of copper to allow the copper to be sweated away from the steel scrap without wetting it significantly, the procedure might offer promise as a recovery process. If thermal treatment below the melting point of copper could induce embrittlement of the copper, this too might form the basis for a reclamation method. The main intent of the work described in this report was to determine to what extent copper could be separated from steel by thermal treatment. Small tests were conducted under closely controlled conditions in laboratory apparatus. It was realized that if copper could not be separated effectively under these favorable conditions, larger scale tests employing actual automobile scrap would very likely be even less successful.
Experimental Procedures and Results
Although the material and apparatus used in this study were not intended to duplicate closely an actual recovery operation, the conditions chosen and the data generated would have to be applicable to processing large tonnages of scrap. Because the amount and distribution of copper in automobile scrap is extremely variable and because it is almost impossible to obtain representative samples for analytical purposes, it was essential to establish procedures that would provide closely controlled experimental conditions for determining the effects of time, temperature, and atmosphere (oxidizing, reducing, or neutral) on the distribution and recovery of copper.
Test specimens were prepared by cutting low-carbon, 18-gage mild steel sheet into panels 5/8 inch wide by 3-½ inches long impurity analyses indicated that the panels contained from 0.03 to 0.04 percent copper. Holes (3/16-inch diameter) were drilled along both edges so that either bare or insulated 16-gage thermoplastic wire (Cornish 4062 type TFF NEC standard wire) could be laced tightly around the steel panels. A picture of untreated specimens is shown in figure 1. The amount of copper wrapped on steel specimens was held at two levels, approximately 4 and 15 to 20 wt pct. No attempt was made to duplicate the average level of copper found in actual automobile scrap; the higher levels of copper were more representative of the isolated and concentrated areas where copper occurs in massive automobile scrap.
The specimens were treated in a 4-inch ID by 35-inch long quartz tube mounted in a vertical position in a Kanthal wire-wound resistance furnace. Stainless steel end plates were used to seal the quartz tube. The top plate was provided with an O-ring sealed shaft so that specimens could be raised or lowered into the central (constant temperature) zone of the furnace. A bottom plate with appropriate inlets and a separate plenum equipped with a gas-mantle

burner were used to provide controlled atmospheres. Gas from the plenum was mixed and passed into the main reactor to provide either oxidizing or reducing conditions. Analyses of the various atmospheres is shown in table 1.
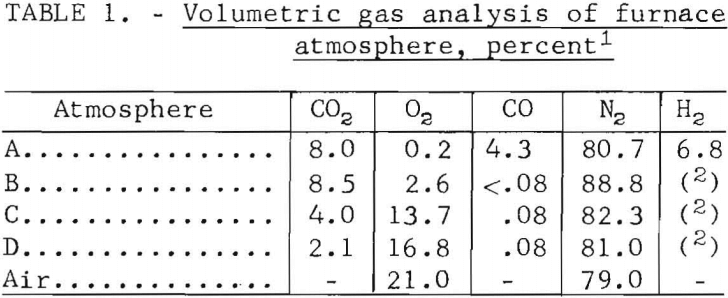
Figure 2 is a schematic diagram of the experimental apparatus.
The procedure followed was to suspend a specimen above the hot zone of the reactor, bring the reactor to the desired operating conditions, lower the specimen into a constant temperature zone for a predetermined period, and then raise the specimen into the upper, cooler portion of the furnace where the specimen was allowed to cool to approximately 300° C before it was removed from the furnace.
