Table of Contents
- Manufacture
- Production of Natural Iron Oxide Pigments
- Mines and Mining Methods
- Size Reduction
- Production of Synthetic Iron Oxide Pigments
- Thermal Decomposition (Dry Process)
- Solution Methods (Wet Process)
- Regenerator Oxide
- Aniline Process
- Operational Factors in Iron Oxide Production
- Relationships Between Iron Oxide Producers and Other Materials Industries
- End Uses
- Physical and Chemical Characteristics of Iron Oxide Pigments
- Chemical and Physical Stability
- Particle Size
- Particle Shape
- Uses and Applications
- Paint
- Building Materials, Drugs, and Cosmetics
- Ferrites
- Catalysts, Gas Absorption, and Foundry Sands
- Transparent Oxides
- Magnetic Recording
- Copy Machines and Wastewater Treatment
- Micaceous Iron Oxide
- Iron Oxides in Relation to Other Pigments
- Trends in Production and Foreign Trade for the United States
- Present and Future Status of the Industry
Until the late 19th century iron oxide pigments were obtained wholly from natural materials, generally with little alteration other than physical purification. In some cases roasting or calcination was also carried out. However, beginning in the first part of the 20th century, chemical methods were developed for synthetic production of commercial iron oxides. Synthetic production offered improved uniformity as well as characteristics not obtainable with natural oxides, and consequently synthetics displaced natural materials for many applications. Today the iron oxide pigment industry produces a mix of synthetic oxides prepared to a range of specifications plus a number of specific natural oxides still desired because of lower cost or uniqueness of color.
This Bureau of Mines publication treats iron oxide pigments as a commodity within the U.S. economy. The various pigments, methods of manufacture, and uses are described, but not in great detail. Further technical information is available from references cited in the text. Statistics pertinent to the domestic industry over the interval from 1955 through 1976 are presented and reviewed. The discussion essentially reflects industry status and outlook as of the end of 1976. Part 2 of this study will cover the location of natural iron oxide pigment deposits worldwide, together with some geologic and economic features. Therefore, these subjects are mentioned only briefly in part 1.
The iron oxide industry is mature, with products matched to end uses according to chemical and physical characteristics, outstanding among which are stability and nontoxicity. Because of low cost and availability, iron oxides constitute one of the most important groups of colored inorganic pigments. Synthetic pigments, frequently produced from wastes or as a byproduct of other industries, are now the more important portion of the iron oxide industry.
Types and Chemical Forms of Iron Oxide Pigments
Development of color in iron oxides stems from the electronic structure of the iron atom, which has the ability to exist in more than one valence state. Valence states encountered in pigments are the divalent (ferrous ion) and the trivalent (ferric ion). The trivalent state is more common and is found in Fe2O3, hematite, which is red, and in hydrated ferric oxide, Fe2O3·H2O, limonite or goethite, which is yellow. The divalent state occurs along with the trivalent state in the magnetic oxide, magnetite, Fe3O4, also written as FeO-Fe2O3, which is black. Thus, iron oxides provide colors ranging from yellow to red to black, as well as intermediate shades and browns depending on method of preparation and impurities. Iron oxides do not give blue or green colors, but pigments with these colors can be produced using other iron-containing chemicals, as in iron blue, ferric ammonium ferrocyanide.
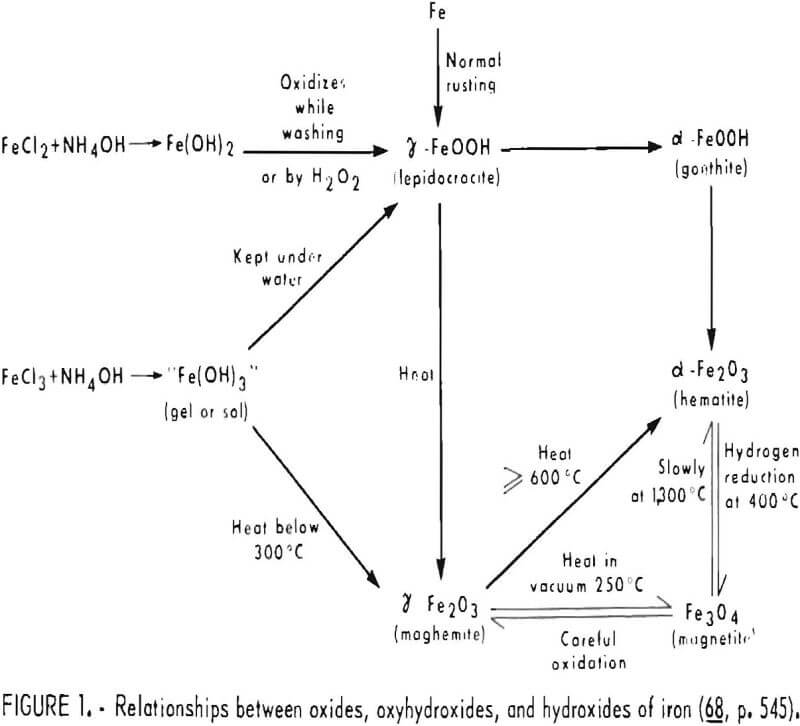
Oxides and hydrous oxides of iron occur or can be made in a variety of forms and physical conditions, and this has resulted in the existence of a number of names for the various compounds, some with only poorly defined chemical nature. However, for pigment purposes it is necessary to consider only five of these compounds: Two forms of ferric oxide, two forms of ferric oxide hydrate, and magnetite. The stable form of ferric oxide, hematite, designated α-Fe2O3, alpha iron oxide, has a rhombohedral structure, is nonmagnetic, and is the basis compound of red iron oxides. A metastable form of ferric oxide, denoted γ-Fe2O3, gamma iron oxide, is a defect spinel which has the magnetic property known as ferrimagnetism. Gamma iron oxide is important mainly for application of its magnetic imaging characteristics in magnetic recording. Heating gamma iron oxide to about 600° C causes permanent transformation to stable alpha iron oxide. Goethite, commonly also called limonite, has the formula α-FeOOH and is the basis compound of both natural (ochers, siennas, and umbers) and synthetic yellow iron oxides. Ferric oxide monohydrate also occurs in nature, or can be formed chemically, as γ-FeOOH, which is known as lepidocrocite. Its greatest significance is that it can be formed as an intermediate in the preparation of magnetic gamma iron oxide. Magnetite, Fe3O4, has inverse spinel structure and, like gamma iron oxide, is ferrimagnetic.
However, to date it has not been as important in magnetic recording applications and is a relatively minor pigment. Both of the ferric oxide hydrates and magnetite can be changed into hematite by heating; calcination of yellow α-FeOOH is one of the main ways of producing synthetic red iron oxide.
The technical literature contains descriptions of the various oxides, oxyhydroxides, and hydroxides of iron, the relationships between them, and the way these relationships can be used in the production of synthetic pigments. A schematic diagram of these relationships for the compounds of main interest to iron oxide pigments is given in figure 1. The compounds in this diagram can be classified in terms of two series, the alpha series for α-FeOOH and α-Fe2O3 and the gamma series for γ-FeOOH and γ-Fe2O3. The stability of the compounds relative to one another, and the variety of ways in which α-Fe2O3, hematite, can be produced are indicated in the diagram. Also shown is the possibility of forming pigments beginning with either a ferrous or a ferric salt. This type of diagram is mainly of interest in the preparation of synthetic oxides, but it also helps in visualizing the natural processes involved during formation of pigment ores.
Terminology
Terminology for iron oxide pigments can be kept simple, on the basis of color, except that a few of the natural colors have names that mostly reflect the places where they were initially obtained. Thus, for synthetic oxides, it suffices to speak of black, brown, red, and yellow pigments. Several types of the synthetic pigments can be distinguished according to method of manufacture. In its tabulations, the Bureau of Mines has differentiated synthetic reds into copperas red, red manufactured by other chemical processes, and other manufactured red iron oxides. Copperas red is oxide produced by calcination of the iron sulfate chemical known as copperas, “red manufactured by other chemical processes” refers to oxide prepared by calcination of precipitated oxide (usually yellow), and “other manufactured red iron oxides” signifies red obtained by direct precipitation.
For natural oxides, a variety of names has been assigned to materials coming from particular sources. A still-prominent example is Spanish red, which as the name implies is a natural red of particular characteristics coming from Spain. Many of the other natural oxides having special names are no longer produced or are of only occasional significance. Therefore, the following terms are adequate to describe modem natural oxide products: Black (magnetite), brown (includes the variety known as metallic brown), Vandyke brown, red, ocher, sienna, and umber. Metallic brown is an oxide obtained by the calcination of siderite, iron carbonate. Vandyke brown is a carbonaceous material containing only a minor amount of iron; it is included here because of tradition and not because it can be clearly identified as an iron oxide pigment.
Ocher, sienna, and umber can be thought of as a group of yellow to brown pigments which more or less, grade into each other on the basis of both chemical composition and color. The terms “ocher” and “sienna” appear to have been used to describe similar material of both domestic and foreign origin. Use of the terra “umber” is more specific because of the manganese content and greenish-brown natural shade of this pigment. In the natural state, the iron coloring agent in all three of these pigments is hydrated ferric oxide, limonite, which tends to give a yellow to brown color. Heating a limonitic material sufficiently will produce a color change as water of hydration is driven off and limonite is converted to hematite (red). Therefore, drying these natural ores must be controlled, usually to temperatures below 115° C, if color change is to be prevented. On the other hand, ores can be calcined deliberately to produce darker shades. Ocher is prized for its natural yellow color and is not heated other than for drying. However, the option of calcining results in there being two main types of both sienna and umber. The term “raw” is used to designate material that has not been heated other than for drying, and the term “burnt” signifies material that has been calcined to produce a color change.
As stated, the dividing line between ocher and sienna is not sharp. General practice is to associate “ocher” with the lighter shades of ores, which are also lower in iron; siennas are usually darker and higher in iron. Siennas may also contain some manganese, because of their tendency to be associated with umber deposits. To illustrate, the iron oxide content (Fe2O3) in a selection of pigments supplied domestically in 1970 was 20%, to 57% for ocher, 35% to 73% for raw sienna, and 23% to 58% for raw umber; the manganese oxide (MnO2) in raw umber ranged from 6% to 25%.
Micaceous iron oxide is another natural form of iron oxide used as a pigment. As suggested by the name, this material is a flaky hematite whose platelike nature is physically similar to that of mica. Chemical similarity does not exist, and micaceous iron oxide does not contain mica. The particle form in micaceous iron oxide appears to give exceptional resistance to weathering. This oxide therefore finds use in coatings for surface protection, and it has been so employed outside the United States for many years, as in painting of the Eiffel Tower in Paris. However, the predominant use of domestic deposits of micaceous iron oxide in the United States has been as ore for iron and steel production, and little, if any, has been used in pigments.
Specifications
Specifications for iron oxide pigments have been developed by the Federal Government. Table 1 lists the Federal specification numbers along with those of the American Society for Testing and Materials (ASTM). The few military specifications indicated by the table are for iron oxides which in some cases are too coarse to be considered as pigments. The U.S. statistical number (Standard Industrial Classification or SIC No.) encompassing mining of crude iron oxide is 1479, while that encompassing manufacture of synthetic oxide is 2816.
Manufacture
The term “pigment” usually carries with it the meaning that the particles giving color are small enough that they cannot be detected individually. This means that one of the objectives in pigment manufacturing is to produce materials with fine particle size. A convenient unit to use in measuring size of pigment particles is the micrometer, which equals 10-³ mm. In a modern pigment, a particle with a dimension of 25 micrometers (about 1/1,000 inch) would be relatively large. Some natural iron oxide pigments have average particle sizes as great as 25 micrometers; synthetic oxides are much finer, most particles being no larger than 1 micrometer and many particles being smaller. These small sizes mean that only for natural oxides is there appreciable retention on a 325-mesh sieve screen as used in powder work, inasmuch as the openings for this screen will pass particles with diameters of less than 44 micrometers. Thus, manufacturers of synthetic oxide typically characterize the size distribution of their product as “99.9% -325 mesh.”
Accordingly, the task before a pigment manufacturer is either to appropriately reduce the particle size of starting materials that are too coarse or to manufacture oxides having particle sizes that are fine enough to begin with. As a rule, size reduction is necessary only for natural oxides, because the processes used to manufacture synthetic oxides yield sufficiently fine particles. The need to achieve small sizes leads to the differentiation of pigment material into “crude” and “finished.” Crude pigment is natural ore which has been dug from the ground like other kinds of ore, but needs to be further treated prior to final use, as in a paint. Washing alone does not produce a finished pigment, but grinding will. Along with size reduction, drying and/or calcining may be required. Finished pigments of iron oxide are part of a larger pigment class termed “dry colors.”
In the iron oxide pigment industry it is rare to find a completely integrated company, in the sense that a single company begins with its own feed stock (natural ore or chemical raw materials) and eventually produces a manufactured good such as paint or a ferrite body. More typically, companies producing natural oxide will confine themselves to mining and selling crude ore, to mining ore and processing it into finished pigments to be sold, to buying ore and processing it into finished pigments, or to buying and processing ore plus making paint to be sold. Similarly, a typical company producing synthetic oxide mainly manufactures pigment. Such a manufacturer frequently blends finished pigments of various kinds with other materials to produce multi component pigments meeting certain specifications. A pigment producer may also make and sell dispersions, which are pigmented liquid vehicles whose form may be convenient for paint manufacturing. Some firms in the pigment industry simply buy finished pigments and do blending or dispersing, or perhaps both.
This review is concerned with the methods and materials used in producing crude and finished iron oxide pigments and with the manufacturers who produce such products. Blending is considered only within the context of pigment manufacture. Dispersion and other operations and manufacturing carried out using finished pigments are not discussed in any detail.
Production of Natural Iron Oxide Pigments
Mines and Mining Methods
Two classes of mines can be distinguished as sources of crude ore for pigment production, “iron ore mines” and “pigment mines.” Iron ore mines are mines operated primarily to produce ore for smelting in the blast furnace. A small amount of the production of these mines may be given additional beneficiation to produce pigment raw material. Although the amount additionally beneficiated is small in comparison to total mine output, this amount constitutes the majority of U.S. crude ore production for pigments. Most of the crude obtained as a coproduct of iron ore mining is red oxide, hematite. Pigment mines are mines operated solely to produce crude for pigment production; the character of this ore makes it unsuitable, or at best uneconomic, for iron smelting. Production from pigment mines has tended to be a relatively stable but lesser part of overall crude pigment production.
The most notable features of crude pigment mining are that mining is underground in the case of ore produced from iron ore mines and is very selective, even to the use of pick-and-shovel methods in pigment mines. Pigment-grade crude ore is presently produced as an adjunct to iron ore mining at two of the six underground iron ore mines in the United States. An earthy hematite is produced at one of these mines; most of it is pelletized, but some is processed as direct-shipping ore. About 1.5% of total production is shipped directly from the mine for pigment purposes. Mining of this ore is by block caving, and the ore is crushed and screened before being brought to the surface. Until December 1977 magnetite ore was obtained at another underground mine by a combination of sublevel stoping, pillar recovery, and sublevel caving. The ore was crushed, first underground and then additionally on the surface, after which magnetic separation and flotation were used to produce a concentrate. Most of the output was pelletized for smelting, but a small amount was specially concentrated into a 71.5% iron-content product sold for pigment-type applications.
