Table of Contents
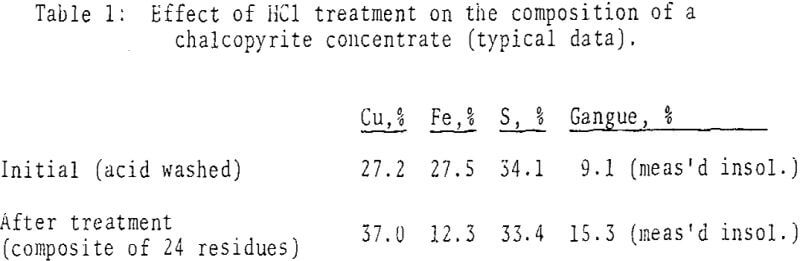
Experimentation with chalcopyrite concentrate in dilute hydrochloric acid, under reflux conditions (in Pyrex flasks) and with an inert purge gas, revealed substantial amounts of H2S production and iron solubilization, with negligible copper solubilization, after 2½-4 days. Under comparable condition the extent of reaction with dilute sulfuric, acetic and phosphoric acids was markedly less.
Pertinent Chemistry and Mineralogy
The chemistry of the Cu-Fe-S-H2O system is marvelously complex, including as it does a variety of oxidation states for each aqueous solute species and ranges of composition (stoichiometry) of the solid sulfide phases. Basically, however, the primary reaction of chalcopyrite with hydrochloric acid in the absence of an oxidant is:
CuFeS2(s) + 2HCl (aq.) → CuS(s) + FeCl2 (aq.) + H2S (aq.)
CuS(s) + 2 HCl (aq.) ↔ CuCl2 (aq.) + H2S (aq.)
H2S (aq.) ↔ H2S (g)
The introduction of oxygen can lead to complete solubilization of copper sulfides. For chalcopyrite and oxidation of sulfide to sulfur, the reaction may be denoted
CuFeS2 + (x+y) Cl- + 4H+ + O2 + 2S + CuCly 2-y + FeClx 2-x + 2H2O
CuFeS2(s) + CuCl2 (aq. ) → 2CuS(s) + FeCl2(aq).
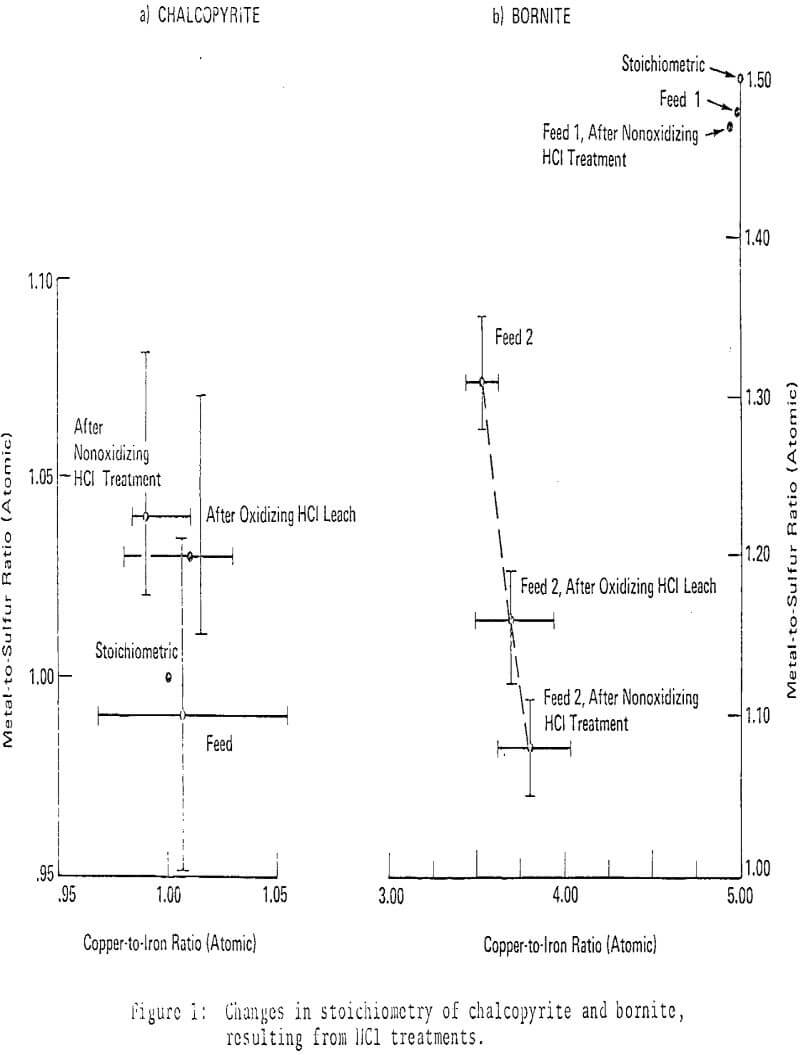
From the above chemical considerations , mineralogical changes can be anticipated. Under nonoxidizing conditions at 140°C, chalcopyrite reacted to form covellite almost exclusively, chalcocite formed rarely as an accessory product. Microprobe analyses indicated approximately theoretical stoichioimetry for chalcopyrite throughout the reaction; observed apparent increases in the metal-to-sulfur ratio were not statistically significant.