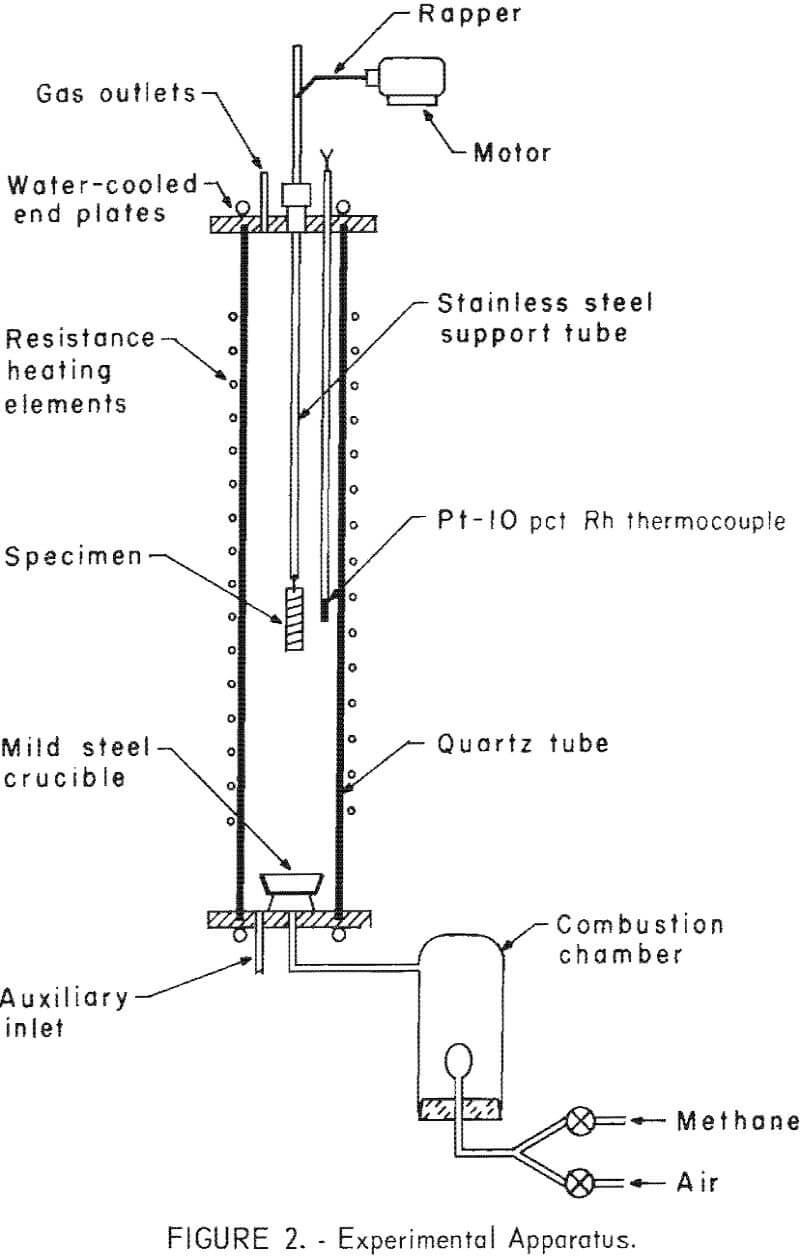
Thermal treatments at 600° to 1,150° C varied from 5 to 60 minutes under oxidizing, reducing, and neutral atmospheres. The extent to which copper could be separated from steel, either by liquation or by scale formation, could then be determined. Oxide scales were removed from the surfaces of the specimens by tumbling in an 8-inch- diameter, cylindrical steel mill provided with three lifts. Steel washers were used as a grinding aid. After the specimens had been tumbled, the scale was separated magnetically into two fractions.
The effectiveness of any given set of operating conditions was determined by visual inspection of the specimens after treatment, weight balances, weight loss after 15 minutes tumbling under controlled tumbling conditions, and chemical analyses of the scale fractions and test specimens.
Static Tests Above Melting Point of Copper
In the initial studies, performed at temperatures above the melting point of copper under oxidizing, reducing, and neutral atmospheres, specimens were suspended in the hot zone of the furnace, and were not rapped or disturbed in anyway to provide an insight into the behavior of copper when automobile hulks are incinerated or thermally treated in furnaces. Tests were conducted with both bare and insulated wire at two copper levels, 4 to 5 wt pct and 15 to 20 wt pct. From the results of these tests, shown in table 2 several conclusions may be derived. The maximum amount of copper removed from the test specimens did not exceed 88.8 percent, and the minimum weight-percent of copper remaining on any of the test specimens was 2.5 percent. Copper removal from specimens wrapped with 15 to 20 wt pct copper wire was much higher than that from the 4 wt pct specimens; this is reasonable because liquation was the major mechanism involved in removing copper. Visual inspection disclosed that the specimens surfaces had been wetted by a thin film of copper for all specimens, whether wrapped with bare or insulated wire.