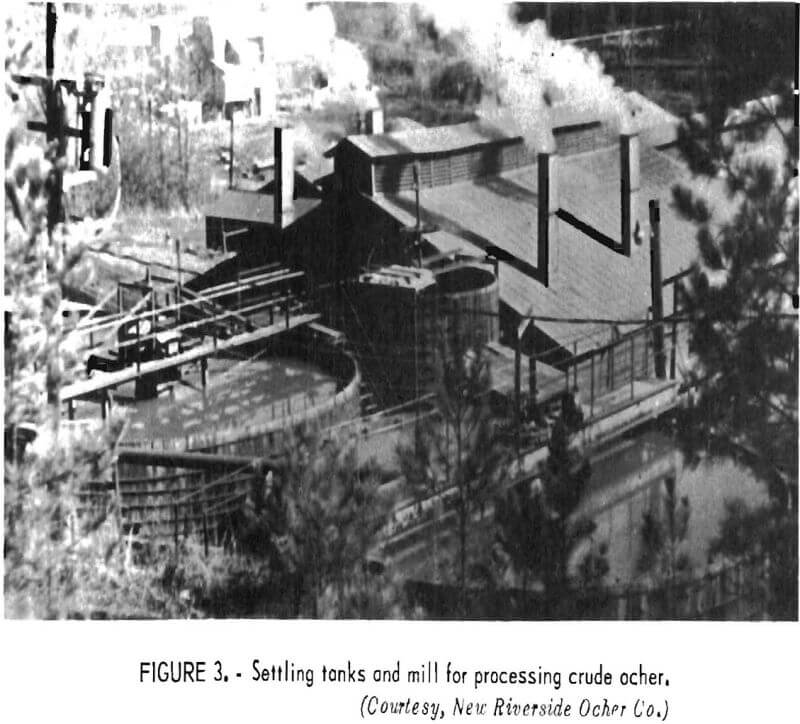
In contrast to massive hematite and magnetite deposits, where the supply of material usable for pigments far exceeds demand and where mass production methods are used to obtain blast furnace feed, deposits of yellow and brown ores suitable for pigments occur in spotty fashion and are mined on a much smaller scale. Underground mining for ocher, sienna, and umber has been practiced in the past, but operations at the two remaining mines are open pit. Depending on the deposits being worked, mining is either by hand or by front-end loader. At one location, a multiple-bench method is employed. Mined material is stockpiled by grade (in the open by one producer as shown in figure 2) and then consumed at the milling plant in accordance with demand.
Ocher is still beneficiated by methods much like those described in detail years ago. Ore dumped at the top of an incline is disintegrated into a slurry by a water spray. The slurry flows down into a log washer for removal of coarse sand and rock particles. Further removal of clay and sand particles occurs as the slurry passes successively through a separator tank and a Dorr bowl rake, shown in an overall view of the plant in figure 3. The ocher leaving the rake as an overflow goes to a settling tank, from which it is withdrawn at a controlled solids content. The slurry is dried by being spattered by an agitator onto a slowly rotating steam-heated drum; the speed of the drum is regulated so that drying is complete in less than one drum revolution. Product is removed by a knife edge located on the side of the drum opposite that where fresh slurry is being added.

Size Reduction
Size reduction of natural iron oxide pigments involves pulverizing and classifying to eliminate agglomerates rather than shattering and crushing to small average particle sizes. Lump ore may be crushed in a hammer mill. As desired or necessary, the resulting powder can be dried or calcined, generally in a rotary kiln. Steam heat can be used to dry ocher, sienna, and umber without producing dehydration and color change; higher temperature calcining yields burnt sienna and burnt umber but is not applied to ocher.
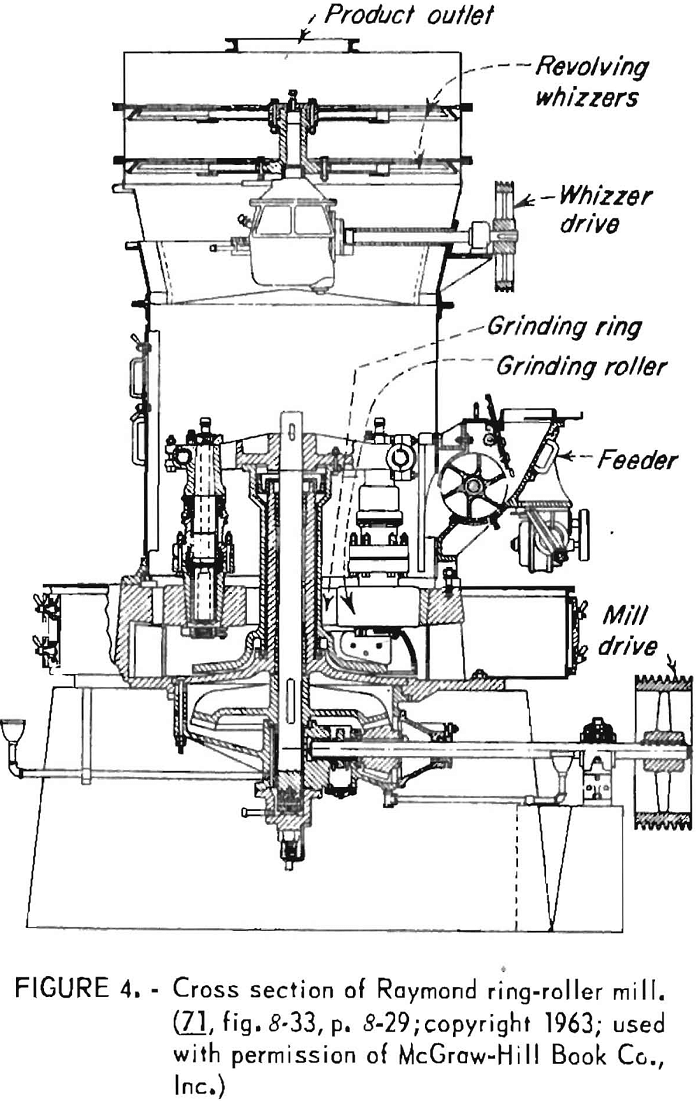
The several kinds of grinding units in use can be distinguished on the basis of operating principle. One of the older units is the Raymond ring-roller mill in which pigment agglomerates are broken down through the crushing action of a roller rotating against a ring (fig. 4). Classification of the product may take place through the action of a “whizzer” mounted in closed circuit on top of the mill. More recently developed grinding units are high-speed hammer and rotor mills and fluid-energy mills. A hammer mill reduces size by impact of pigment particles with very rapidly moving hammers within a confined circular space, followed by integral air classification. Fluid-energy mills use steam or compressed air to generate an autogenous grinding action by impact of pigment particles with each other as they travel at high speed about a circular path, with classification occurring as a consequence of machine design. Introduction of fluid energy mills has led to the use of the terms “micronizing” or “jet milling,” one of the main advantages of which is reduction in the number of larger particle agglomerates.
Grinding alone or in combination with calcination may be enough to produce a
finished pigment containing only one component. In this case, ground material can go directly to a bagging unit, generally automatic. However, intermediate storage and handling steps are required if blending is to be done. Ribbon blenders are commonly used to make mixtures; these mixtures may be passed through a small centrifugal mill to break up any lumps formed during blending, and then the mix can be bagged.
Production of Synthetic Iron Oxide Pigments
As evident from the foregoing, the methods used to produce natural iron oxide pigments can all be considered “dry” methods. Processing steps for natural pigments serve mainly to produce the desired physical characteristics in dry solids, and chemical solutions are not involved. The sense in which “dry” is being used here includes calcination, a step which is used also to produce synthetic oxides from dry chemicals. However, the majority of synthetic oxides are produced basically by manipulating chemical solutions, suspensions, or slurries; and the term “wet process” can be used to refer to synthetic oxides made in this way. In considering synthetic oxides, it is well to recall that their color depends on the oxide formed, by whatever process or sequence of steps: Hematite, ferric oxide, γ-Fe2O3, is red; goethite, hydrated ferric oxide, α-Fe2O3·H2O, is yellow; and magnetite, Fe3O4, is black.
Thermal Decomposition (Dry Process)
Iron oxide can be produced from a number of iron salts by heating them sufficiently in air. This procedure converts iron carbonate in siderite ore into hematite; this in one of the older methods of producing iron oxide (so called metallic brown) from natural materials. Oxide thus produced from siderite and other natural materials, such as pyrite, is classed as natural oxide because of the origin of the starting material. On the other hand, oxide produced by calcination of iron salts is regarded as synthetic.
Iron oxide has been produced this way for many years from iron sulfate. The iron sulfate commonly used is the heptahydrate of ferrous sulfate, FeSO4·7H2O, copperas, and the oxide so produced is referred to as copperas red. A two-step process has been developed for calcination, in which the heptahydrate is first dehydrated to the monohydrate and then decomposed at temperatures in excess of 1,200° F to the oxide. This process can be conducted so that the sulfurous offgases can be used to generate sulfuric acid; the sulfuric acid is reacted with scrap iron to produce, with subsequent processing, more copperas. Oxide particles produced by calcining copperas have a spheroidal shape.
Venetian red is a pigment obtained by methods similar to those used for copperas red, a major difference being inclusion of a lime compound in the furnace charge. This effectively introduces calcium oxide into the system and causes the product to contain calcium sulfate (gypsum) as well as iron oxide. The calcium sulfate forms by reaction between calcium oxide and sulfur-containing gases generated by decomposition of the copperas. While Venetian red has been produced for many years, today its production is quite small in comparison with that of other synthetic red oxides.
Ferrite tans constitute another type of iron oxide pigment that is obtained by forming a compound between iron oxide and another oxide. The second oxide may be either magnesium or zinc oxide and is typically reacted at an elevated temperature with ferric oxide hydrate. This procedure can be considered as a dry method and is similar to methods often followed in making ferrites for magnetic applications. In the case of ferrite tans, reaction between the two component oxides is expedited by including a small amount of magnesium or zinc chloride, respectively, in the starting mixture as a reaction catalyst. The advantage of the ferrite tans, which are nonmagnetic, is that they provide a pigment with yellow shading that is heat stable.
Thermal decomposition methods are used also to prepare small quantities of high-purity iron oxide, generally as Fe3O3. Such oxide is made mainly for chemical rather than for pigment purposes; one of its uses is in preparation of specialty ferrites. Oxide is obtained by first preparing a highly pure form of an only moderately stable compound, which is then heated to yield iron oxide. For example, chemical-grade iron carbonate is readily decomposed into iron oxide and gaseous carbon dioxide.
Solution Methods (Wet Process)
The solution methods most generally used in the domestic production of synthetic iron oxides trace their origin to procedures disclosed by R. Penniman and N. Zoph beginning in 1920. The use of their approach and subsequent modifications thereof is commonly referred to as the Penniman-Zoph process. Alterations and variations of the original technology have made possible controlled production of oxides differing In color and physical specifications. A representative listing of relevant patents is given in appendix A; the 1960 patent by Martin reviews the then existing state of the art. Also, at about the same date the diverse oxides and hydroxides of iron and methods by which they can be produced synthetically were described in a German article. General features of the various synthetic processes can be obtained from the patent and technical literature. However, a thorough presentation of process chemistry and engineering does not appear to be available; this sort of information Is looked upon as proprietary by producers.
The Penniman-Zoph process can be thought of as pigment production through the controlled rusting of iron. Iron in the form of scrap is reacted with a solution from which iron is simultaneously precipitated as iron oxide. Also, by methods developed later, it is feasible to produce pigment by similar chemistry but without the need for scrap iron. In commercial practice, both kinds of processing, with and without the use of scrap iron, are employed. As generally carried out, the Penniman-Zoph process is a two-step operation. In the first step, a seed solution is prepared by precipitating fine nuclei of ferrous hydroxide. Common practice for preparing this precipitate is to add an alkali such as sodium hydroxide to a ferrous sulfate solution. The ferrous sulfate solution can be obtained by making an aqueous solution of the same chemical, copperas, which can be calcined to iron oxide by dry methodology. Air injected into the seed solution oxidizes the nuclei to ferric oxide hydroxide, γ-FeOOH or α-Fe2O3-H2O, synthetic goethite. The process then passes to a continuous-growth phase, in which the seed solution or a portion of it is added to a reactor containing a ferrous sulfate solution in contact with scrap iron. Circulation of the solution over the scrap Iron along with air injection causes iron to pass into solution at the same time that iron oxide is depositing out of solution onto the growing nuclei. Product is obtained by filtering the solution and washing the recovered precipitate.
The process described produces a yellow iron oxide whose particles are acicular or needlelike. At the time of initial particle formation, the solution has a bluish-green color. As growth continues, this changes into a yellow-brown color which gradually deepens with the development of reddish shades. How long the operation is carried out depends on what shade is desired. Some shades may be produced in a few days; in other instances the run may last for several weeks. Even so, the average particle size of the product remains quite small, generally less than 1 micrometer. The reaction is usually carried out in large cylindrical tanks which typically may contain 15,000 gallons of solution, similar to that shown in figure 5. Construction and design of the tanks vary, as does the method of placing the scrap iron. The scrap iron used is clean low-carbon steel with high surface area, such as punchings and trimmings.
The chemical reactions taking place can be written as follows:
Nucleus or seed formation:
a. 4 NaOH + 2FeSO4 = 2 Fe(OH)2 + 2 Na2SO4
b. 2 Fe (OH)2 + ½O2 = Fe2O3·H2O + H2O
Growth:
a. Solution of iron:
2Fe + 2H2SO4 = 2FeSO4 + 2H2
b. Precipitation onto seed:
2FeSO4 + 3H2O + ½O2 = Fe2O3·H2O + 2H2SO4
The course of the reactions and nature of product depend on a number of variables, including rate of circulation of the solution, manner and quantity of oxygen injection, type of precipitant, temperature, iron quality and quantity, acidity, amount and character of seed particles, and presence of other ions in solution, either accidentally or from deliberate addition. Zinc and aluminum salts are prominent among additives that have been recommended for controlling the character of the product.
An important variation of the process is elimination of use of metallic iron, which can be accomplished by careful control of precipitation conditions, including through use of gaseous ammonia. Also, it is possible to use iron solutions other than those of the sulfate. An example is the use of ferrous chloride solution, as described in a recent patent. In this
instance, conditions are maintained such that during both seed formation and growth stages the iron compound formed is synthetic lepidocrocite, γ-FeOOH.
As with its natural counterpart, synthetic yellow iron oxide can be heated and its water of hydration driven off, thus yielding red oxide, α-Fe2O3. This is an important way of producing synthetic red iron oxide. This introduces another consideration into control of preparation of synthetic yellow, because procedures depend on whether the process is being used to prepare yellow oxide as final product or yellow oxide that is to be calcined to red. Red oxide produced by dehydrating synthetic yellow retains the needlelike shape of the yellow, and this gives properties differing from those of spheroidal copperas red.
Precipitation methods can be used to prepare red, black, and brown pigments directly, by careful control of factors such as temperature and extent of reaction. Black oxides can also be produced from red and vice versa. Hydrogen reduction of red hematite can be used to make black magnetite; conversely, calcination of magnetite will yield red iron oxide. Although brown oxide can be made directly, the more usual procedure is to blend a mixture of red, yellow, and black.
