Pennsylvania has ample reserves of high-alumina clays which are potential sources of alumina, but many of these clays contain large amounts of iron, which makes them unsuitable for treatment by acid processes.
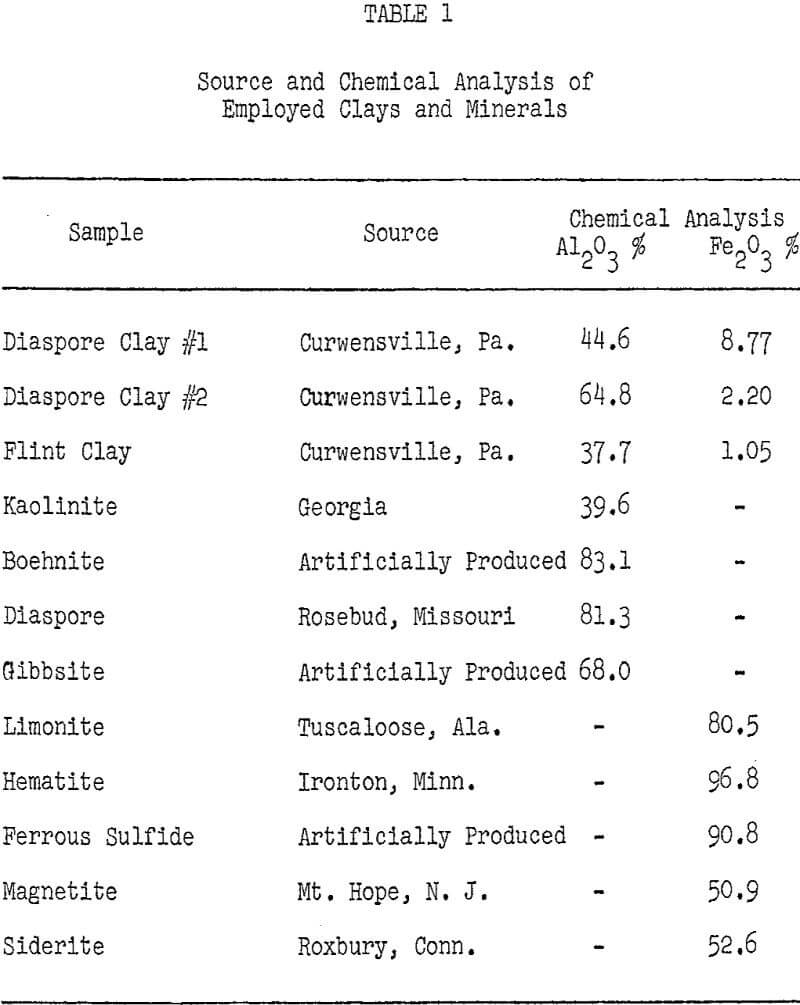
Of all the clay minerals tested, only gibbsite exhibits high acid solubility. Kaolinite, diaspore, and boehmite, which are the major alumina-bearing minerals in Pennsylvania clays, all display alumina losses of 1-2 percent for a one hour hydrochloric acid leach. This leads to the prediction that only 2-5 percent of the alumina in a Pennsylvania clay would be extracted by a hydrochloric acid leach for one hour at 104° C. The difference in acid solubility among the tested clay minerals has been attributed to their disparity in crystal structure.

Sulfuric Acid Leaching of Iron from Clay: Tests were performed to determine the effects of pulp density, temperature, acid concentration, and leaching time on the sulfuric acid extraction of iron from a Pennsylvania diaspore clay.
Iron extraction increases with decreasing pulp density. This is due chiefly to the higher acid to clay ratios at lower pulp densities. The optimum concentration of sulfuric acid is 40%. More concentrated solutions of acid are not completely ionized, and consequently are less effective In Ion extraction.
The iron content of Pennsylvania aluminous clays can be appreciably lowered by means of hydrochloric acid leaching. The optimum leaching conditions are: 19% hydrochloric acid at the boiling point of the acid, 104 °C; 10% pulp density; and 10-30 minutes leaching time. The alumina losses are relatively small.