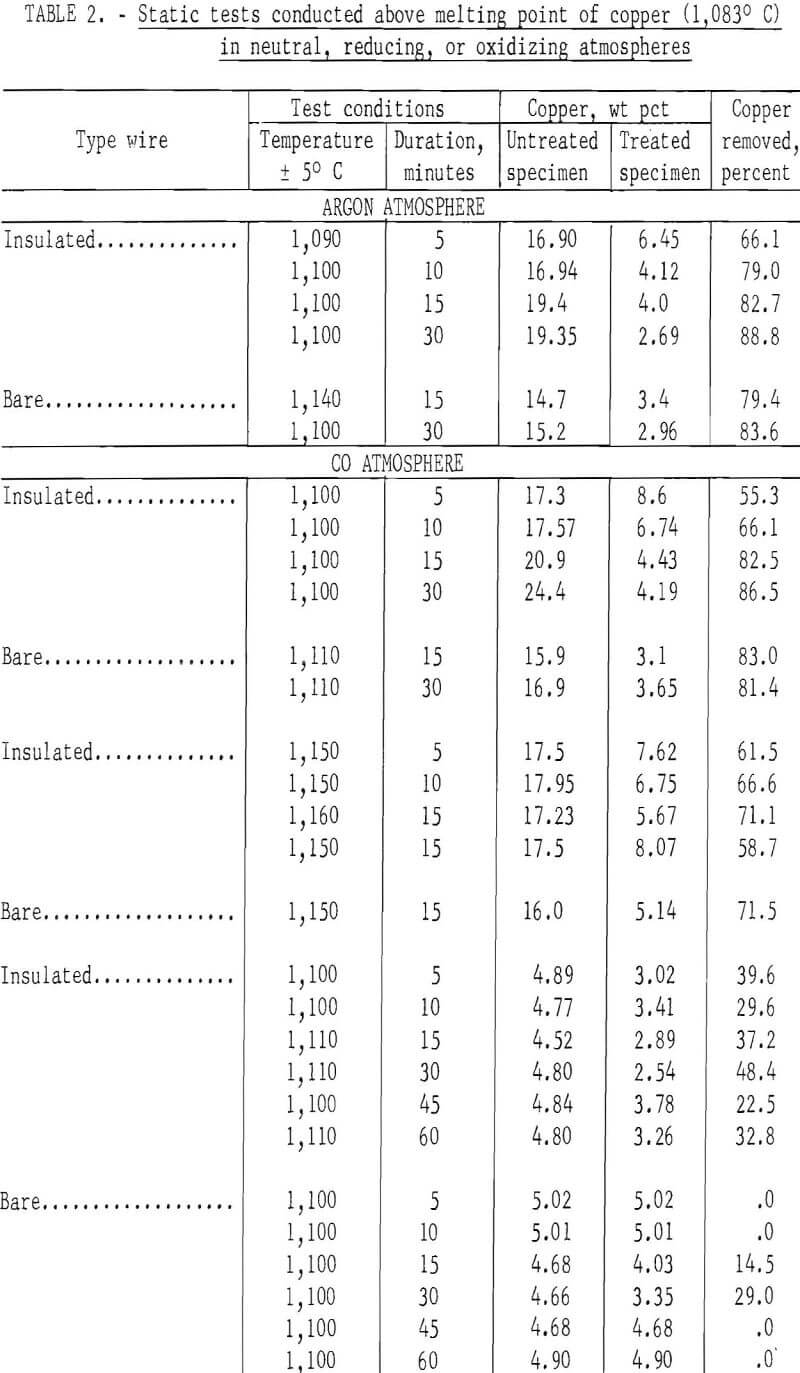
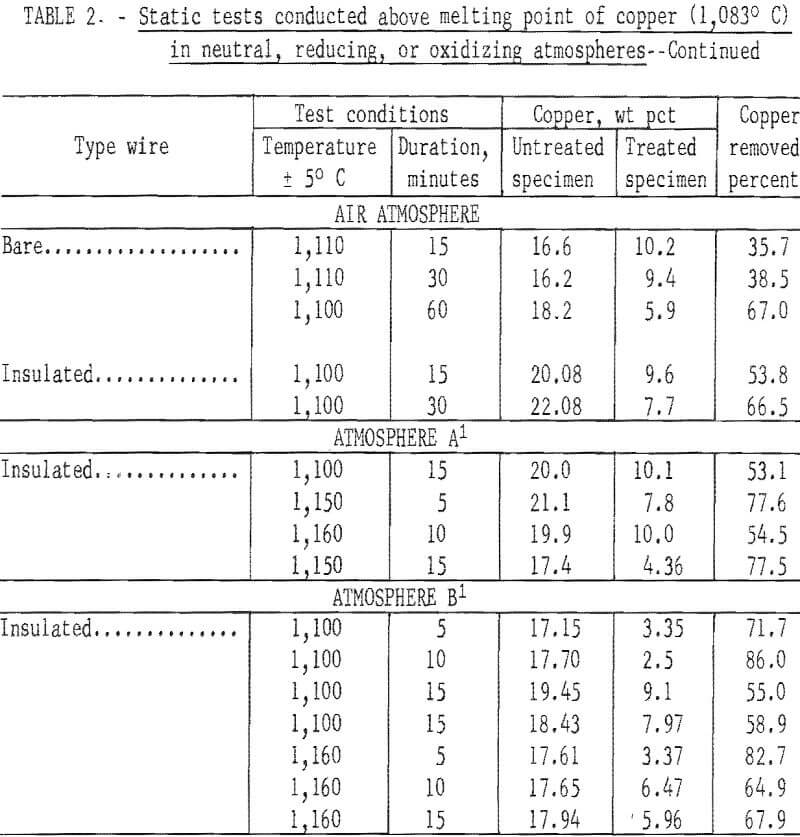
When specimens contained 15 to 20 wt pct copper, copper removal usually increased with time; however, no time pattern was observed in the specimens containing 4 wt pct copper. It is probable that during treatment of specimens containing bare wire the copper first tended to wet and spread over the steel panels, then some of the copper diffused into the steel, some was removed by liquation, and the balance was held by surface forces. Visual inspection of the reactor after treatment of insulated wire specimens gave clear evidence that some type of transport mechanism was occurring, since copper was found not only on the surface of the treated specimens but also on the reactor’s internal surfaces. Presumably, copper reacted with the wire insulation to form volatile species that were subsequently reduced or decomposed to metallic copper.
The spread in the data shown in table 2, especially for those tests conducted with the 4 wt pct copper specimens, was partially caused by the tendency of copper droplets to adhere (viscous drag) to the specimens rather than to coalesce and drop off (liquation). All specimens contained relatively large amounts of copper after thermal treatment regardless of the amount of copper originally present. The results of this series of tests strongly indicate that adequate removal of copper cannot be effected by thermal treatment of automobile scrap above the melting point of copper under static conditions.