An important brown iron oxide produced for its magnetic rather than its coloring properties is gamma iron oxide, γ-Fe2O3. Gamma iron oxide has a spinel type of crystal structure and is widely used in magnetic recording applications. This oxide can be considered a derivative of the synthetic yellow iron oxide industry and is usually produced by the following sequence of steps (1) Production of synthetic yellow, α-Fe2O3·H2O; (2) dehydration of yellow to red, α-Fe2O3; (3) reduction to magnetite, Fe3O4; and (4) oxidation to gamma iron oxide. This sequence produces magnetic particles whose shape and physical properties are both desirable. The synthetic yellow oxide produced as starting material in step 1 has an acicular shape with a high ratio of length to width, and this particle geometry is retained throughout the balance of processing. In step 2 water is removed to give temperature-stable ferric oxide. In the alpha form, however, the oxide is nonmagnetic (antiferromagnetic). Reduction with hydrogen, step 3, produces magnetite, which has a spinel lattice and Itself finds use in magnetic applications although it is deficient in chemical stability. In step 4, oxidation Is conducted so as to preserve the spinel structure of magnetite; the end result is an oxide with desirable magnetic characteristics and good stability. Gamma iron oxide is unstable with respect to alpha iron oxide, and heating to about 600° C can cause a permanent reversion to the alpha form.
Precipitation techniques can be controlled so as to produce “transparent” iron oxides. These oxides derive their insignificant hiding power from very fine particle size, measured in hundredths of a micrometer, or an order of magnitude finer in size than ordinary synthetic oxides. Transparent oxides can be produced in yellow, red, and intermediate shades. They constitute a relatively small portion of total synthetic production; the chief applications are automotive finishes and plastics used in vinyl upholstery.
Regenerator Oxide
Regenerator oxide is byproduct iron oxide produced when hydrochloric acid is regenerated from liquid waste from the pickling of steel. Pickling is a chemical descaling process for cleaning the surface of steel sheets and other intermediate shapes which have become covered with an oxide film, usually as a result of heating or annealing. Formerly sulfuric acid was the main chemical used for pickling, but now the majority of pickling is done with hydrochloric acid. An estimated 40 million tons of steel per year are pickled with hydrochloric acid.
Spent pickle liquor containing ferrous chloride and some unused hydrochloric acid presents a pollution and disposal problem. A few steel plants have installed facilities for treating spent liquor by “reversing” the pickling reaction. Hydrochloric acid is thereby regenerated for reuse in pickling, and iron oxide is obtained as a byproduct. This oxide, which may require further treatment to decrease residual chlorine, has found use primarily as a raw material for production of hard ferrites used in permanent magnets. Regenerator oxide produced in Canada has been particularly prominent in U.S. consumption of this form of oxide. Most regenerator oxide has been produced by a spray-roasting process. A facility of this type operated by a U.S. steel company is shown in figure 6. However, other types of regeneration processes have been installed, or are under development as pollution control methods, and have potential for furnishing forms of oxide suitable for traditional iron oxide markets.
Aniline Process
The aniline process has not yet been used commercially in the United States. Its use to make iron oxide pigments has been unique to Germany, where it has been employed for many years as the main method used by a major maker of synthetic pigments. The process entails reduction of an aromatic nitro compound to an amine in the presence of iron and acid. The reaction known as Bechamp reduction, when applied to production of aniline from nitro-benzene is nominally
4 C6H5NO2 + 9 Fe + 4 H2O = 4 C6H5NH2 + 3 Fe3O4
While this reaction was originally utilized to manufacture aniline, the process can be conducted so that byproduct oxide sludge yields valuable pigments.
The technology for making pigments by the aniline process was developed by I. G. Farbenindustrie A. G. not many years after the Penniman-Zoph process was introduced in the United States. The aniline process is practiced at Uerdingen in North Rhine-Westphalia, West Germany, by Bayer A. G. , a successor of I. G. Farbenindustrie, and accounts for most of Bayer’s large output of synthetic pigments. Iron chips of controlled size are used; they may be of cast iron. To produce yellow pigments, the reaction is carried out in the presence of aluminum chloride; ferrous chloride is added when black pigments are desired. Red pigments may be obtained by calcining either yellow or black products. A description of the aniline process as practiced at the end of World War II is given in appendix B.
Operational Factors in Iron Oxide Production
Because of relatively low product prices, natural oxides cannot carry a large cost component for transportation of raw material. Therefore, milling operations are located close to mines for natural pigment, and additional crude sources are sought in the immediate vicinity. On the other hand, crude material from iron ore mines is shipped considerable distances to existing mills, rather than being ground at plants sited nearby. As ocean freight rates rise, processing and use of foreign natural oxides can be expected to encounter increasing competition from domestic material.
Blending is a secondary operation carried out both by crude processors and by other firms handling only finished pigments. In blending, pigments, and perhaps additives, are combined to achieve a particular shade or physical property. Blends may be mixtures of wholly natural or synthetic pigments or mixtures of the two kinds of pigments. For example, a small amount of synthetic oxide may be blended with predominantly natural oxide to obtain a brighter shade. Blending is used to manufacture synthetic brown pigments from mixtures of synthetic reds, yellows, and blacks. Some blends are rather complex with as many as a dozen constituents, none of which is dominant in the mixture.
Iron oxides are usually produced in batch or intermittent fashion, although the processing and milling of natural oxide could be carried out continuously. Milling can be readily started and stopped, and therefore can be done conveniently on a single-shift basis. However, calcining and carrying out wet-process methods require a prolonged period of continuous operation. Also, energy inputs for heating are needed for both of these operations. Therefore, it is desirable to conduct calcining and synthetic oxide manufacture for extended periods, or else to shut the plant down, rather than to run on a stop-and-start basis.
As with many other industries, iron oxide producers have had to take steps in recent years to limit air and water pollution. Problems with pollution have been least for dry operations such as grinding of natural oxide. For the natural oxides, relatively simple equipment suffices to keep airborne emissions within acceptable amounts. However, care and some expense are also involved in avoiding pollution of adjoining waterways by runoff from mill property. More extensive facilities are required to cope with potential water pollution from wet-process production of synthetic oxides. This technology requires handling of acid solutions containing solid fine particles. Meeting environmental standards has meant extra expense for installation of water treatment facilities mainly designed to control suspended solids and iron levels in effluent. Waste water treatment facilities at a major U.S. producer of synthetic pigments are shown in figure 7. The Iron oxide industry has been included In a study of waterborne wastes of the Inorganic pigments industries but has not been deemed of sufficient concern by the U.S. Environmental Protection Agency to warrant a specific investigation. One producer has funded study of uses for iron oxide plant wastes containing gypsum.
Relationships Between Iron Oxide Producers and Other Materials Industries
The majority of iron oxide production ties in at least indirectly with some other industry or form of minerals processing, as shown schematically in figure 8. Only the portion of iron oxide production derived from natural pigment mines can be thought of as completely independent of other mineral and materials-producing industries.
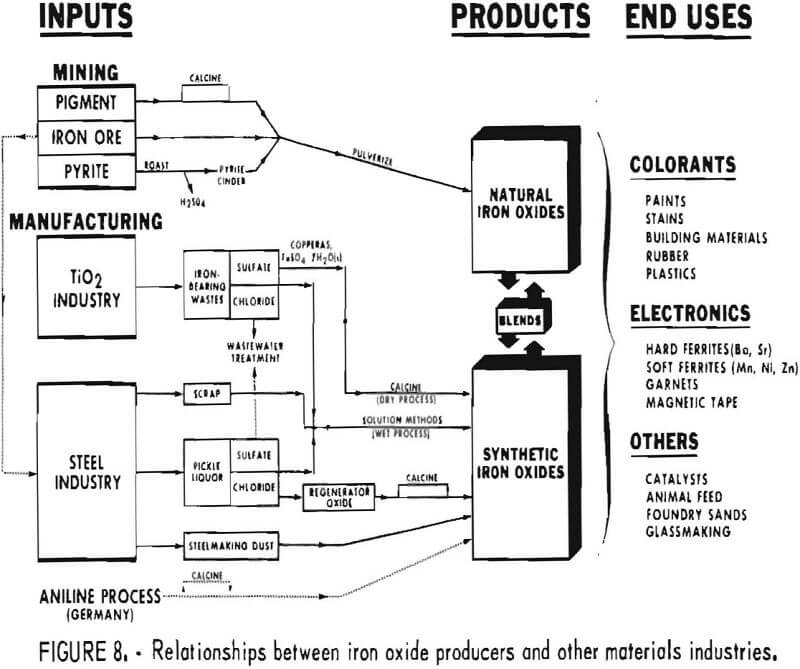
Both the titanium pigment and the steel industry play a very Important role in providing iron-containing materials from which iron oxides are produced. Various possible interactions between these industries and the iron oxide pigment industry are shown in figure 8. Not all the interactions are practiced currently. The sulfate-process portion of the titanium pigment industry is a key source of copperas, FeSO4·7H2O, which can either be calcined directly to iron oxide or used in solution methods for production of precipitated forms of iron oxide. Wastes from newer chloride processes for titanium pigments also are potential starting material for iron oxide production.
The steel industry, which processes and produces iron-bearing materials in a variety of forms, is also a significant source of feed materials for iron oxide production. Light steel scrap with high surface area is used in producing synthetic oxide according to the process originated by Penniman and Zoph. Acid pickle liquor from cleaning steel surfaces has been used to prepare ferrous sulfate, which when roasted has been an important source of oxide used as polishing rouge. Within the past decade the steel industry has turned at many plants to hydrochloric acid instead of sulfuric acid for pickling steel. Several processes have been developed for regenerating hydrochloric acid pickle liquor, and in them a byproduct iron oxide is produced. Some of this so-called regenerator oxide has found application outside the steel plant, particularly for manufacture of hard ferrites used in making permanent magnets. The steel industry produces a great quantity of dusts; those collected from certain steelmaking operations can be used in some low-grade pigment applications.
The steel industry has a potential for being a much larger source of iron oxide powder and in the future could conceivably produce a volume much greater than the market is capable of absorbing. This can be seen by projecting the total amount of regenerator oxide that would be produced if all pickle liquor were processed for recycle. However, even if regeneration technology were applied universally, it is unlikely that this would significantly displace established methods of manufacturing iron oxides, because oxides from steel plants would not possess the characteristics required by end applications. More likely, these oxides will eventually be recycled via the blast furnace.
Figure 8 also indicates that, sulfate and chloride wastes from the titanium pigment and steel industries are usable as agents for treating wastewaters. This is a market for bulk chemicals that has been projected to grow at about 7% annually. Thus, wastewater applications may prove able to absorb sulfate and chloride wastes, only a small portion of which can be consumed in manufacturing iron oxides.
Production of natural iron oxides has less interdependency with other industries. Production of oxides using crude from pigment mines relates to other industries only to the extent that other materials, such as synthetic iron oxides, are blended with natural oxides. The same is largely true for pigment material derived from crude from iron ore mines. However, it should be noted that mining for iron ore is, to a very great extent, intended to provide feed for blast furnaces, and therefore production from such mines is scheduled with that as the major objective. Similarly, iron oxide from pyrite is a byproduct of an operation whose major function is to produce other materials, such as sulfuric acid.
Another industry in figure 8 relates to iron oxide production in Europe. This is the organic chemical intermediates industry, one portion of which, via the aniline process, makes iron oxides as a coproduct in West Germany. This technology has not been used in the United States, but is scheduled to be applied within the next few years at a new facility.
End Uses
Physical and Chemical Characteristics of Iron Oxide Pigments
Uses of iron oxide pigments depend on properties of the pigment particles, individually and in relation to the medium in which they are used. Ordinarily, pigment particles can be considered insoluble in, and unreactive chemically or physically with, the vehicle into which they are dispersed. Among the properties or characteristics that: can affect selection of a pigment and the manner in which it is used are mass color (color at full pigment strength), tinting strength, hiding power, oil absorption, particle size and shape, particle size distribution, and cost. Selection of a pigment further depends on optical, magnetic, and chemical properties. In what follows, properties of iron oxide pigments will be discussed in general terms and their main areas of application Indicated. Published literature and data from pigment manufacturers should be consulted for specifics on pigment properties.
Chemical and Physical Stability
Chemical and physical stability along with relatively low cost are important advantages of iron oxide pigments. Both natural and synthetic oxides are generally characterized by resistance to alkaline and acid environments. This helps impart high bleed resistance; that is, resistance to attack from solvents in coatings applied over coatings pigmented with iron oxides. Natural and synthetic oxides are both light fast and nontoxic; the latter is especially a property of synthetics. Red oxides and calcined natural oxides deriving their color from ferric oxide (Fe2O3) are heat stable. Other oxides in which coloration is based on hydrated ferric oxide (Fe2O3·H2O) or on ferrosoferric oxide (Fe3O4) are stable under normal ambient conditions but can withstand only a limited amount of heating. Thus, natural yellow oxide, ocher, raw sienna, and raw umber are subject to color change on heating above 220° F, natural black oxide (magnetite) is not color stable above 300° F, and synthetic yellow and black do not withstand heating above 350° F.
Particle Size
The particle size of iron oxide pigments depends on the origin or method of preparation and on whether size reduction procedures were used. The largest particle sizes are found in natural pigments, which may contain agglomerates as coarse as 30 to 100 micrometers (about 1/1,000 to 4/1,000 inch). Pulverized natural pigments and synthetic pigments are finer, typically having particle sizes of 1 micrometer or less on down to particle sizes of only hundredths of a micrometer in the case of transparent oxides. Thus, iron oxide pigments span the size range normally associated with all kinds of pigments from relatively coarse down to colloidal dimensions. Particle size can be used to control shade, because development of a stronger red shade is favored as particle size decreases. Smaller particle size also gives increased hiding power, provided pigment particles are not so small that hiding power disappears, as in transparent pigments. The range of particle sizes in a pigment, that is, the particle size distribution, is also important because a tighter size distribution gives greater color purity and brilliance.
