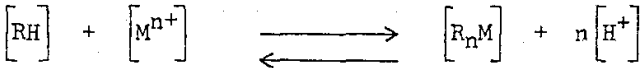
Kelex 100 is a single organic compound designed to selectively extract copper ions from leach liquors having acidities in the pH range of one to three. In the process of extraction, as indicated by the forward direction of the following equilibrium expression, an extremely stable copper complex is formed.

The unusual stability of [R2Cu] org in the above equation forces the reaction to the right, permitting the reagent to function well at low pH.
Reversal of the above equilibrium by contacting the copper-loaded organic phase with an aqueous strip solution containing 120 to 150 grams per liter sulfuric acid can produce a concentrated copper sulfate solution of sufficient ionic purity to serve as feed to electrolysis .
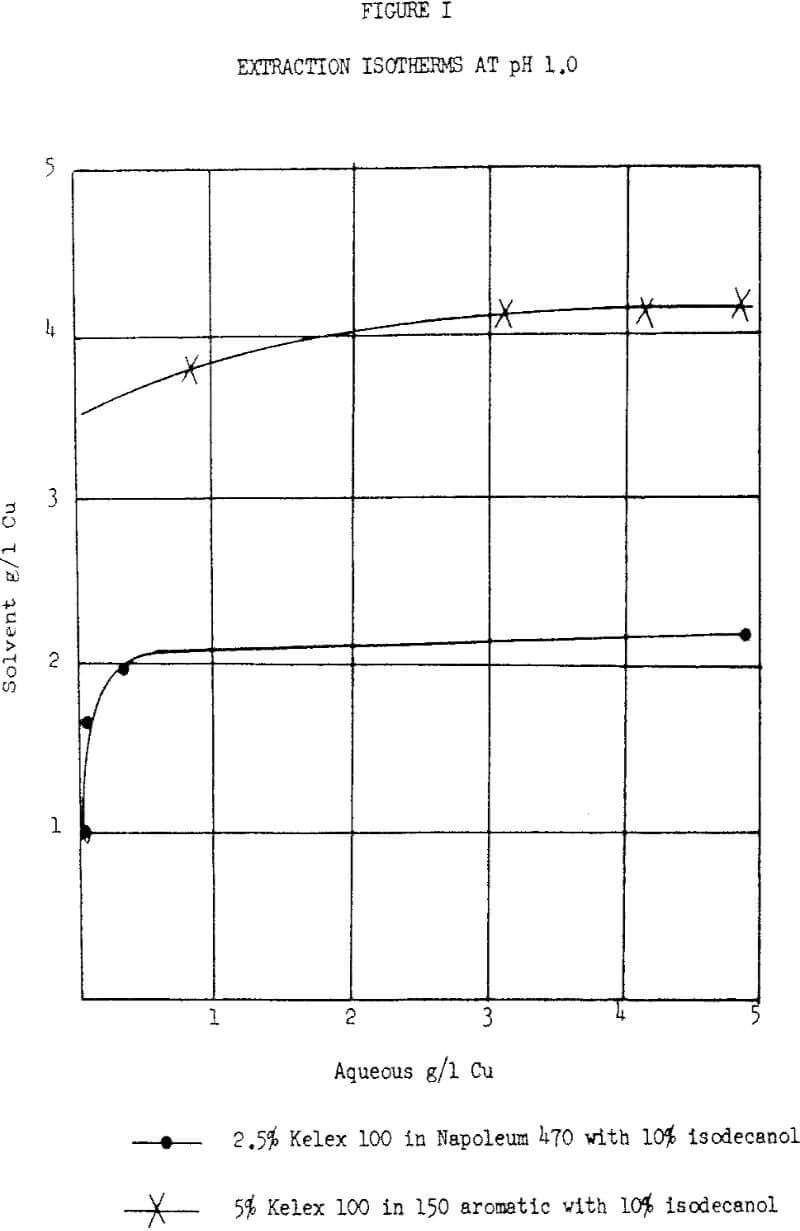
The Ashland copper reagent, Kelex 100, has been evaluated at several different levels of concentration in kerosene and in an aromatic 150 petroleum solvent, the latter having a flash point of 150°F and a distillation range of 370°F to 410°F. The extraction characteristics are the same in both solvents. The maximum loading capacities of 5% and 2.5% solutions are 4.0 gpl and 2.0 gpl Cu, respectively.
