For several years work has been carried on by the U.S. Bureau of Mines at Rolla, Mo., to develop improved methods of concentrating the oxidized ores of lead and zinc. Various samples have been investigated, and results on some have been published.
Preliminary Examination: The sample on which the following experimental work was conducted consisted principally of quartz sand grains, a clay mineral of the illite group and limonitic iron oxide, with some cerrussite and manganese oxide and very small amounts of altered feldspar and pyromorphite. A white, earthy, amorphous lead-bearing mineral which could not be identified was later detected in some of the test products. Galena was not present.
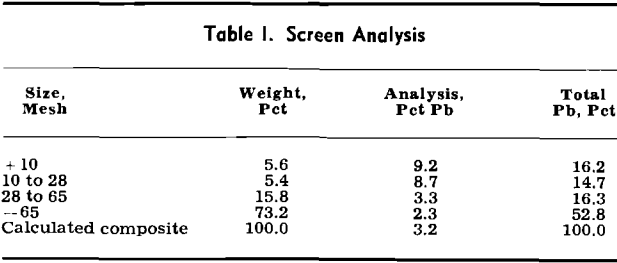
Detailed chemical analysis of the sample upon which these tests were made showed the following percentages: Pb, 3.2; Fe, 11.7; SiO2, 54.3; Al2O3, 9.3; S, 0.24; and P, 0.08.
Gravity Concentration: Accordingly, a sample of the Mine La Motte oxidized lead-bearing material was crushed and ground to pass 10 mesh, slurried to a pulp density of 10 pct solids, and deslimed in a 5 in. liquid-solid cyclone. During some early experimentation the overflow and underflow openings of the cyclone were set at 1.25 and 0.50 in., respectively. These settings, under a feed pressure of 20 psi, resulted in a slime overflow of 6 pct solids and a sand underflow at 59 pct solids. The overflow, amounting to 52 pct of the weight, was less than 2 pct + 400 mesh in size.
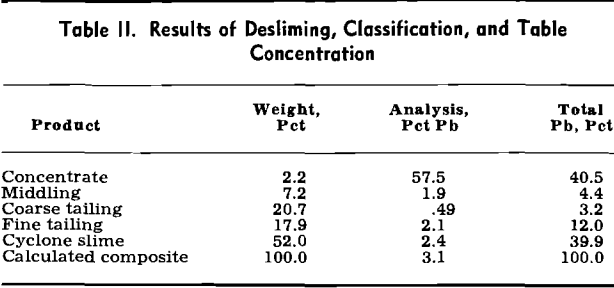
The grade of concentrate at 57.5 pct lead was very satisfactory, but recovery was low. In terms of granular cerussite, however, the 40.5 pct recovery probably represents most of what is available.
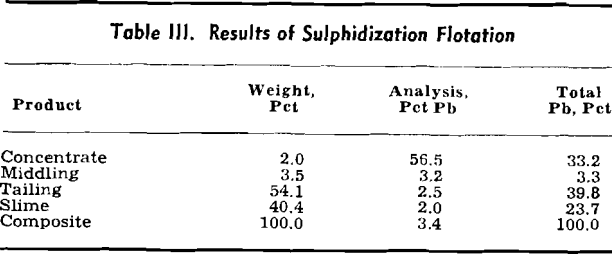
Sulphidization Flotation: Numerous references to the flotation of lead carbonate, both on a laboratory and plant scale, have appeared in the technical press.
The Mine La Motte material, therefore, was not readily amenable to the sulphidization process. Adsorption of sodium sulphide by gangue minerals made it necessary to use a considerable quantity of that reagent to coat the cerussite during flotation of the natural pulp.
Anionic Flotation: A third means of attacking the problem of lead carbonate recovery is flotation with such anionic promoters as fatty and resin acids, fatty acid soaps, amine soaps, sulphonated oils, and petroleum sulphonates. This approach has apparently received little consideration in the past, primarily because it is usually less selective than sulphidization flotation.
Nevertheless, the method was found to possess certain advantages which made it applicable to the treatment of Mine La Motte cerussite. No gangue carbonates were present, and a means of controlling the floatability of the iron oxides was developed. Finally, although this type of flotation was also adversely affected by the presence of large quantities of clay slimes, it was less sensitive in this respect than the sulphidization flotation..