Particle Shape
Pigment properties are also dependent upon shape of pigment particles. This is perhaps most evident for synthetic oxides and micaceous iron oxide. In large measure, synthetic yellow iron oxides have an acicular or needlelike shape as precipitated. Such oxides and the reds prepared from them tend to be higher in oil absorption than oxides having particles with more regular shapes. On the other hand, an elongated shape is more desirable in oxides for magnetic applications. All types of synthetic pigments except yellow are available in varieties whose particles have a spheroidal or cubic shape rather than an acicular shape. Red oxide from calcination of copperas is spheroidal, and this is significant in determining powder and sintering characteristics when this oxide it used in preparation of ferrites. Particle shape of natural oxides is not controllable beyond the effects of size reduction and calcination treatments. Micaceous iron oxide particles have a relatively large plate like or wafer shape which can be utilized to give a coating with enhanced protective properties. In general, with the exception of micaceous oxide, particles of natural oxide have irregular shapes but are not highly asymmetric
Uses and Applications
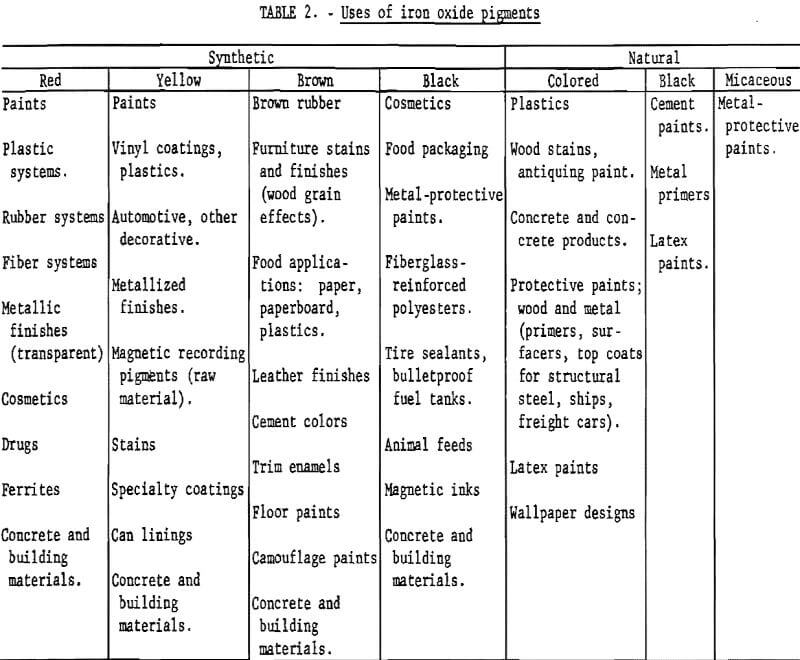
The variety of uses to which iron oxides are put is indicated in table 2, which is based mainly on information given in volumes I and II of the Pigment Handbook. Coloring applications cover a wide range of materials, such as paint, plastics, and rubber. Iron oxides are chosen in certain instances because of the particular shades which they provide, as in stains for wood. Detailed information on relative amounts going to various end uses is not available. For synthetic oxides in 1971 in the United States, it has been estimated that one-half went into paint, paper, plastics, and rubber applications; one-fourth into magnetic tape and ferrites; one-eighth into roofing granules and cement; and the remaining one-eighth into catalysts, rouge, drugs, cosmetics, and miscellaneous uses. By comparison, a distribution of consumption for coloring purposes only as reported in a German publication in the early 1960’s was as follows: Lacquer, emulsion, and glue colors, 21%; linoleum, tile, and wall and floor coverings, 21%; cement building materials, 20%; stone and hard plaster, 17%; polishing agents, 13%; and asbestos slate, enamel, and rubber, 8%.
Paint
Paint is a major area of application for iron oxides because of their attributes, with limitations as given, of high hiding power, high tinting strength, color fastness, heat resistance, bleed and chemical resistance, ease of dispersion in all vehicles, high infrared reflectance, high ultraviolet absorption, and low price. The mass colors available are red, purple, yellow, brown, and black. Within this range of colors, the main reason for selecting a pigment other than iron oxide is that iron oxides do not have high chroma or color purity, which means that an organic pigment may offer greater brightness or richness of color.
With the growing trend towards water-reducible paints to minimize solvent emissions, the question arises as to whether iron oxides will prove satisfactory for use in aqueous paint systems. If not, this major end use will be taken by some other appropriate pigment, and iron oxide consumption will be depressed, it appears that most synthetic oxides function as well in water as in solvent paint systems but that use of natural oxides will be more limited in water-reducible paints. Tests of aqueous industrial paint systems for primer and top coat applications indicated that synthetic oxides and those natural oxides with high iron content and low water soluble salts can be expected to have wide use, with certain limitations. Oxides such as siennas and umbers are likely to be restricted to use in particular applications.
Building Materials, Drugs, and Cosmetics
The high stability and the nontoxic nature of iron oxides make them obvious candidates for coloring purposes in building materials and in drug and cosmetic applications. Red, yellow, brown, and black oxides all show good fastness to light, cement, and lime, and red oxide has high temperature resistance; these features have caused these pigments to be the most important for producing the respective colors in building materials. Both synthetic and natural iron oxides are used in construction materials. Because of their nontoxicity, synthetic oxides can be used without certification in drugs, cosmetics, and pet food and in plastics for food packaging; raw sienna and burnt umber also may be used in food packaging.
Ferrites
The use of iron oxides in ferrites is an important end use and one in which color has no more than an incidental role. Rather, the interest in iron oxides for this application is due to the magnetic and electronic properties of the compounds or bodies produced. Ferrites are manmade mixed oxide compositions containing iron and one or more other cationic elements and can be classified into four main groups:
- Soft ferrites, also known as linear ferrites, are chiefly manganese-zinc and nickel-zinc ferrites corresponding to the general formula MO-Fe2O3. They are an important part of the ferrite industry, comprising television deflection yokes, high-frequency transformer cores, antenna rods, and recording heads.
- Magnetic memories and switches, also known as square-loop ferrites and structurally similar to linear ferrites, are mainly magnesium-manganese and copper-manganese ferrites.
- Microwave ferrites, used in telecommunication devices in small quantities but at high unit value, include nickel-copper and manganese-magnesium ferrites, garnets, and some hexagonal ferrites.
- Hard ferrites, mostly barium and strontium ferrites according to the general formula MO-6Fe2O3, are used as permanent magnet materials in loud-speakers, motors, generators, and flexible magnets.
Commercialization of ferrites is a post-World War II development which by 1974 grew into a market of over $100 million for soft ferrites (including memory cores) and to about $40 million for hard ferrites. Strongest future growth is expected in hard ferrites, because the trend is for gradual displacement of ferrite memories by silicon devices and other technology.
The vast majority and the greatest volume of ferrites are prepared by powder methods similar to those used in powder metallurgy and ceramics. Heating causes intimate mixtures of the component oxides to react and form ferrite grains, which are shaped, usually by pressing, and then densified in another heating operation. The result is a polycrystalline body which is never perfectly uniform because of variations in grain size and porosity that cannot be eliminated. While certain intrinsic properties of ferrites have little to do with microstructural details, such other properties as mechanical strength, initial permeability, and coercive force depend on microstructure and therefore may occasion considerable quality control and development efforts to insure reproducible attainment of optimum properties.
A generalized diagram of the steps involved in making ferrites is shown in figure 9. In the preparation of ferrites from oxides, particle size and shape are important in determining which oxides are the most suitable. In the less demanding applications, regenerator oxide from steel plant pickle liquor and natural magnetite containing silica impurity are satisfactory, low-cost raw materials. Copperas red oxide is used at higher cost when circumstances require. As
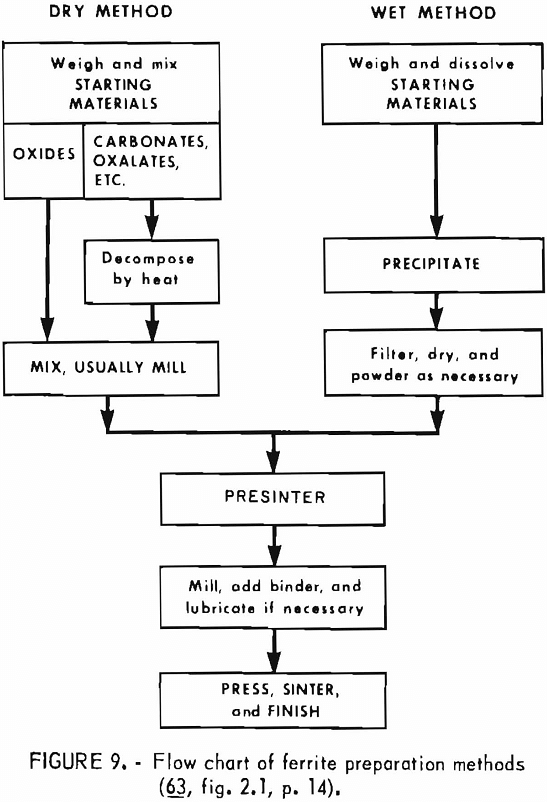
indicated in the diagram, mixtures other than oxides, such as carbonates, hydroxides, or oxalates, may also be used as starting materials. Another approach by which intimate mixtures of two or more components may be prepared in specified proportions is to precipitate or otherwise cause to separate from a multi- component solution fine particles of the constituents. As with carbonates or oxalates, heating converts these mixtures into oxide ferrites. For specialized applications and research purposes, a small quantity of ferrites is made in the form of single crystals by such techniques as flame fusion, gradient solidification, and flux melt.
Catalysts, Gas Absorption, and Foundry Sands
Besides ferrites, other noncoloring uses to which pigment-quality iron oxides are put because of their chemical nature are catalysts, gas absorption, and foundry sands. Catalytic properties of iron in oxides come about because iron is a transition element of variable valence, usually 2 or 3, which also forms defect oxides. Many oxide catalysts have been developed containing iron, chromium, aluminum, and other elements; they are used in several petroleum-refining reactions. Iron oxides have an affinity for sulfur-containing gases such as hydrogen sulfide (H2S). In the form known as “iron sponge” they were used to purify manufactured gas prior to the popular usage of natural gas. More recently, iron oxides have shown promise for removing hydrogen sulfide from hot gases generated in coal gasification. In foundry sands, it has long been known that small additions of iron oxide improve hot strength, for example, in core sand mixes for heavy sections. Natural iron oxide is used as an additive in foundry sand, in which reaction between iron oxide and impurities takes place when the sand is subjected to the heat of iron and steel casting. This action helps avoid a variety of casting defects. Additions of 0.5% to 0.8%, are the rule, although as little as 0.25% is effective in eliminating porosity defects in iron castings made using urethane-bonded sands. The amount of iron oxide consumed annually in the United States for these purposes, as well as to color-code certain sand mixes, is estimated as about 2,000 tons.
Transparent Oxides
Because of the very fine particle size, about 0.01 micrometer, transparent iron oxides can provide both color and transparency. Particularly notable use of this combination of optical properties has been made in recent years in finishes for automobiles. Utilization of the additional property of absorbing ultraviolet radiation is being investigated to determine the potential of transparent oxides in containers and packaging for food and wherever else durability, transparency, and ultraviolet absorption are desired in a pigment.
Magnetic Recording
The most important application of iron oxides based on magnetic and electrical properties is in magnetic tape in the form of gamma iron oxide, γ-Fe2O3. Production of gamma iron oxide typically begins with synthetic production of yellow iron oxide, which is converted to the red oxide, hematite (α-Fe2O3), by heating and then under carefully controlled conditions reduced to magnetite (Fe3O4) and finally reoxidized, while retaining the spinel crystal arrangement of magnetite, to gamma iron oxide. Gamma iron oxide is metastable with respect to hematite and transforms irreversibly to hematite when sufficiently heated. However, under normal conditions of use, gamma iron oxide remains unchanged and functions as the key component in recording apparatus whose value is many times that of the oxide itself. An advantage of preparing gamma iron oxide as described is that this method automatically gives elongated particles, which have much better recording characteristics than regularly shaped particles of the same oxide. Only a few companies produce synthetic oxide for magnetic recording purposes; it is marketed either as finished gamma iron oxide or as the yellow oxide precursor. The amount of oxide produced for this use is not known, but it is a significant part of total synthetic production.
Copy Machines and Wastewater Treatment
The magnetic properties of iron oxides also lend themselves to certain applications in copy machines and in filtering for water treatment. Iron oxides in the form of ferrites can be used as the carrier for toner particles in electrostatographic copiers. Soft ferrites such as nickel-zinc and manganese-zinc varieties are preferred, because they are magnetically attractable but easily magnetized and demagnetized. Ferrites produced for this use are prepared with a particle size somewhat larger than that normally encountered in pigments. Potential use of iron oxide to facilitate filtering of waste waters is under development. High-gradient magnetic separation techniques are being applied, especially for removing fine particles. In this application, seeding with a magnetic material and a flocculant are used to enhance removal of nonmagnetic or diamagnetic particles. Also, improved filtering characteristics have been obtained by incorporating iron oxide with polymer particles as a filter aid. Magnetite is the oxide used both for magnetic seeding and as a filter aid; in both applications the process is operated so that oxide is recovered and recycled.
Micaceous Iron Oxide
Micaceous iron oxide is a pigment material whose good protective coating properties relate to the flaky shape of the oxide particles, most of which are only a micrometer or so thick but from 10 to 60 micrometers long. This shape, when utilized properly in a coating, helps confer outstanding durability. Micaceous oxide is specular hematite, that is, ferric oxide, the same as in red iron oxide, and therefore is both chemically inert and high in heat resistance. However, micaceous oxide is dark gray and not red. The main source has been Austria, where in the 1970’s “eisenglimmer” has been mined at the rate of about 9,000 tons per year. Because of the lack of suitable domestic deposits, micaceous oxide has not seen much use in the United States. Recently the Japanese have developed a wet-process method of manufacturing a synthetic micaceous iron oxide, using byproduct copperas as feed. Applications similar to those of natural micaceous oxide are visualized, but apparently are still being tested.
