In the chemical and hydrometallurgical industries, solvent extraction is a well established and widely used extractive metallurgical process by which desired metals are loaded onto an organic phase from an aqueous solution. However, the recovery of such metal values is conventionally carried out by suitable fluid stripping, whereby metal ions in the loaded organic are extracted, usually by acidic solutions. Consequently, the products obtained from this simple transport of mediums are neither pure, nor in a directly usable form.
Materials. Equipment and Experimental Methods
The aqueous phase used in loading the organic phase consisted of reagent grade cupric sulfate or nickel nitrate dissolved in distilled water, to obtain 36 gram per liter Cu and 58 gram per liter Ni. Unless otherwise indicated, the organic phase consisted of a mixture of as received Kelex 100 (30% by volume) and a low vapor pressure kerosene (70% by volume). Reagent grade ammonium hydroxide, NH4OH, was used for pH adjustment where necessary. Distilled water was used and nitrogen gas of high purity (less than 2 ppm oxygen) was used as an inert medium for purging the extraction system.
Experimental Methods
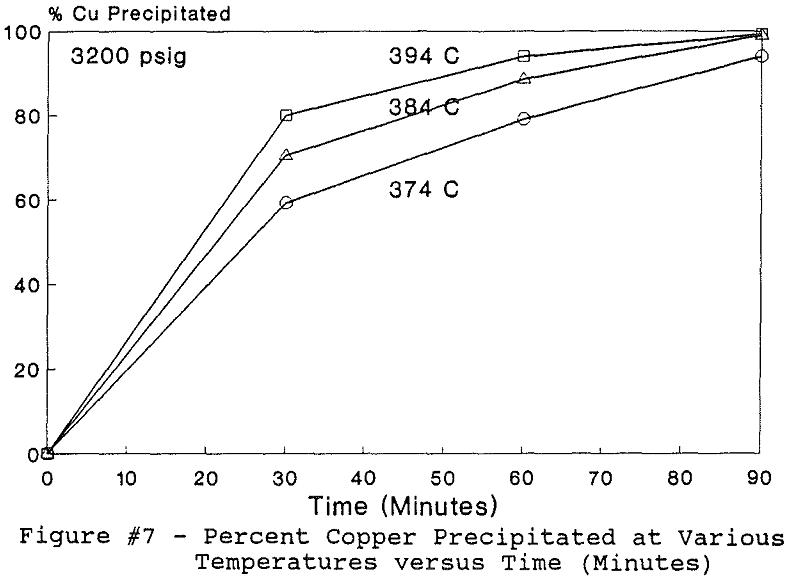
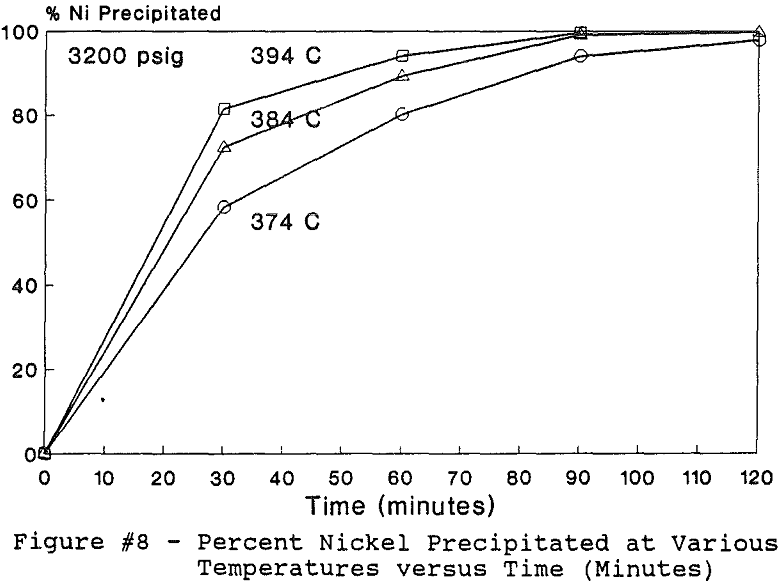
All of this work reported in this paper was performed under the batch operation mode. Equal volumes of organic mixture (Kelex 100 + Kerosene) and aqueous copper sulfate and/or nickel nitrate solutions were contacted under appropriate agitation and temperature until equilibrium was established. Upon phase disengagement and analysis, 50 ml of the loaded organic and a known volume of distilled water were introduced into the reaction vessel. After nitrogen purge, pressure was generated, up to 3500 psi (238 atm), by heating up the system beyond 374 °C.