The relative degree of extraction of cations other than copper can vary substantially for any given system, depending on the variables which enter into the general equation:
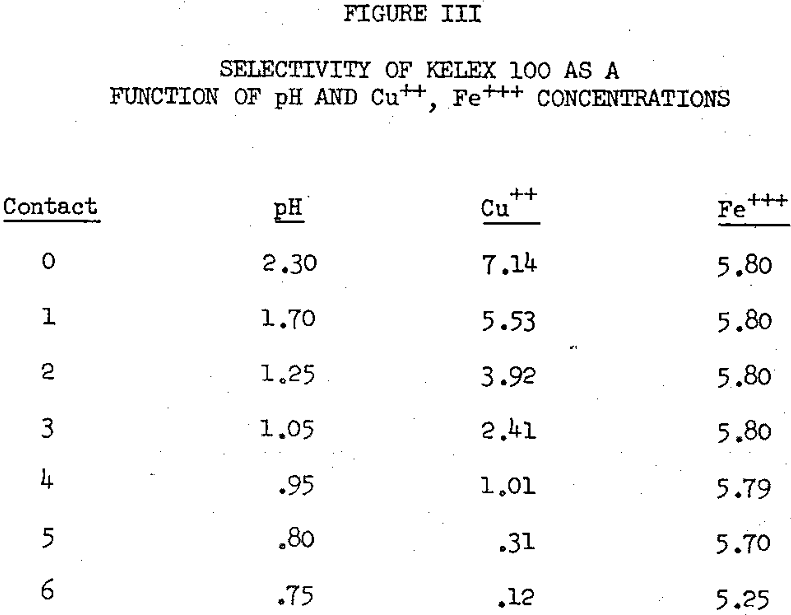
The primary factors which affect the selectivity are pH, relative ionic concentrations, nature of the cations (Mn+) present in the aqueous phase, and the amount of reagent (RH) present in excess of extractable copper ions. Of all the factors listed above, the most important one for consideration when acidic leach streams are being extracted with Kelex is the amount of reagent available in excess of extractable copper ions.
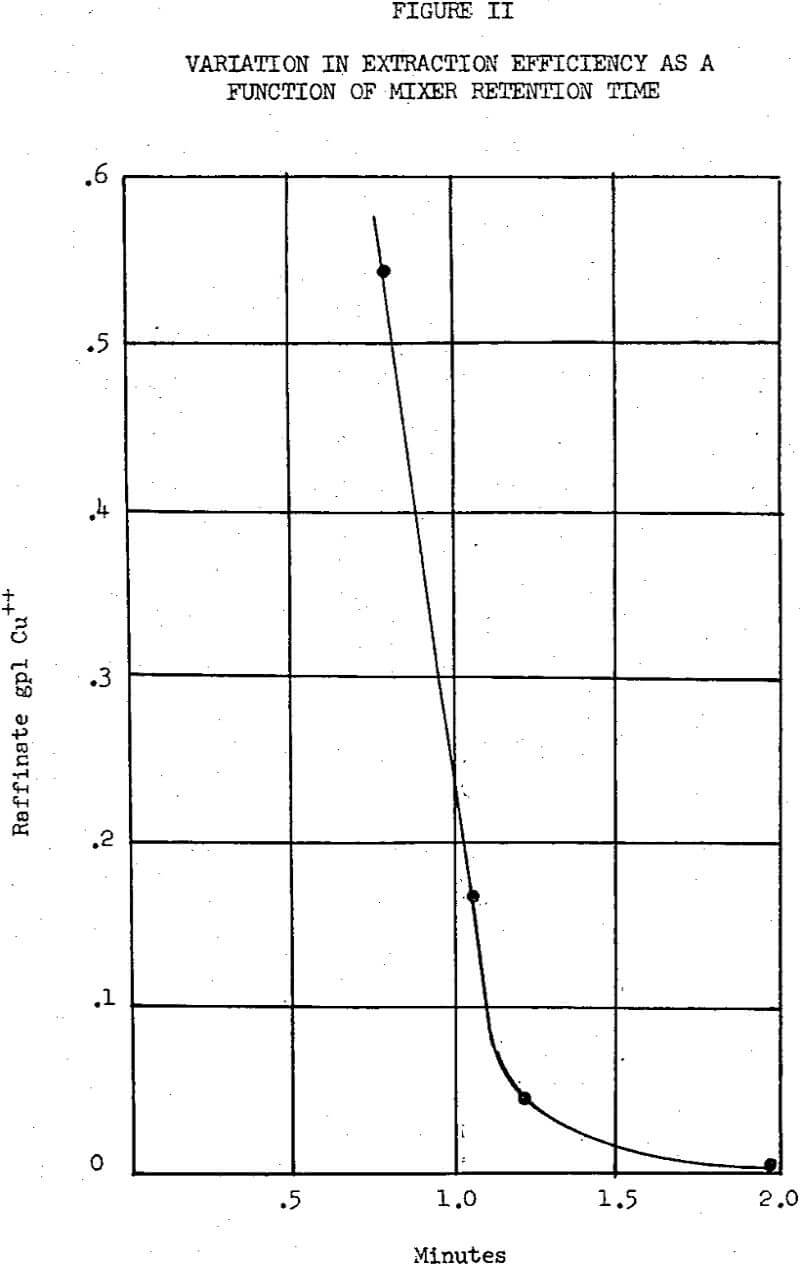
Because Kelex 100 is such an unusually strong chelation extractant, many of the operating parameters for its use are governed by conditions in the stripping circuit.Any sulfuric acid that is dissolved in the organic phase during the stripping operation is washed out in the next stage of extraction and is eventually circulated to the dump in the raffinate solution. Sulfuric acid thus transferred from the stripping circuit to the leach stream can be considered as an additional cost if sufficient sulfuric acid is produced in the pile to maintain adequate leaching efficiency, but it may also be a convenient way to add sulfuric acid to the leach solution in those operations where more sulfuric acid is neutralized in the pile than produced.