Iron Oxides in Relation to Other Pigments
In today’s technologically based society, market and application niches of iron oxide pigments depend on their attributes compared with those of other pigments. The position of iron oxides in the overall pigment industry is shown in figure 10, on the basis of 1974 data. In that year, iron oxides accounted for 12% of the volume and over 5% of the value of production of all pigments. The most important pigment was white titanium dioxide, which accounted for 67% of the volume and 54% of the value of production. Among inorganic pigments, iron oxides and chromates followed titanium dioxide in order of importance ahead of zinc oxide. Iron oxides do not compete with titanium dioxide because all iron oxides are colored. Of the colored inorganic synthetic pigments, oxides and chromates comprised the greatest amount. Oxides were produced in greater volume than chromates but at lower value. Iron oxides were the only representatives of colored inorganic pigments of natural origin. The data presented in figure 10 illustrate the
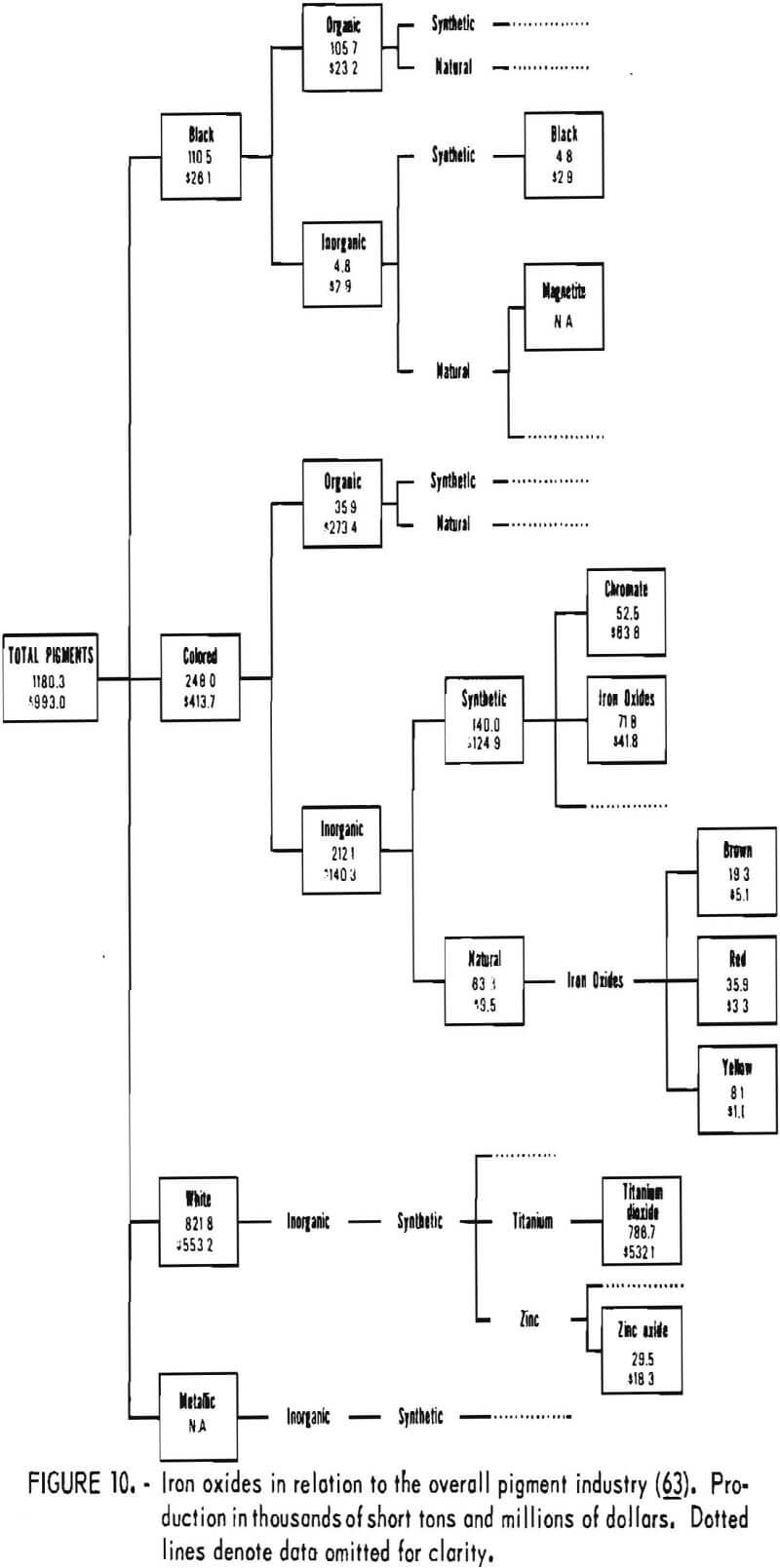
relatively low unit value of iron oxide pigments, especially for natural oxides, and the relatively high cost of colored organics.
Trends in Production and Foreign Trade for the United States
Collection and compilation of iron oxide pigments data by the Bureau of Mines began during World War II, so that this portion of the Bureau’s program on mineral commodities currently spans some 30 years. Shipment figures for crude and finished pigments are a major part of the annual tabulations. The list of companies participating in the Bureau’s canvass in 1976 is given in table 3; only a few companies produced crude material, but a number produced finished pigments, either natural or synthetic or both.
The discussion that follows considers trends for finished pigments only. Shipments are treated as equal to production; data are not collected on production directly or on stocks held by either producers or consumers. For long-term trends, data will be utilized only as far back as 1955; data for earlier years do not appear to be sufficiently comparable. Furthermore, for direct comparison with modern industry, only data from 1964 and later years can be considered. In 1964, producer reclassification of reporting base occurred, affecting primarily red oxide; that product, formerly reported as manufactured oxide, was henceforth reported as natural oxide. This change is consistent with modern concepts of what constitutes “natural” and “synthetic” pigments. Unfortunately, 1955-63 data cannot be revised to the same base as has applied since 1964.
Domestic Shipments
Overall (Natural Versus Synthetic Oxides)
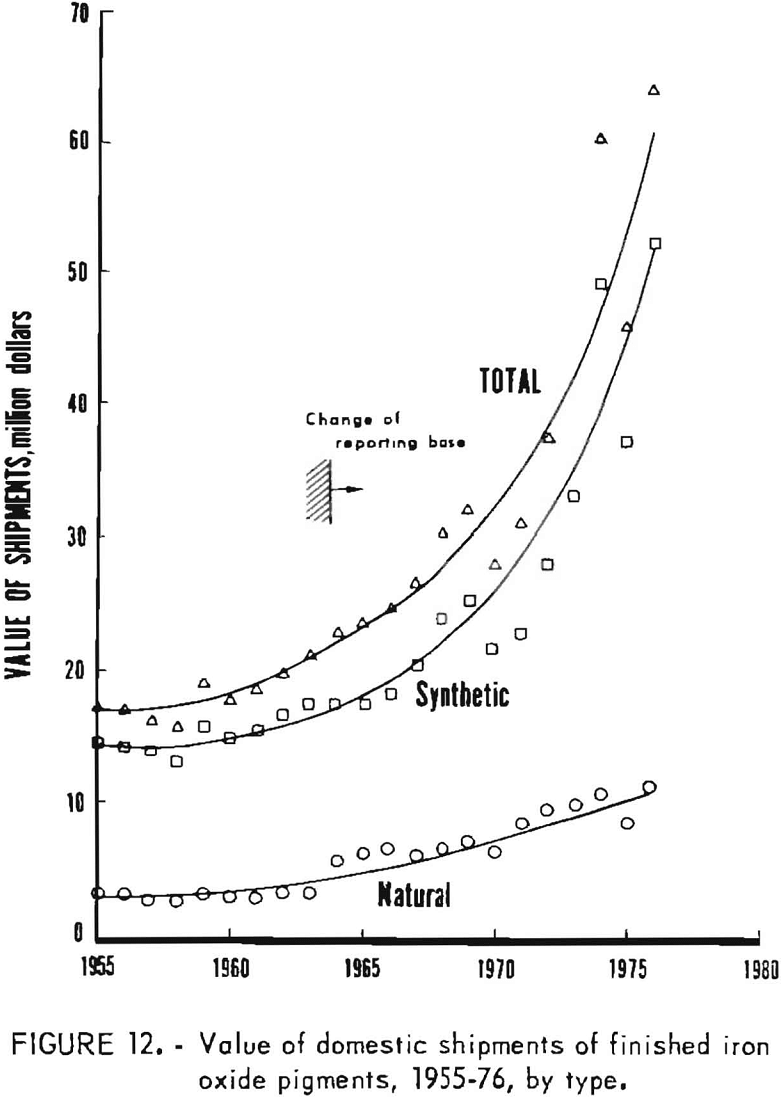
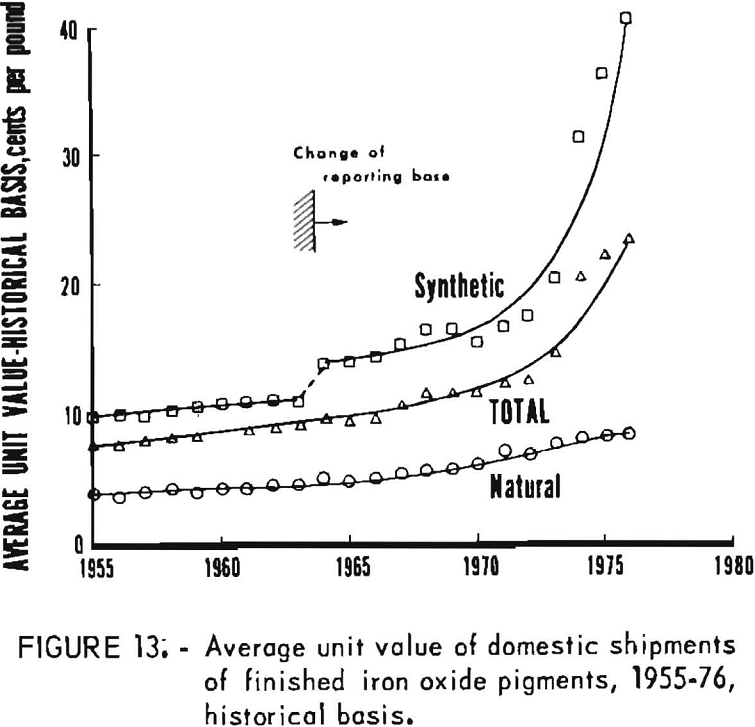
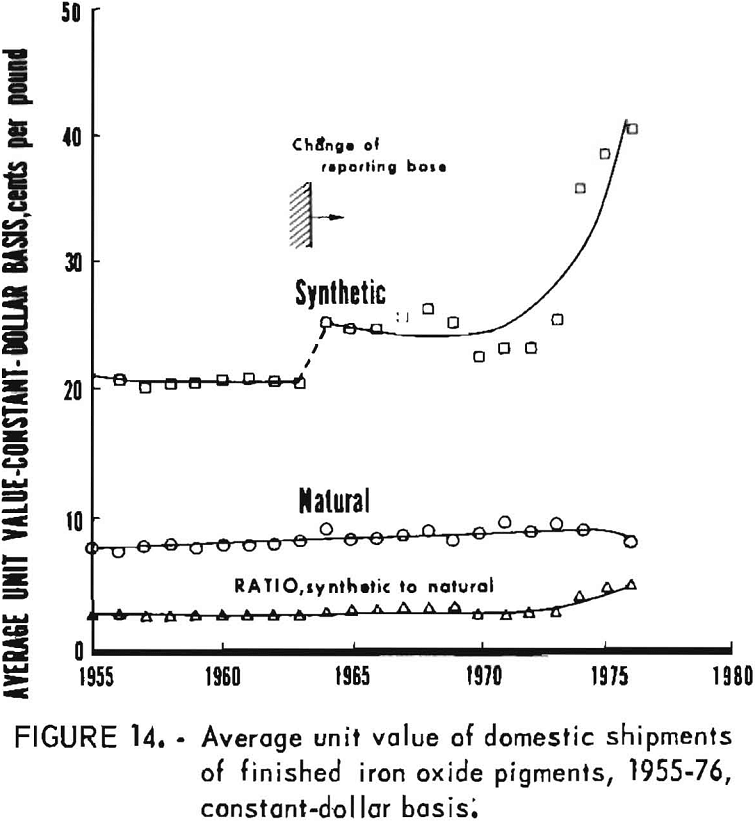
Data for 1955-76 for overall domestic shipments of finished iron oxide pigments are given in table 4 and presented graphically in figures 11 to 14. Data were obtained from the 2-year table of shipments data in the Iron Oxide Pigments chapters of the Bureau of Mines Minerals Yearbook. Revised data were selected whenever they appeared in the chapter for the year following original publication. The listing in table 4 and the graphs based on it contain an adjustment made in order to redistribute values for the “Mixtures and Other” category as listed in the Yearbook chapters into simplified totals for only natural and synthetic pigments. This adjustment was made by assigning one-third of the quantity and one-seventh of the value in the “Mixtures and Other” category to “Natural,” with the balance assigned to “Synthetic.”

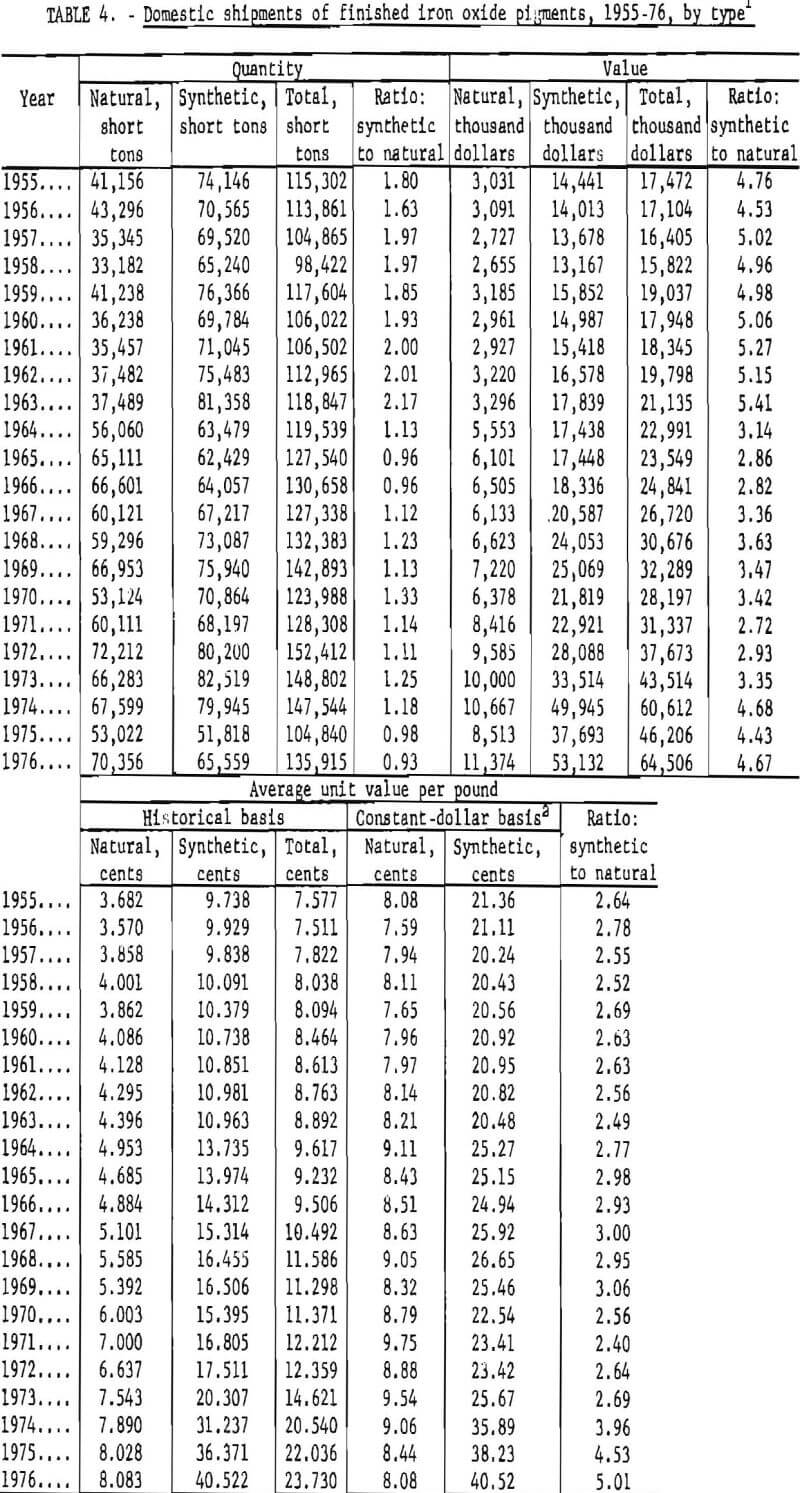
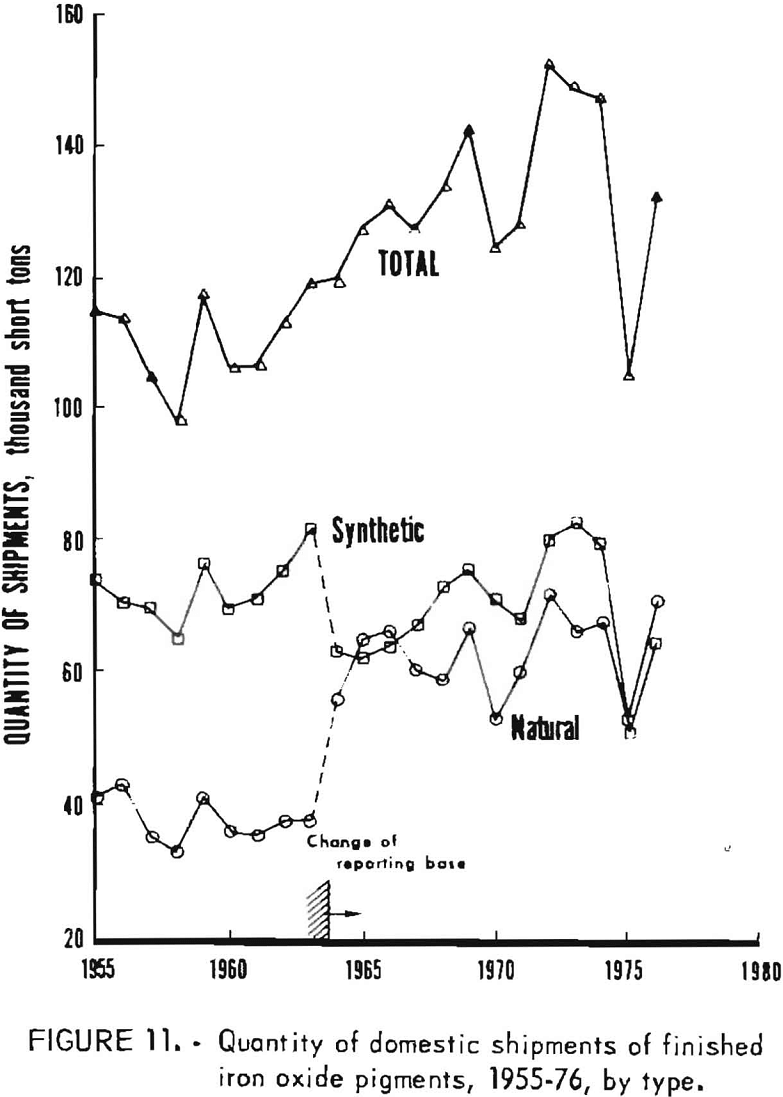
As shown in figure 11, total shipments grew nearly 50% from an annual total near 100,000 tons in the mid-1950’s to about 150,000 tons in the mid-1970’s. Shipments growth has been particularly uneven in the 1970’s, with recovery in 1976 not enough to compensate for a steep decline in 1975. Synthetic pigments have been responsible for most of the growth trend; natural pigments have fluctuated without much long-term progression or recession. Synthetics have accounted for both greater volume and value; the volume of synthetics since 1964 has been about 11% greater than that of naturals. It can be surmised that the disparity between the amounts of synthetics and naturals shipped has averaged about the same as this since World War II.
