- To participate in the 911Metallurgist Forums, be sure to JOIN & LOGIN
- Use Add New Topic to ask a New Question/Discussion about Mineral Processing or Laboratory Work.
- OR Select a Topic that Interests you.
- Use Add Reply = to Reply/Participate in a Topic/Discussion (most frequent).
Using Add Reply allows you to Attach Images or PDF files and provide a more complete input. - Use Add Comment = to comment on someone else’s Reply in an already active Topic/Discussion.
Free Cyanide Determination by Titration (4 replies)
Hello Daniel,
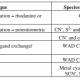
Rhodanine titrations can be conducted with a 5 ml sample, two to three drops of indicator and titrated directly with 0.05 M silver nitrate using a Metrohm 665 Dosimat (for constant addition rate) until the end point was observed.
Potentiometric titrations can be conducted using a Metrohm 716 Titrino auto-titrator with 0.01 M silver nitrate solution. Samples (1 - 5 ml) are diluted with deionised water to achieve sufficient volume for the measurement set-up.
http://www.onemine.org/document/document.cfm?docid=150380
“Free” Cyanide Analysis Methods
A 0.0103 M silver nitrate solution was used for titrations. Potentiometric titrations were conducted using a Schott Instruments TitroLine easy titrator interpreted by SI Analytics TitroLine Chart v1.5.1 software. The volume of analyte was varied according to the expected analyte cyanide concentration. The potential of the silver wire was measured against a Ag/AgCl reference electrode. When copper cyanide solutions were titrated the inflection point was not automatically recognisable by the titrator, and therefore had to be manually calculated. Potential change data produced by the titrator are placed to plot a graph with a best fit equation (R2 value >0.99) and calculate the inflection point by Calculus (the equation was differentiated twice and set to zero). For copper cyanide solutions where there are multiple inflections, the endpoint in the potential region of -350 to -200 mV was used for the cyanide determination. The calculated end point volume was used to calculate the analyte cyanide concentration.
Rhodanine titrations were conducted using a Metrohm 702 SM Titrino titrator. Six drops of rhodanine indicator were added to 50 ml analyte. The end-point was marked by a colour change from yellow to light pink. If the end-point was over-shot, the solution changed from clear and pink, to a cloudy pink colour, due to precipitation of AgCN. Calculation of the cyanide concentration (mg/l CN) for a 50 ml sample is 10 times the volume (ml) of silver nitrate titrated.
http://www.onemine.org/document/document.cfm?docid=219951
I hope someone else can jump in and complete this.
Use the Social Share Bar on the Left. Tell everyone you can about https://www.911metallurgist.com/metallurgy/ It's FREE & GOOD.
I brought up a couple of documents via Google Search and both say that the lower level of sensitivity for simple titrations is ~1ppm (i.e. 1mg/L). Colorimetry, however, is sensitive down to .02 ppm. This requires an instrument - a spectrophotometer, or a colorimeter, which is basically an cheap spectrophotometer. Another caveat: If sulfides are present, you have to get rid of them before doing the titration; sulfides interfere with the titration reaction(s). Here's one of 2 references (only one can be attached at a time):
A friend tells me the Standard silver nitrate solution, 0.0192 N: Prepare by crushing approximately 5 g AgNO3 crystals and drying to constant weight at 40°C. Weigh out 3.2647 g of dried AgNO , dissolve in distilled water, and dilute to 1000 mL (1 mL = 1 3 mg CN).
This is a standard solution. Very easy to prepare. In this particular technique, you have to take care of some interferences such us: Cu.
It is very important to validate the right stoichiometry of the Standard Silver Nitrate solution. You can do that by titration of 2 or 3 stock solutions with known concentration of Free Cn.
As a result, you will validate the relation 1mL of AgNO3 (0.0192N) = 1mg CN
https://www.911metallurgist.com/laboratory/preparation-of-silver-nitrate-solution/
Use the Social Share Bar on the Left. Tell everyone you can about https://www.911metallurgist.com/metallurgy/ It's FREE & GOOD.
I have used the colorimetric method for some time. you can purchase a B & L Spec 20 for around $200.00 on Ebay. The Spec 20 works well.

Hi guys,
Our company has been operating 3 years now. We are a CIL plant hence we are handling cyanide. Our solution sample is being analyzed with Free cyanide by ISE method. However, the instrument has reached its limit and decided to break down. Our only option right now before we can purchase a new instrument is to titrate it with silver nitrate. Do you have any idea/s on the right concentration of silver nitrate to be used for a low free cyanide concentration (max of 0.1 ppm)?
Thanks