Dynamic Tests Above Melting Point of Copper
Because of the wide scatter in the data obtained during static tests, additional tests were performed during which the specimens were activated by having a 1/4-inch-diameter bent rod rotating at 6 to 8 rps rap the support shaft, approximating the conditions that would prevail if fragmentized scrap were treated thermally on a traveling grate or in a rotary kiln. This series of tests, performed above the melting point of copper in a carbon monoxide reducing atmosphere, produced more consistent data than the static tests.
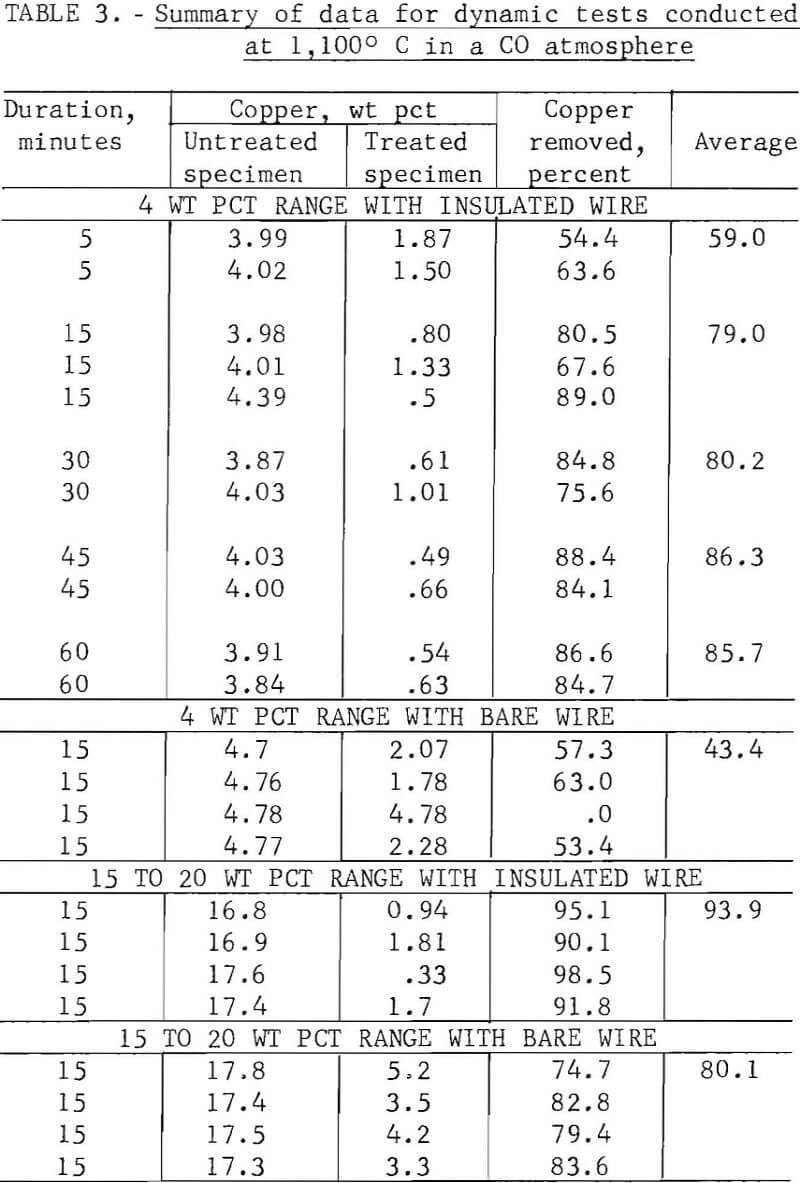
The maximum copper removal, 98.5 percent, occurred with insulated wire at the 17 wt pct copper level; however, the treated steel specimen still contained 0.33 wt pct copper. Using bare copper wire, 2 to 5 wt pct copper remained with the treated specimens, indicating that copper removal by liquation will vary considerably, depending on the ratio of surface contact between copper and steel. It appears that for a liquation process to be successful, the liquated copper must be separated while contacting a minimum surface area of scrap; for this, a traveling grate system probably would be more favorable than a rotary kiln operation. In a rotary kiln the liquated copper would wet the surface of and tend to adhere to the additional scrap.
