During roast, virtually all the iron in the concentrate feed is converted into zinc ferrite which remains almost inert in the neutral-leach stage. In order to recover zinc incorporated in ZnO·Fe2O3, a follow-up treatment of the leach residue is required.
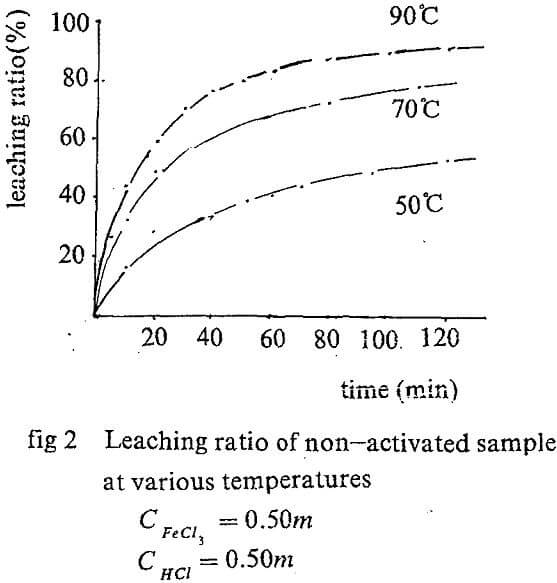
Ferric chloride is one of the ideal agent for the direct leaching of sphalerite. But the disadvantages lie in the long time and high temperature for leach.(To leach the concentrate completely, the temperature upto 90 °C and leaching time of more than two hours is necessary).
Leaching Kinetic of Sphalerite Experiment
Material
The sphalerite concentrate used was provided by Zhuzhou Smelter, Hunan, China. Prior to leaching, the concentrate was treated with Na2S solution to clean and sulfurise the surface of the mineral. Chemical analysis shows the concentrate containing (wt %) Zn 53.12, Fe 9.58 and S 31.94. ; FeCl3 · 6H2O, hydrochloride acid of chemical pure and distilled water are used.
Operation
800ml FeCl3, HCl solution of certain concentration was used for each leaching experiment. When the temperature has reached the schedule value, add 1.0g sample to it and record the time simultaneously. The leaching ratio was obtained by sampling timely and the zinc content in the solution was determined by ion exchange chromatography analysis.
Result and Discussion
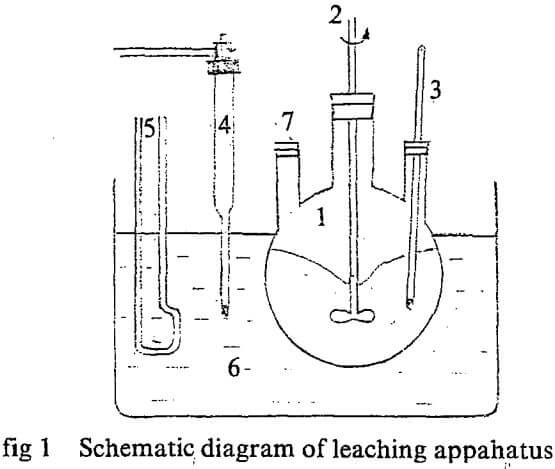
- Three-necked flask
- Stirrer
- Thermodetector
- Contact thermodetector
- Electric heater
- Thermore const, bath.
- For feeding and sampling
Leaching kinetic of original concentrate
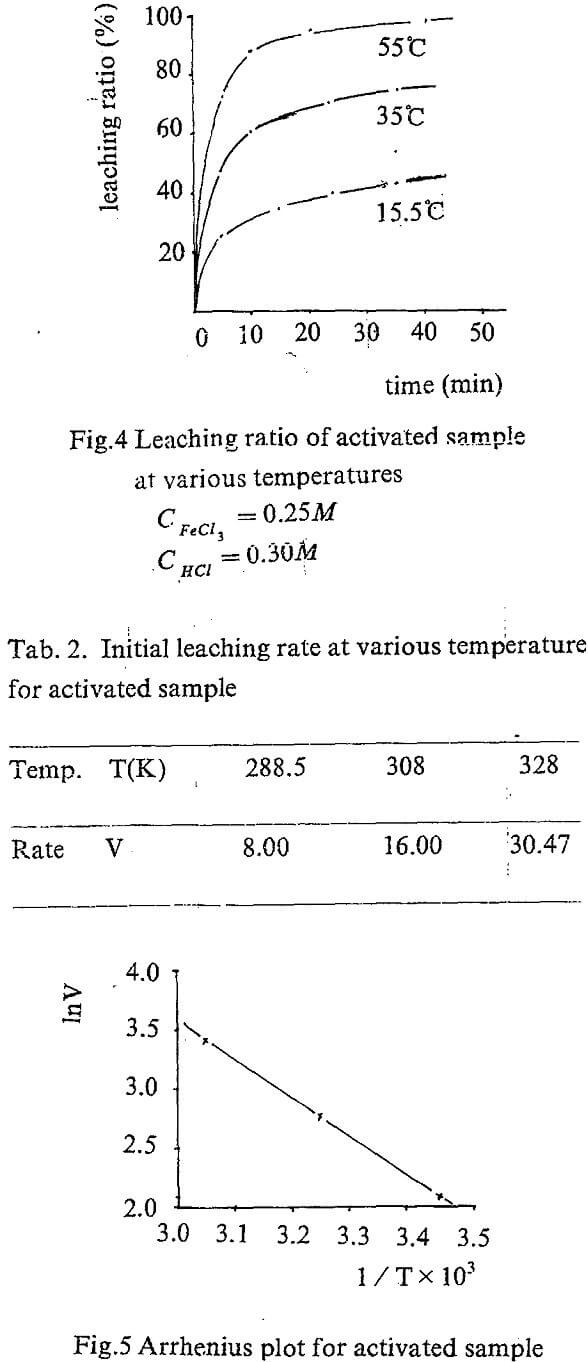
The equation A=f(t) can be obtained by regression the experiment data. The slope of the curve for, t=0 is actually the initial rate of the leaching. The values are showed in Tab. 1.
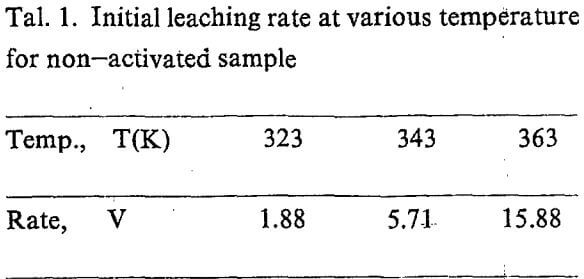
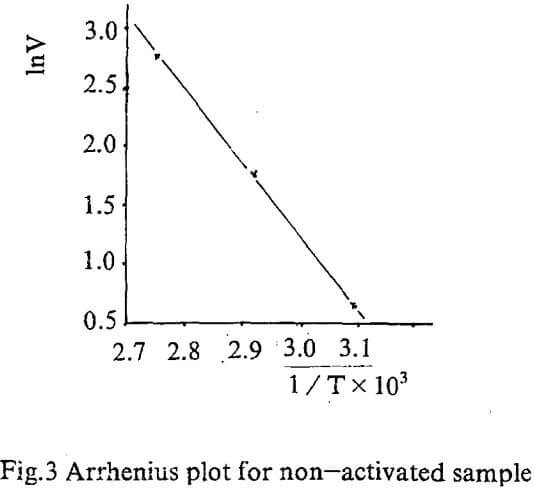
Temperature dependence on reaction rate was obtained by correlating initial rate for various temperature according to Arrhenius equation.
Leaching kinetic of the activated concentrate