Reaction of bornite with HCl produced predominant chalcocite with subordinate covellite, as expected. Microprobe analyses indicated that stoichiometric bornites did not change composition significantly during the course of reaction, while optically homogeneous but non-stoichiometric bornites grew increasingly metal-deficient. The compositional variations of non-stoichiometric bornites were statistically significant. Chalcopyrite exsolution from bornite was frequently observed in partly reacted, non-stoichiometric bornites.
Preliminary Process Considerations
Three major process routes were identified for treating chalcopyrite concentrates:
Treatment with hot, strong hydrochloric acid under autogenous H2S pressure to convert the primary copper sulfides to secondary sulfide and to solubilize the combined iron; separating the solids (mainly covellite, pyrite and acid insoluble gangue) and extracting the copper by known processes.
Subsequent steps in a process based on an O2/HCl leach would not be significantly different than for known hydrometallurgical processes whereby the oxidant is ferric or cupric chloride. Consequently, experimental investigation of process option c. was comparatively limited. In the discussions of HCl treatments to follow, the absence of an oxidant may be assumed, unless otherwise stated.
Experimental Data
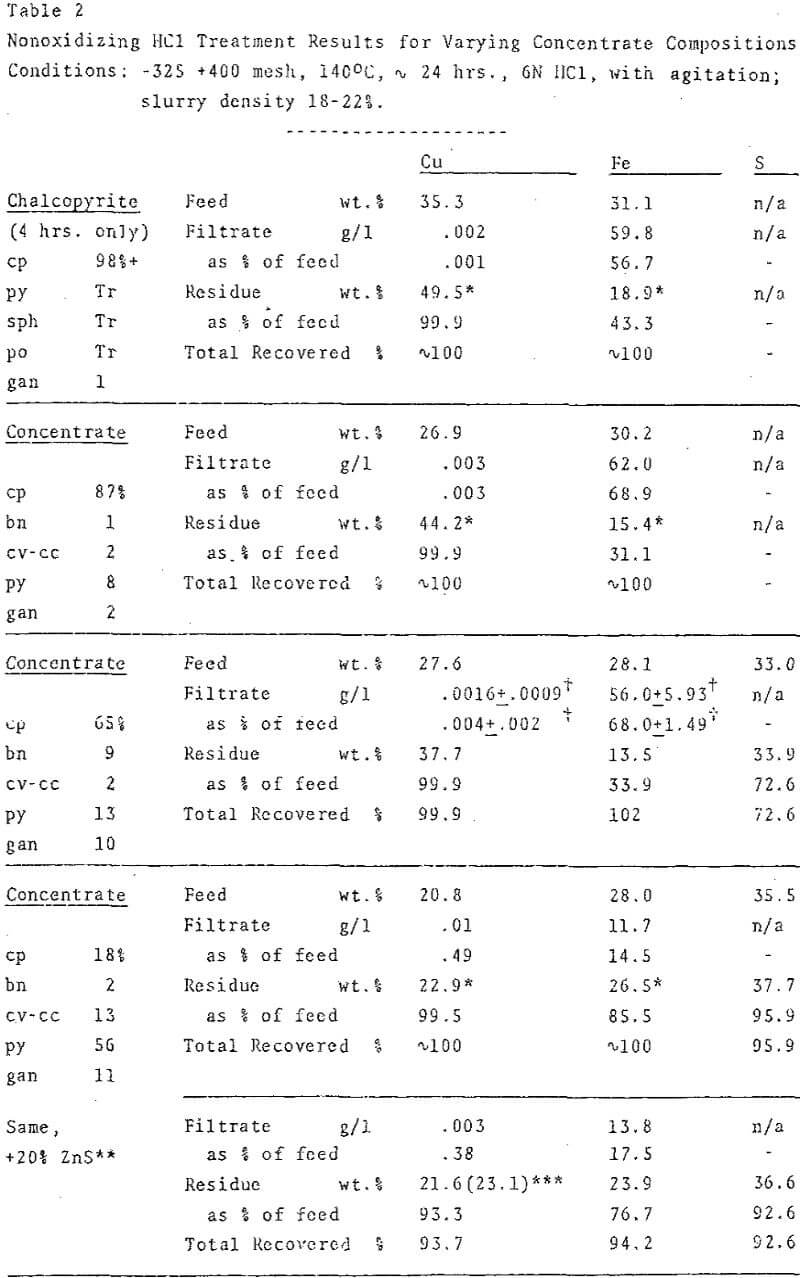
Experimental conditions for nonoxidizing HCl treatment of concentrates were adjusted to effect rapid and complete reaction of primary copper sulfides and to suppress copper solubilization. The extent of reaction was determined by calculating the percentage of iron solubilization. Preliminary studies with mineral separates had demonstrated that the order of reactivity of the major sulfides was: chalcopyrite a bornite >> chalcocite ≅ covellite >> pyrite > molybdenite.