It was concluded that even under the most favorable conditions a significant amount of copper would remain with the steel after thermal treatment of automobile scrap above the melting point of copper. The cost of providing the energy required to heat large tonnages of low-economic-value scrap above 1,083° C, the melting point of copper, also argues against this approach.
Thermal Treatment Below the Melting Point of Copper
Tests were conducted below the melting point of copper to determine if copper could be embrittled and subsequently removed by tumbling. Basically, the test procedures and apparatus (fig. 2) were the same. Test specimens were treated at 600° to 900° C under various oxidizing atmospheres and for varying periods. A minimum amount of oxidation occurred in tests conducted at 600° and 700° C, and over 90 percent of the copper remained on the test specimens. When insulated wire was used, the polyvinyl chloride insulation tended to char, but the underlying copper wire was only slightly oxidized, remained ductile, and could not be removed by tumbling.
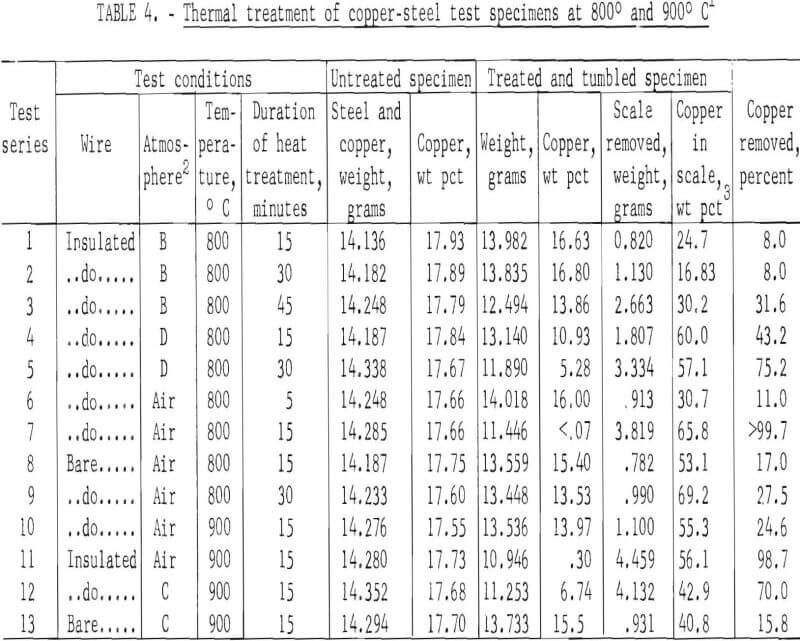
Much more encouraging results were obtained when tests were conducted at 800° and 900° C. The data shown (table 4) for each test series are average values for four individual tests conducted under identical conditions. Atmospheres labeled B, C, and D are those obtained by burning methane (CH4) along with various amounts of excess air. In all cases, the treated specimens were tumbled for 15 minutes to remove the scale fractions.