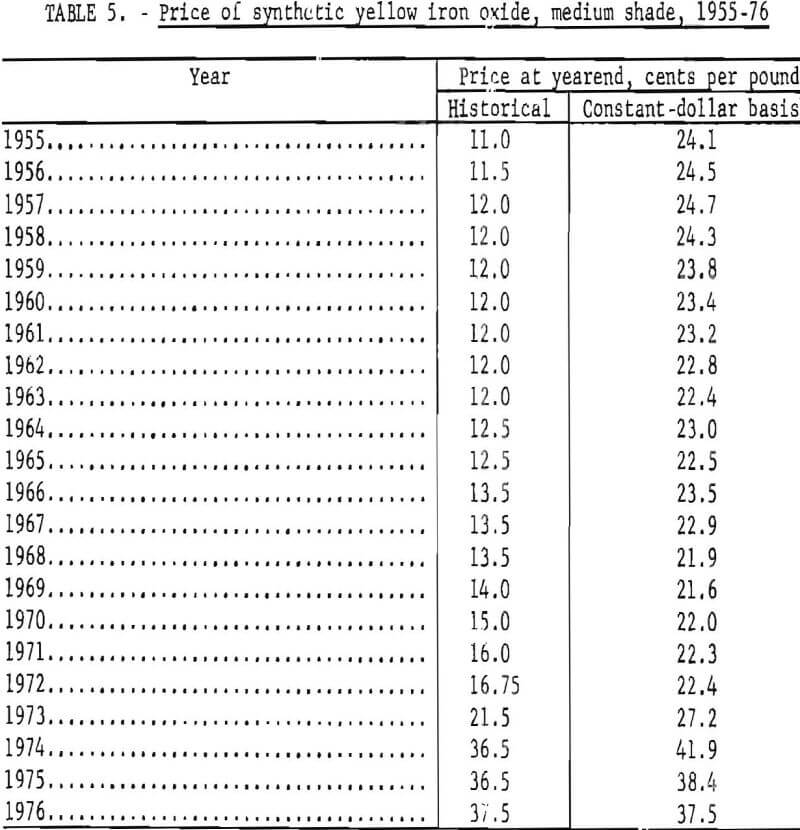
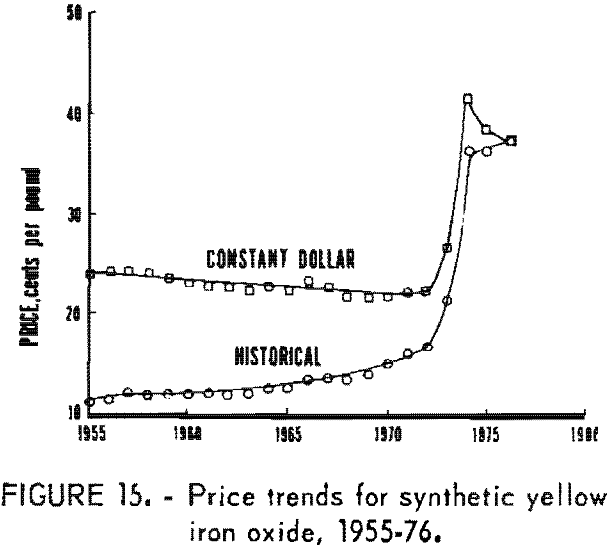
Synthetic oxides have dominated over natural oxides in value. This is evident in figures 12 through 14 for both total value of shipments and average unit value. Since 1964 the synthetic-to-natural ratio for both total value and average unit value has been about 3:1, and since 1974 this ratio has been markedly higher. Value of domestic shipments of iron oxides, which more than tripled over the past two decades to around $60 million annually in the mid- 1970’s, has increased more rapidly than volume of shipments, mainly because of inflation. On a historical basis, average unit value has been steadily increasing for both natural and synthetic oxides. However, on a constant-dollar basis, it appears that little or no change in average unit value, has taken place for natural oxides as far back as 1955. The same holds for synthetic oxides, except for unusual value increases beginning about 1974, on both a historical and a constant-dollar basis. This is a reflection of price trends, as given in table 5 and shown in figure 15 for synthetic yellow iron oxide, medium shade. On a constant-dollar basis, the price of synthetic yellow declined gradually by about 10% from 1955 through 1972. However, price increased significantly in 1973 and 1974, mostly between October 1973 and July 1974. In this 9-month interval, actual prices increased nearly 100% in several steps.
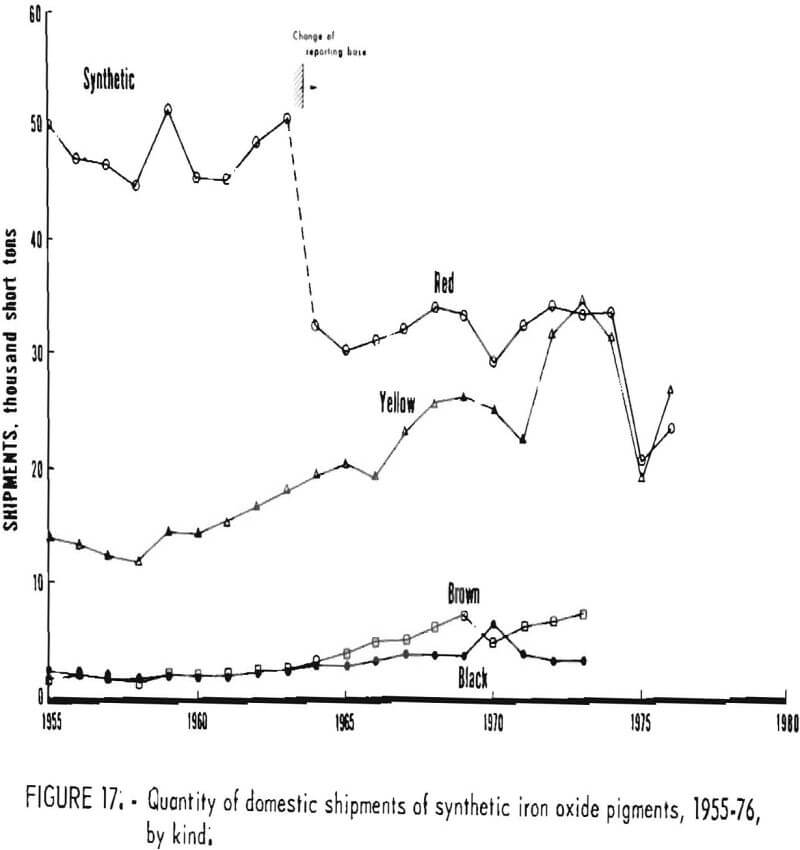
Individual Oxides
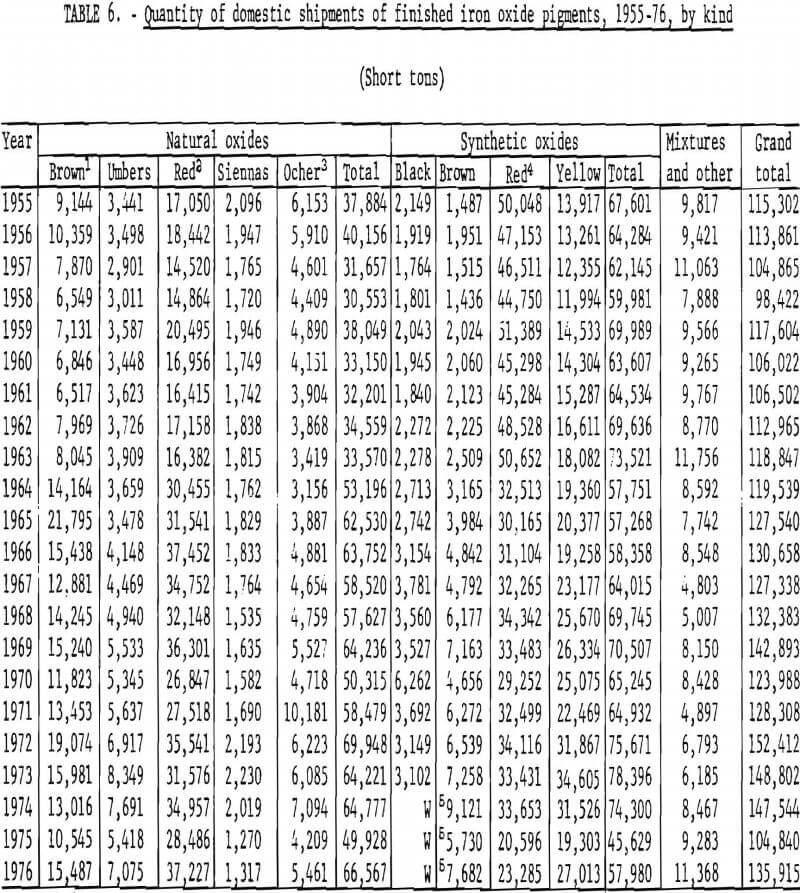
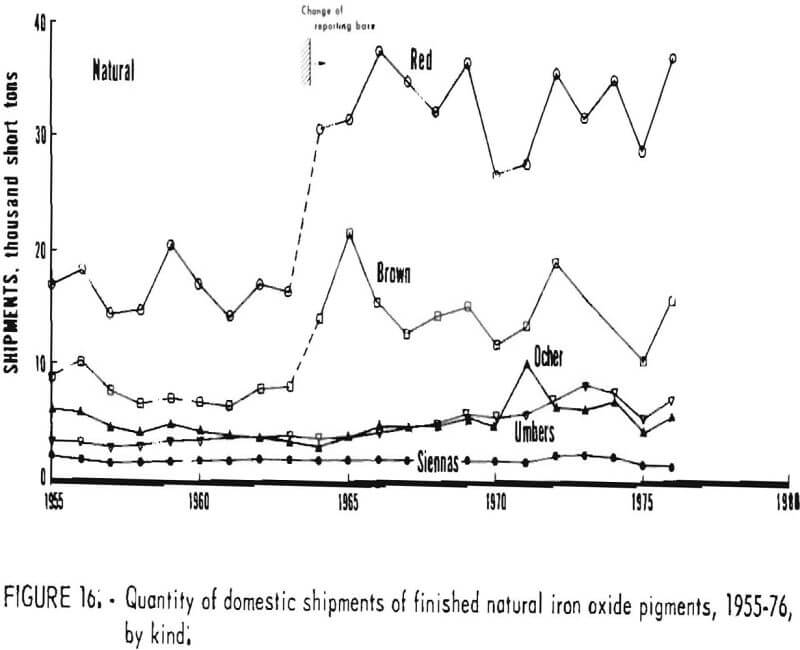
Trends in shipments of the various natural and synthetic pigments during 1955-76 are listed in table 6 and plotted in figures 16 and 17; it can be seen that the most significant effect of the 1964 change in reporting basis was for red oxides. While red oxides have been shipped in largest quantity in both natural and synthetic varieties, little, if any, growth has taken place for at least the last 10 and possibly the last 20 years. Shipments of natural brown oxides also appear to have reached a plateau. Growth in shipments of natural oxides has been confined to umbers, ocher, and siennas, although the amount of these three oxides taken together is still only about half that of natural red oxide. Among synthetics, growth trends can be noted for all shades except red Shipments of yellow oxides have advanced especially and in the past few years have drawn even with those of red oxides.