For the majority of experiments, which required closed reactors to maintain H2S pressure, bombs with self-sealing 25-ml teflon cups (Parr Instrument Co., 4745 Acid Digestion Bomb) were employed. The construction of the bombs precluded direct measurement of internal pressure and temperature. Agitation was provided by teflon-encapsulated magnetic stir bars. Optimal reaction conditions were bracketed by varying particle size, slurry density, temperature and acid concentration.
Even though excess HCl (in relation to the stoichioimetry was used in all experiments, acid concentration was a critical factor. At 1N and 3N HCl, the extent of reaction was limited, while at very high acid concentrations (9-12N) , copper solubilization rather than secondary sulfide formation was the dominant reaction. An apparent optimum near 6N HCl permitted both rapid reaction and quantitative production of secondary sulfide.
Microscopic examination of the residues from chalcopyrite-rich concentrates showed that 95-98% of the chalcopyrite had been converted to aggregates of minute covellite grains. In spite of their small average particle size, the slurries required no prefilter or filter-aid for separation. Pyrite remained essentially unreacted, up to 5% of it may have transformed to marcasite.
Total solubilization of copper sulfides can be achieved in an O2/HCl leach system. In our experimental work, 90% of the Cu contained in chalcopyrite mineral separates and mill-run concentrates was solubilized by 6N HCl and oxygen under reflux condition (105-108°C) within 6 hours. Experiments with oxygen and nitrogen purge gas, under identical conditions, showed 1) that dissolution of copper sulfides was 25-50% more rapid in the presence of oxygen.
Elemental sulfur was the predominant sulfur product of an oxidizing HCl leach, in contrast with nonoxidizing HCl treatments, in which sulfide remained virtually the sole oxidation state of sulfur.
Sulfuric acid leaching of the nonoxidizing HCl treatment residues under oxygen pressure was chosen as the most attractive means of copper recovery. The technical goal on the O2/H2SO4 leach was production of an electrolyte suitable for direct electrowinning.
Recovery of Mo, Ag, and Au can be a significant credit in the treatment of copper concentrates, while the buildup of As, Sb, Bi, Se, Te, etc. in a leaching or electrowinning circuit can cause serious problems. The partitioning of Mo, Ag, Au, As, and Se among the products of the processes described above was studied in order to anticipate the treatments their recovery or elimination might require. Analytical uncertainties are necessarily large in much of the trace element data, due to small sample size and because high acid concentrations cause extreme interferences..
The most significant distinguishing features of hot, strong hydrochloric acid for treating copper concentrate are the small number of product species and the relatively sharp demarcation between reactive and unreactive minerals. As we have seen, under conditions sufficient to transform chalcopyrite essentially completely into secondary sulfide, pyrite is virtually unattacked. This feature of the chemistry represents an important advantage over other concentrate treatments, in that reagent consumption and regeneration requirements are minimized.
Because of limitations of equipment, it was not possible to quantify the H2S pressure requirement. However, autogenous H2S partial pressure was sufficient, provided (a) a reasonably large proportion of primary copper sulfide (the source of the H2S) is present in the concentrate and (b) any oxidizing or H2S-fixing capacity of the system – whether derived from ingress of air, pre-existing oxidation products in the concentrates, or whatever – is insufficient to consume a major fraction of the H2S produced. If, for a specific concentrate, autogenous H2S partial pressure is inadequate for complete retention of copper in the solids, the reactor can be pressurized with this gas. No significant economic penalty would be incurred, since the process gives a net production of hydrogen sulfide