Copper removal was greatest from specimens that were treated in an air atmosphere. In these tests over 98 percent of the copper was removed from the insulated wire specimens, whereas the maximum amount of copper removed from the specimens wrapped with bare wire did not exceed 27.5 percent. This was attributed to the formation of a protective oxide film on the wire that greatly retarded the rate of oxidation, which in turn reduced embrittlement.
The data also indicate that in all cases the scale fractions contained high concentrations of copper, 16.8 to 69.2 percent. Recovery of copper from the scale fractions by solution treatment would be a straightforward procedure that might improve process economics for scrap subjected to thermal treatment.
Magnetic Separation of Scale Fractions
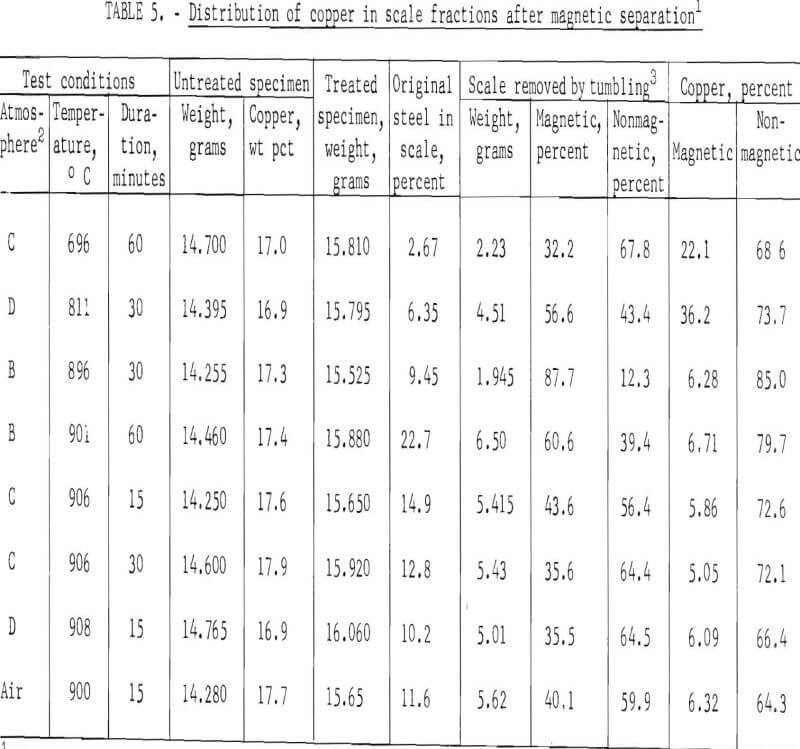
Oxide scales removed from treated specimens after tumbling for 15 minutes were separated into magnetic and nonmagnetic fractions. In addition, the magnetic and nonmagnetic scale fractions were subjected to X-ray diffraction and chemical analyses to determine the compounds present and the amount of copper in each fraction.
It was hoped that format ion of strongly magnetic cupric ferrite (CuO·Fe2O3) would be favored under one or more of the test conditions employed. Unfortunately, since formation of nonmagnetic cuprous ferrite (Cu2O·Fe2O3) is favored above 800° C and reduced oxygen pressure, both cuprous and cupric ferrites were formed, making magnetic concentration impractical. X-ray diffraction analyses showed the presence of trace quantities (1 to 10 percent) of both cupric and cuprous ferrites; the major compounds present were CuO, Cu2O, FeO, Fe2O3, and Fe3O4. In scale formed under strong oxidizing conditions above 800° C, most of the iron was present as magnetite (Fe3O4) with only trace amounts of Fe2O3.
Results obtained by magnetic separation and the amount of copper in the scale fractions (table 5) show that most of the copper was present in the non-magnetic scale fractions. The data strongly indicate that adequate removal of copper cannot be effected from the magnetic scale fraction, since copper in these fractions was not reduced below 5 percent.