Foreign Trade
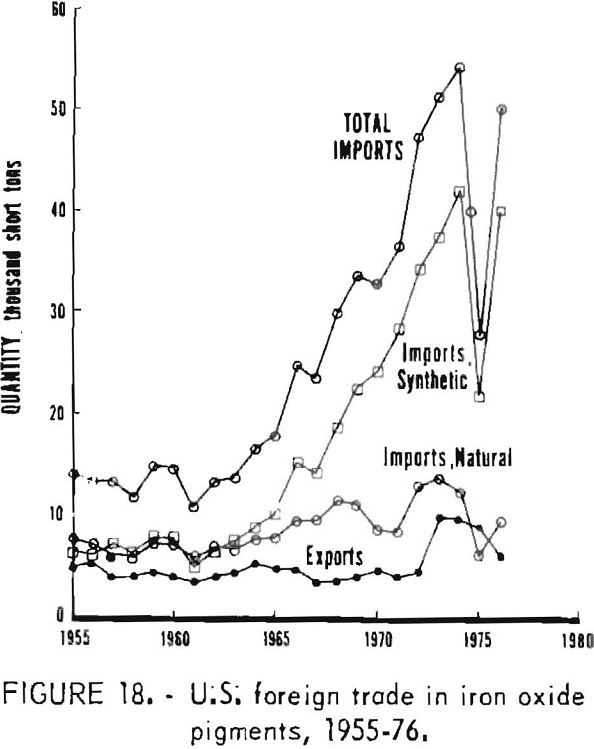
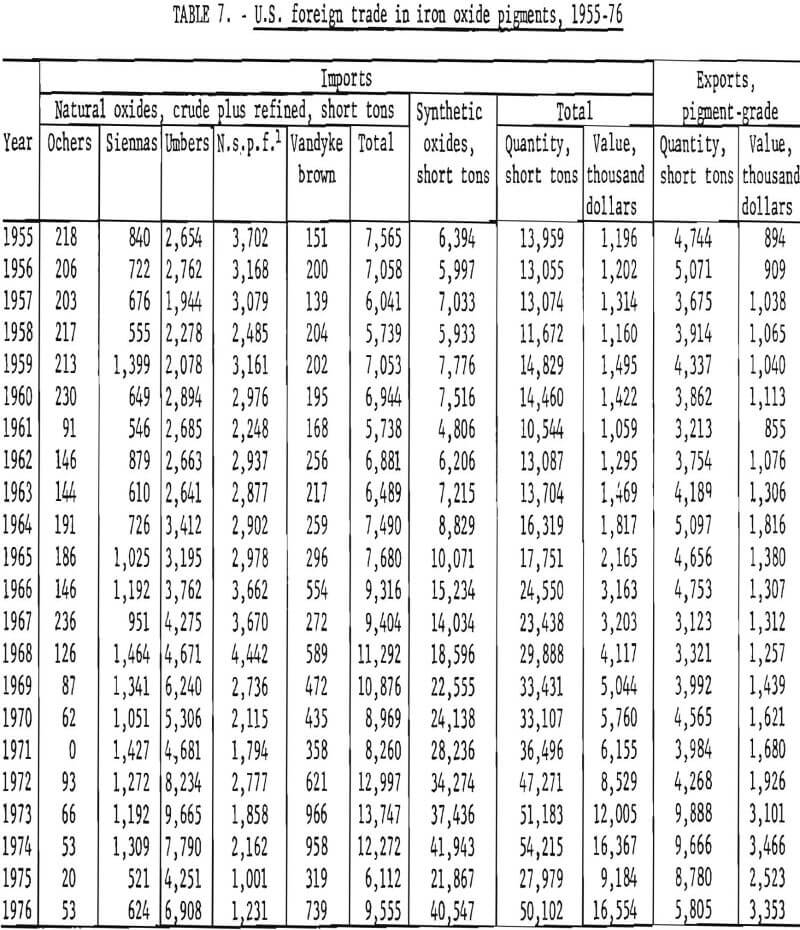
U.S. foreign trade in iron oxide pigments for 1955-76 is tabulated in table 7 and plotted in figure 18, taking imports of natural oxides as the sum of those for crude and refined material. Both forms eventually find their way into use, and data for making a distinction between ground and unground material are not readily available for the years prior to 1963. On this basis, the historical record shows that during 1955-63 annual imports of both natural and synthetic pigments each fluctuated about an average of 6,500 tons, while exports of pigment-grade oxides were running about 4,000 tons annually. As of approximately 1964, the rate of importation began increasing, with growth in imports concentrated in synthetic oxides. Between 1964 and 1974 the volume of imports increased nearly sixfold for synthetics and doubled for naturals, giving an overall fourfold increase. Value of imports rose overall by a factor of nearly 8. In the same period, volume of exports showed no gain until 1973, when exports approximately doubled abruptly in both volume and value. Imports of both synthetic and natural oxides fell sharply in 1975 and then rebounded, but not fully, in 1976.
Imports of Natural Oxides
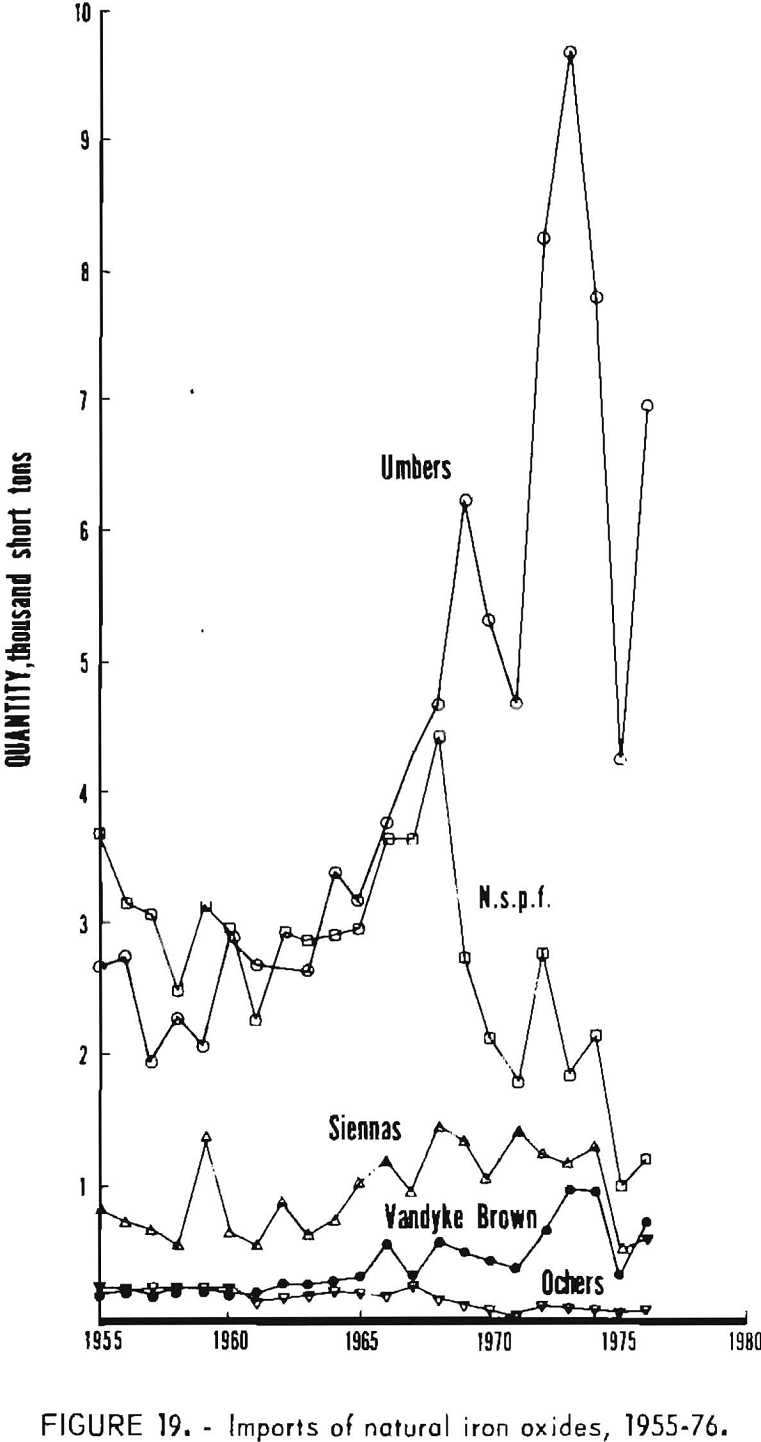
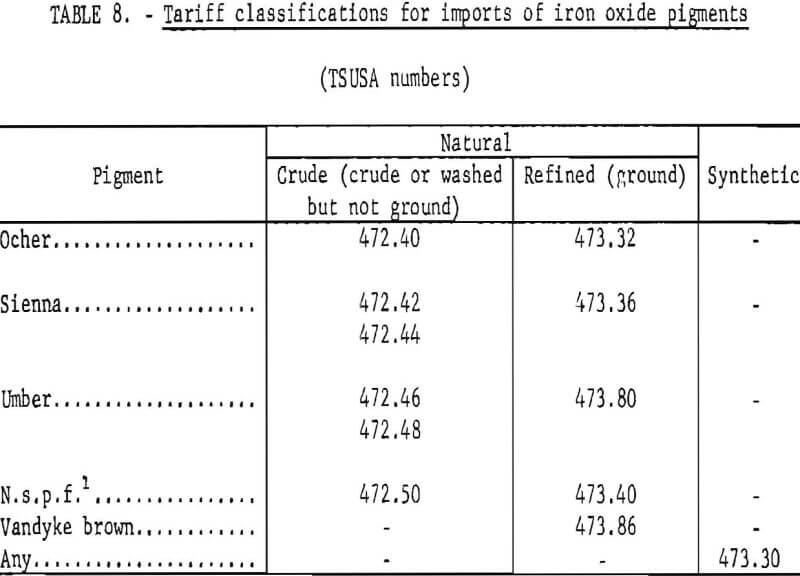
The classification scheme for imports of iron oxides according to kind under the present Tariff Schedules of the United States (TSUSA) is given in table 8. It can be seen that details on imports of the various natural iron oxides are available, and these have been listed in table 7 and plotted in figure 19. Of the natural oxides, umbers have been imported in greatest quantity and have shown the strongest import growth trend. The “not specifically provided for” category, which by elimination and by country of origin chiefly corresponds to natural red oxide, has been second to umbers in import volume but has declined significantly. Of pigments imported in lesser amounts, in the past decade imports of siennas have grown moderately, those of Vandyke brown have shown strong growth, and those of ochers have nearly ceased. In recent years the main sources of imports of natural pigments have been Cyprus for umber, Spain for natural red oxide (n.s.p.f.), Italy for Sienna, West Germany for Vandyke brown, and the Republic of South Africa for ocher.
Imports of Synthetic Oxides
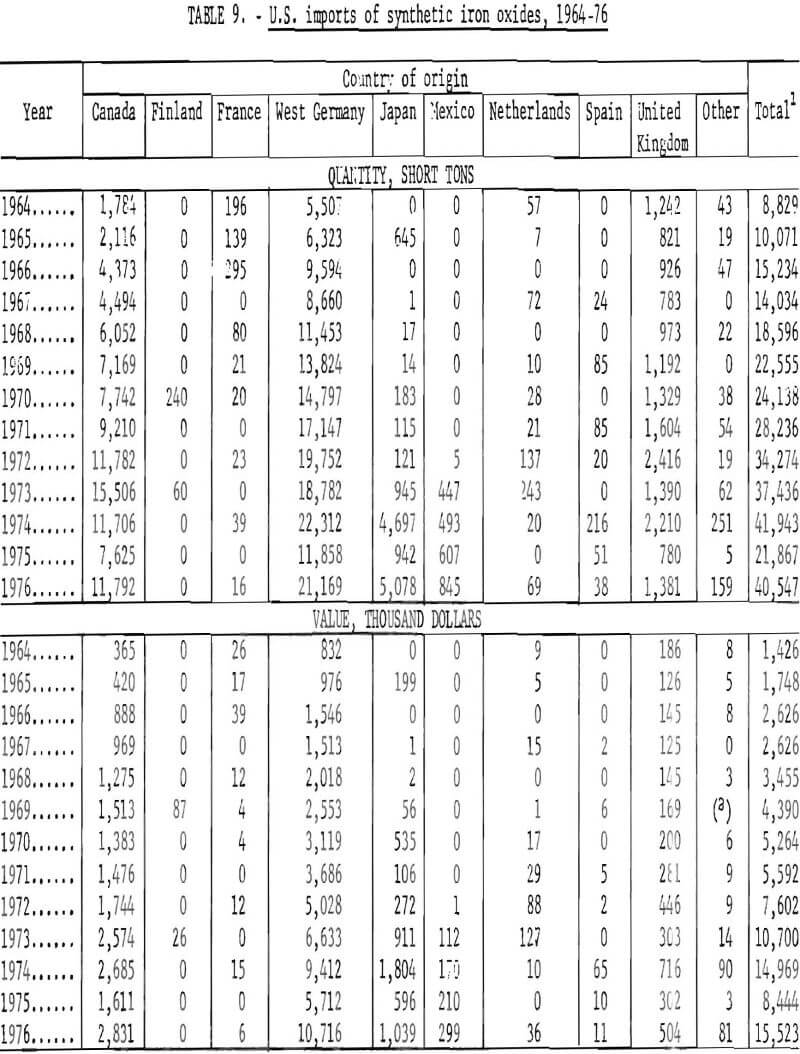
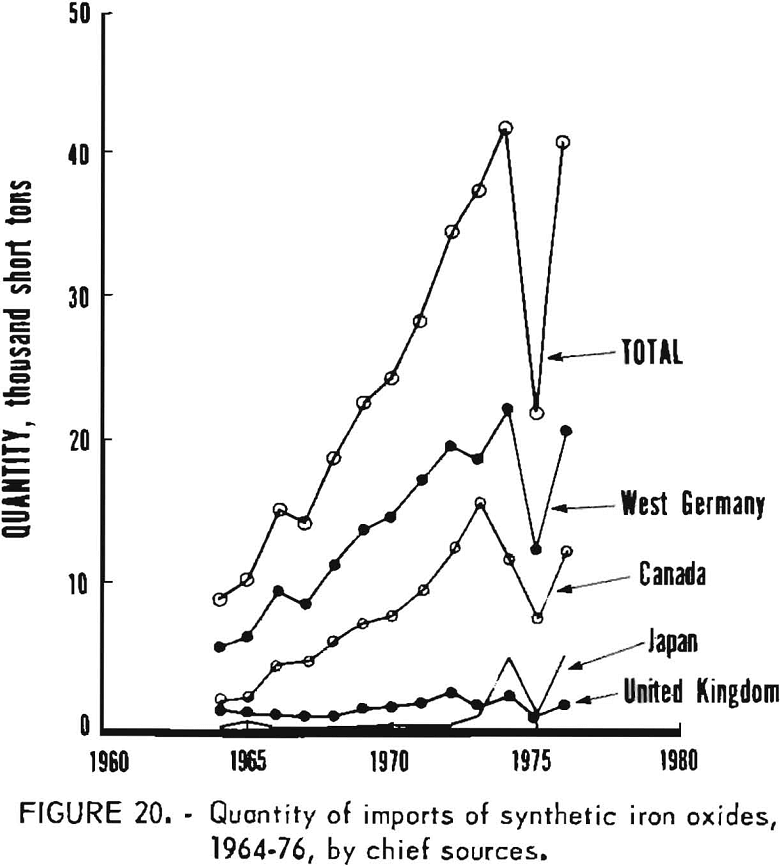
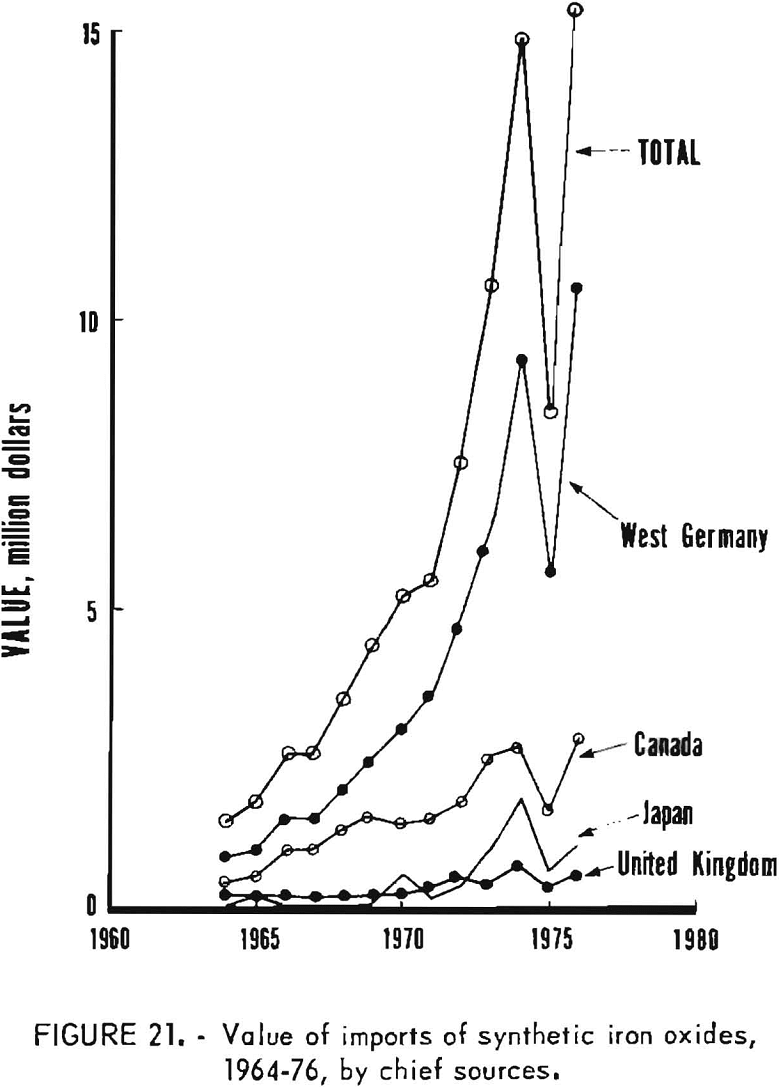
Data are not similarly available on kinds of synthetic oxides imported, because under the tariff schedule all imports of synthetics are lumped into a single reporting category. Therefore, the amounts of imports of synthetics which have been red, yellow, brown, or black oxides are not known. Available information relates to country of origin, as given in table 9 and graphed in figures 20 and 21, commencing with 1964. West Germany and Canada have been the first and second most important sources of synthetic imports, and have accounted for the majority of such imports. The United Kingdom and Japan have also been relatively significant sources of foreign oxides. However, imports from West Germany and Canada have obviously been largely responsible for growth in imports in the past decade or so from the structure of the iron oxide industry in West Germany, it is evident that most of the oxide from West Germany has been produced by the aniline process. Most of that from Canada has been obtained as a byproduct from regeneration of steel plant pickle liquor and has been consumed domestically by the ferrite industry.