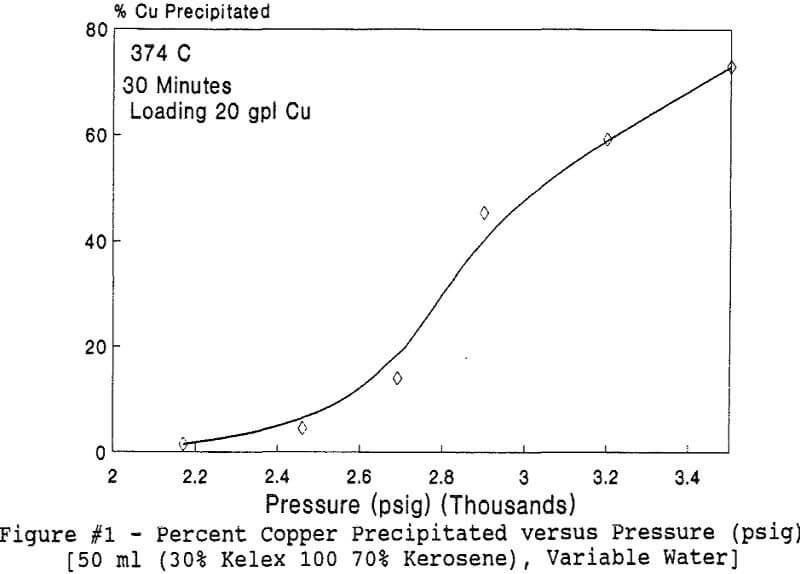
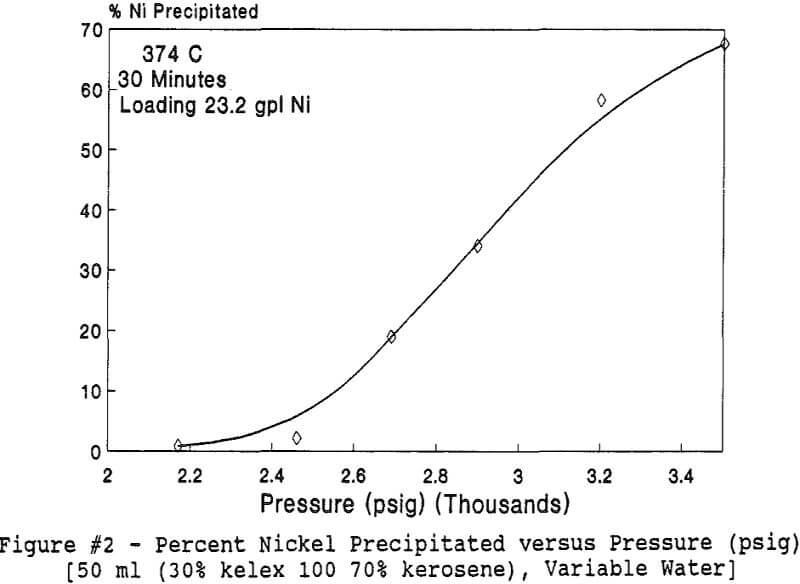
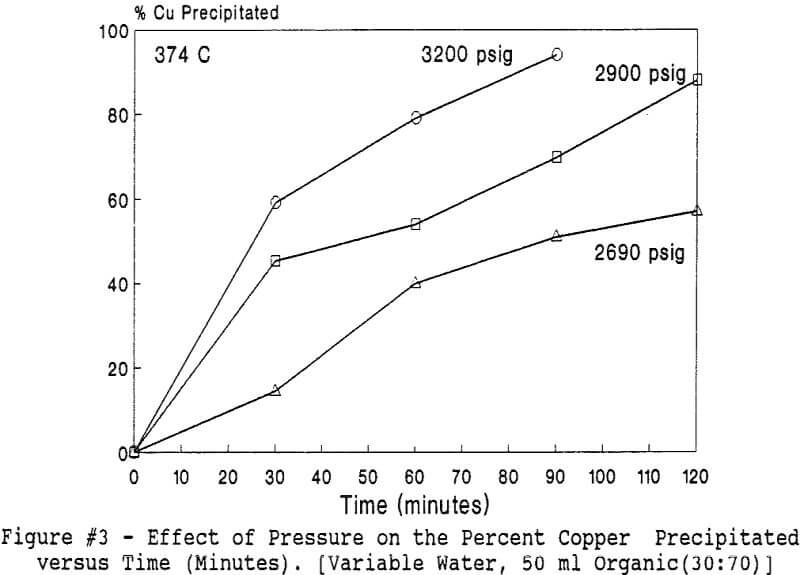
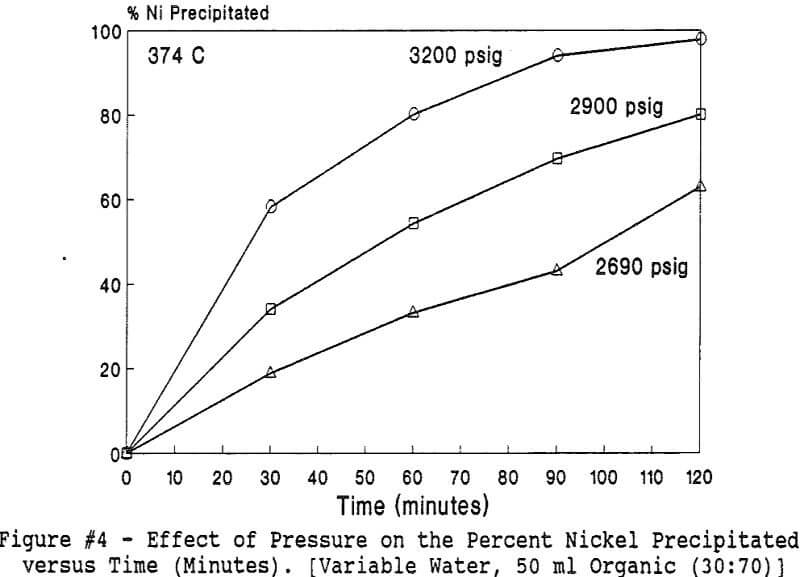
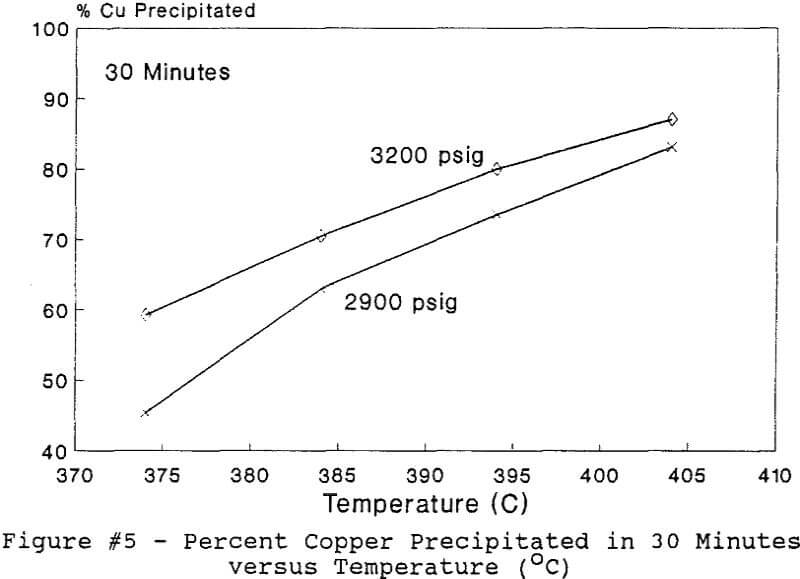
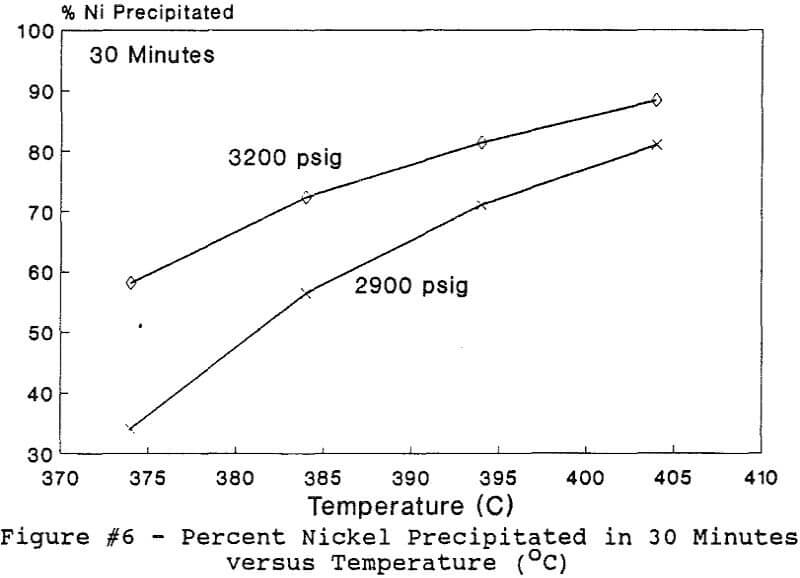
Experiments to investigate the effect of total pressure on the process were conducted by varying the volume of water in the reactor. Using water volumes of 20, 30, 40, 50, 70, and 8 0 milliliters, pressures of 2170, 2460, 2690, 2900, 3200 and 3500 psig were generated and used to study the effect of pressure. In this series of tests, temperature and holding time were kept at 374 °C and 30 minutes.
The use of temperature to generate pressure made it difficult to independently vary temperature alone without affecting the resulting pressure. In order to keep the pressure constant at 3200 psig (218 atm), an ideal gas normalization factor was introduced to correct for the pressure variation from the critical pressure upon small changes in temperatures of the system.
Discussion about Hydrolytic Precipitation of Fine Oxide Powders by Supercritical Fluids
The potential for precipitation of metals from their aqueous solutions may be described by the use of Eh-pH diagrams. Unfortunately, such thermodynamic treatment cannot be applied to the present system due to the low dielectric constant of the inert kerosene solvent.