Data in tables 4 and 5 suggest that most of the insulated copper present in fragmented automobile scrap might be removed at 800° to 900° C, but that only 10 to 27 percent of bare copper (and presumably copper plate) could be removed by this treatment. Although most of the separated copper could be collected in the nonmagnetic scale fraction, both the magnetic and nonmagnetic fractions would have to be treated chemically to recover copper and to produce a high-iron scale suitable for recycling into the iron and steel industry. Depending on the conditions of treatment, 6 to 15 percent steel scrap might be expected to report in a scale fraction. (See column 7 of table 5.)
Miscellaneous Embrittlement Tests
Inspection of the data shown in table 4 (tests conducted at 800° and 900° C) discloses that there was a large and significant difference in the amount of copper removed, depending upon whether the specimens were wrapped with bare or insulated wire, suggesting that copper embrittlement was related to the plastic (generally polyvinyl chloride), plasticizers, or pigments incorporated in the insulation.
To determine if embrittlement varied with different wires, test panels were wrapped with plastic-coated wires of seven different colors from three manufacturers. Treatment in an air atmosphere at 900° C indicated that copper in black, white, and orange insulated wire specimens were embrittled to the same general extent as shown in table 4 for insulated wire (orange insulated wire used in all tests shown in table 4); however, only minor copper embrittlement occurred with yellow, blue, green, or brown plastic insulated wire. When black, orange, and white insulated wire was used, approximately 98 percent of the copper was removed during tumbling; however, only 15 to 20 percent of the copper was removed in the tests involving the other insulated wires.
Because of these differences a limited study was made to determine whether any specific chemical constituent was responsible. Accordingly, residues of the insulation stripped from seven different-colored wires and ignited in a muffle furnace were analyzed spectrographically. Major constituents present in all residues included aluminum, calcium, and silicon. Residues from orange, black, and white insulation contained approximately trace amounts of aluminum, 34 percent calcium, 2 percent silicon, and from 3 to 30 percent lead, whereas residues from yellow, blue, green, and brown insulation contained from 12 to 25 percent aluminum, 27 to 33 percent calcium, 7 to 12 percent silicon, and no lead.
The presence of lead in black, orange, and white insulation and its absence from other insulations implied that lead oxide may be involved in the embrittlement of copper. This conclusion was partially verified by wrapping a small quantity of lead wire around a test panel wrapped with bare copper wire. When this specimen was treated at 900° C in an air atmosphere for 15 minutes and subsequently tumbled, almost complete removal of copper (over 99 percent) resulted.
A limited number of tests were also made to embrittle copper by coating it with various solutions or chemicals prior to thermal treatment. This involved coating bare-wire specimens in various solutions by spraying or dipping, heating to 150° C to drive off water, treating at 900° C in an air atmosphere for 15 minutes, and tumbling for 15 minutes to remove resulting scale. Tests were made with solutions containing NaCl, Na2CO3, CaCl2, CaCO3, and waterglass (Na2Si4O9). With the exception of waterglass, little or no copper embrittlement occurred above that normally obtained with specimens which were not pretreated chemically.
Relatively large amounts of waterglass were needed to effectively embrittle bare copper wire. The coating method involved dipping the specimens into the waterglass solution, drying at 150° C to drive off water, and then repeating the procedure to build up different amounts of sodium silicate on the specimen. On the basis of limited tests it appears that approximately 0.4 to 0.5 gram of sodium silicate (Na2Si4O9) per gram of copper is required to embrittle copper sufficiently so that over 90 percent of the copper can be removed. With lesser amounts of sodium silicate embrittlement and removal dropped off rapidly to very low values. Much more work is needed to determine the effects on copper embrittlement caused by different types of insulation and by chemical treatment.
Conclusions
These major conclusions were derived:
- Clean scrap, free of copper cannot be produced by thermal treatment of automobile scrap above the melting point of copper.
- Copper present in some types of insulated wire can be removed from scrap by thermal treatment below the melting point of copper.
- Bare copper or copper coated on scrap as a thin plate can be only partially removed by treatment below the melting point of copper.
Additional tentative conclusions, based on limited study, are that embrittlement of copper is promoted by the presence of lead compounds in copper wire insulation and by pre-treating bare copper with waterglass. Results indicated that a clean-cut separation of copper cannot be achieved by magnetic treatment of mixed iron-copper oxide scales, presumably because of the formation of both nonmagnetic cuprous ferrite and strongly magnetic cupric ferrite.