Apparent Domestic Demand
Apparent domestic demand for finished iron oxide pigments in the United States is listed in table 10 and is presented in graphical form in figures 22 and 23. This estimation of apparent demand makes no allowance for stocks and stock changes in the absence of appropriate data, and includes only the refined or ground portion of imports of natural pigments. Apparent domestic demand has trended upwards since 1964 except for distinct setbacks in 1970 and 1975, of which the 1975 reversal was much more pronounced.
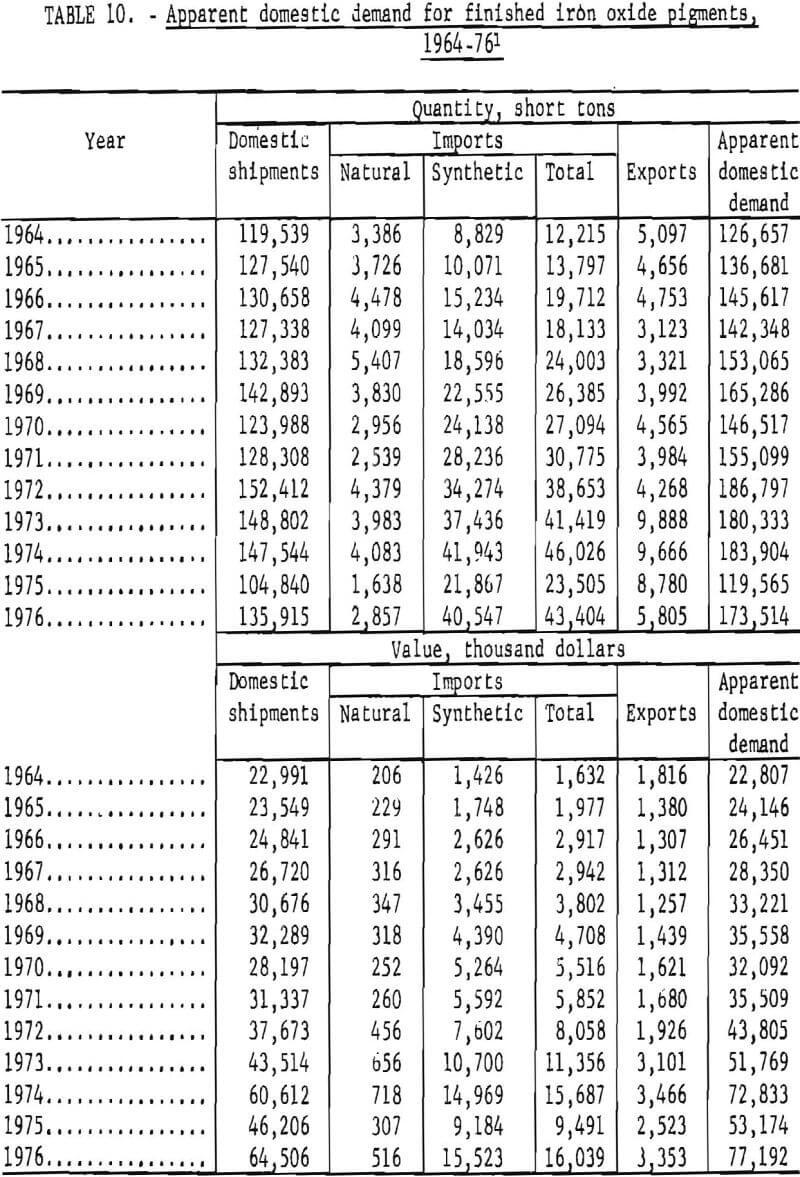
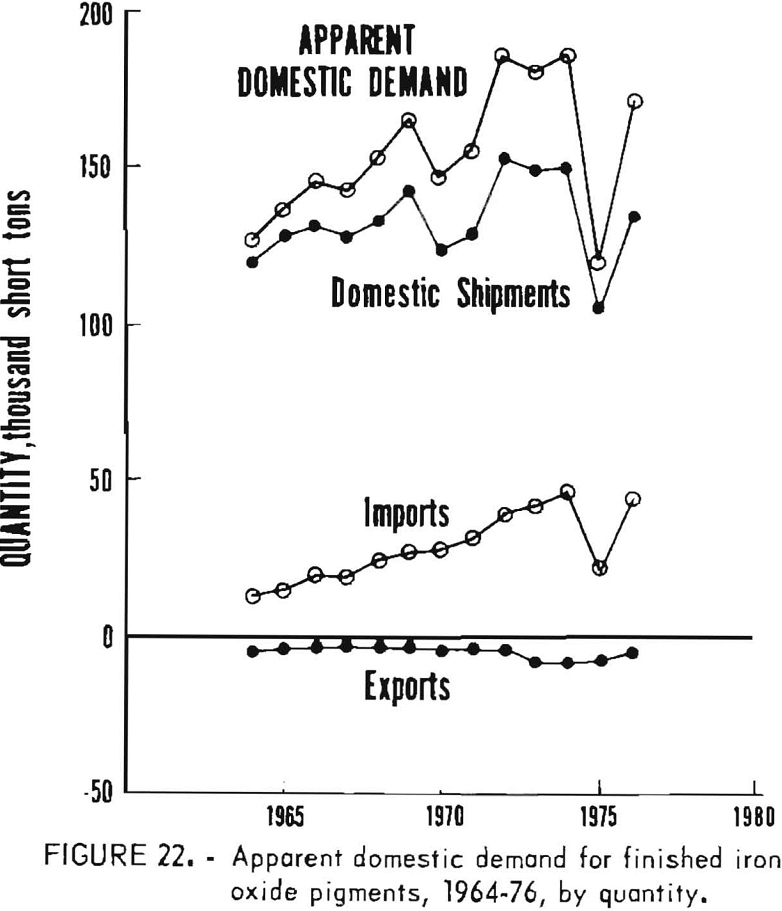
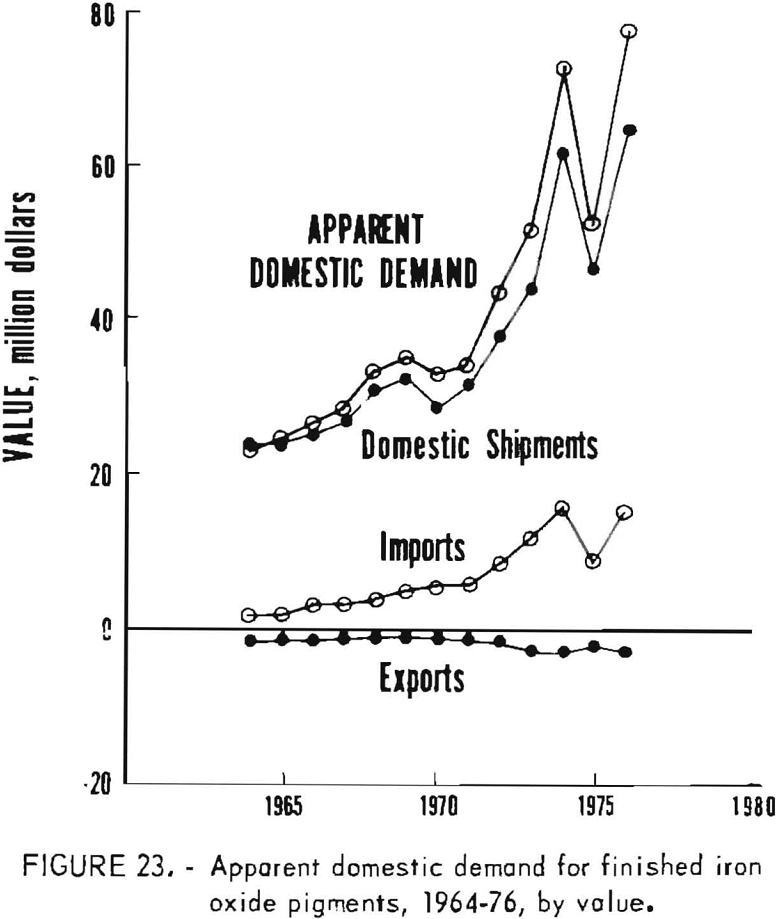
From 1964 through 1974, apparent demand grew from 127,000 to 184,000 tons per year, an increase of 45% that corresponds to a moderate annual growth rate of 3.6%. This was about the same growth rate in that period as for U.S. production of titanium dioxide pigment, 85% of that for the Federal Reserve Board industrial production index (FRBIPI) for total production, and about 50% of the FRBIPI for chemicals and products. By comparison, population increased 1% annually. The quantity of domestic shipments and of imports of iron oxides both increased by about 30,000 tons. The rate of growth was higher for imports, however; annual growth rates for imports were 20% for synthetic oxides and 0.3% for finished natural oxides, whereas growth rates for domestic shipments were 1.1% for natural oxides, 2.7%, for synthetic oxides, and 1.9%, overall. Value of domestic demand rose from $23 million to $73 million. Average unit value grew more rapidly for imports than for domestic shipments, but because of lower unit values, imports increased in value by less than half the amount that value of domestic shipments increased.
Both quantity and value of apparent demand decreased markedly in 1975, and neither returned to the trend level in 1976. The extent to which apparent demand and domestic shipments recovered in 1976 from the reverses of 1975 can be judged by comparison of 1976 actual quantities with those projected from the 1964-74 trend. Demand, shipments, and imports all approached 90% of projections, which is about the same as noted in trends for titanium dioxide production but about 77, below FRBIPI performance both for total production and for chemicals and products. Continued recovery is required to fully offset the 1975 decline; average annual growth rates for apparent demand and for domestic shipments decreased to 1.9% and 0.6%, respectively, when calculated for 1964-76.
Present and Future Status of the Industry
The iron oxide pigments industry is a mature industry. Its ancient roots continue to nourish production of oxides from natural sources, but chemical methods developed in the 20th century have made synthetic oxides the dominant part of the industry. Iron oxides constitute a small but important portion of the overall pigment industry, representing perhaps one-tenth of the total market. Low cost and good chemical and physical stability would seem to guarantee that iron oxides will remain in demand for pigment purposes. Annual domestic use of iron oxides has grown to well over 100,000 tons at a value of around $50 million. Growth in usage has been modest, 3.6% annually in recent years. While this rate exceeds that of population growth, it lags that of the chemical sector generally. Imports have grown more rapidly than domestic shipments of pigments during the last decade and now correspond to about one fourth of domestic demand.
Present trends in natural oxides are for a continuation of demand for certain oxides whose characteristics are desired and otherwise unattainable at comparable cost. There is some indication that the trend to aqueous paint systems may retard rather than enhance the use of natural pigments. Deposits at mines operated solely to produce pigments appear to be in no danger of exhaustion. However, mining of black magnetite has just recently been stopped, and radical curtailment of supply of natural red oxide within a few years appears possible due to the impending cessation of operations at the iron ore mine that is presently the sole domestic source. Should production of natural red oxide cease and no substitute source be available, production of pigment oxide from domestic sources would be confined to relatively small amounts mined by only two companies. Foreign red oxide is not a direct substitute for domestic material and is unlikely to be imported in a quantity comparable to the amount that has been produced domestically. Also foreign sources of natural pigment are subject to uncertainty because of political, labor, and management factors.
The future of synthetic oxides seems more secure. No real raw materials problems are evident, although costs may increase because of technological change in the industries that have traditionally provided feed material. Methods used to manufacture iron oxides will be expanded when the aniline process is introduced from Germany into the United States. This development is expected to have a significant effect on foreign trade in synthetics, because West Germany has been the main source of imports. As with any synthetic product, it is possible that some new discovery or advance could cause a major change in the industry. New sources could arise through application of technical innovation, leading to marketing of iron oxides as a desirable byproduct as has already occurred with the aniline process.
Synthetic oxides can be expected to remain competitive with other pigments because of continuing sophistication in control over manufacture and properties of the products. A possible area for large future growth is yellow oxide in combination with organic pigments as a substitute for pigments such as chrome yellow that have environmental or ecological disadvantages. Use of iron oxides in ferrite permanent magnets appears likely to grow in the future, but declining use of oxides for square-loop ferrites in memory devices is foreseen, Transparent iron oxides are a small part of overall iron oxide production and consumption; however, these oxides possess a number of unique properties favoring future growth. In a technological world, nonpigment uses of iron oxides are most susceptible to change, because new developments may cause demand to increase dramatically or drop abruptly. However, low cost and ready availability are important advantages that should cause iron oxides to be considered for any application where their properties may be useful.

Related:
Best Super Glue for Metal
Magnet Fishing Kit
