Bacterial and fungal populations were observed in samples taken from the White Pine Copper Company tailing pond and mine, and isolates were prepared of the dominant species. The isolates were then screened for solubilization of copper from tailing, ore, and concentrate during incubation in a carbon and nitrogen supplying medium. Certain Penicillium fungi solubilized significant amounts of copper under neutral to slightly acidic conditions. Leaching apparently was accomplished by release into the medium of metabolites which chelated with the copper. The process was found to be highly dependent upon the medium and the concentration of copper in the source material.
Methods
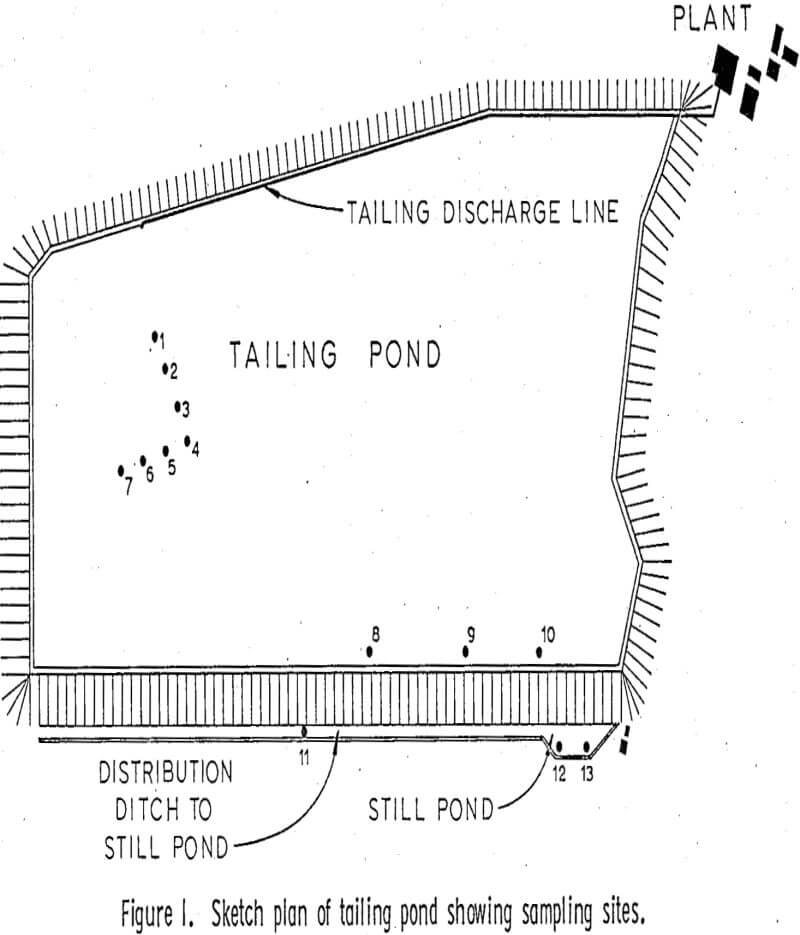
The White Pine tailing disposal area covers about 1700 acres adjacent to the plant; the holding pond takes up about 150 acres. The samples were sealed in sterile one-half pint glass jars and refrigerated at 4° C. until they could be plated within the following 24 hours. After
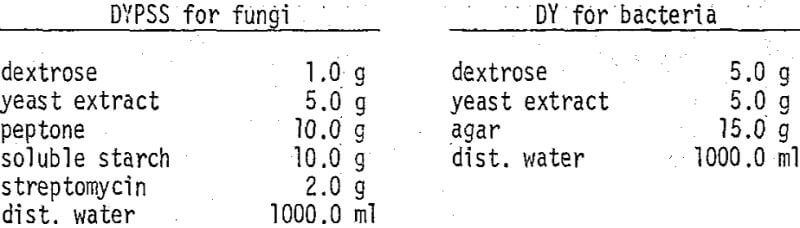
vigorous shaking of the sample jar, one milliliter of fluid and suspended solids was pipetted into a sterile petri-dish and 25 milliliters of agar medium at 48° C. was added. The contents were mixed by swirling, and after the mixture hardened the dish was stored at room temperature and exposed to light for several days. Agar media of the following compositions were used:
lthough the original objective was to study biological processes for leaching copper from tailing and other wastes, it was found necessary to use ores and concentrates of higher copper content in the work so as to have leaching effects that were significantly greater than the experimental error White Pine flotation plant feed and concentrate were obtained for the study, also native copper jig concentrate was obtained from the Ahmeek mill of Calumet & Hecla, Inc.
A primary screen of the pure cultures was carried out to determine their activity on the copper-bearing materials. Czapek’s broth was used as medium for the fungi and nutrient broth for the bacteria. One gram of the copper-bearing material was sterilized in a 125 millimeter flask and 50 milliliters of sterile broth was then added. Triplicate flasks were inoculated with a fungus or a bacterium taken from the isolates by the sterile loop technique, and a control flask was prepared in the same way without fungus or bacterium. The flasks were then stored for 15 – 21 days at room temperature.
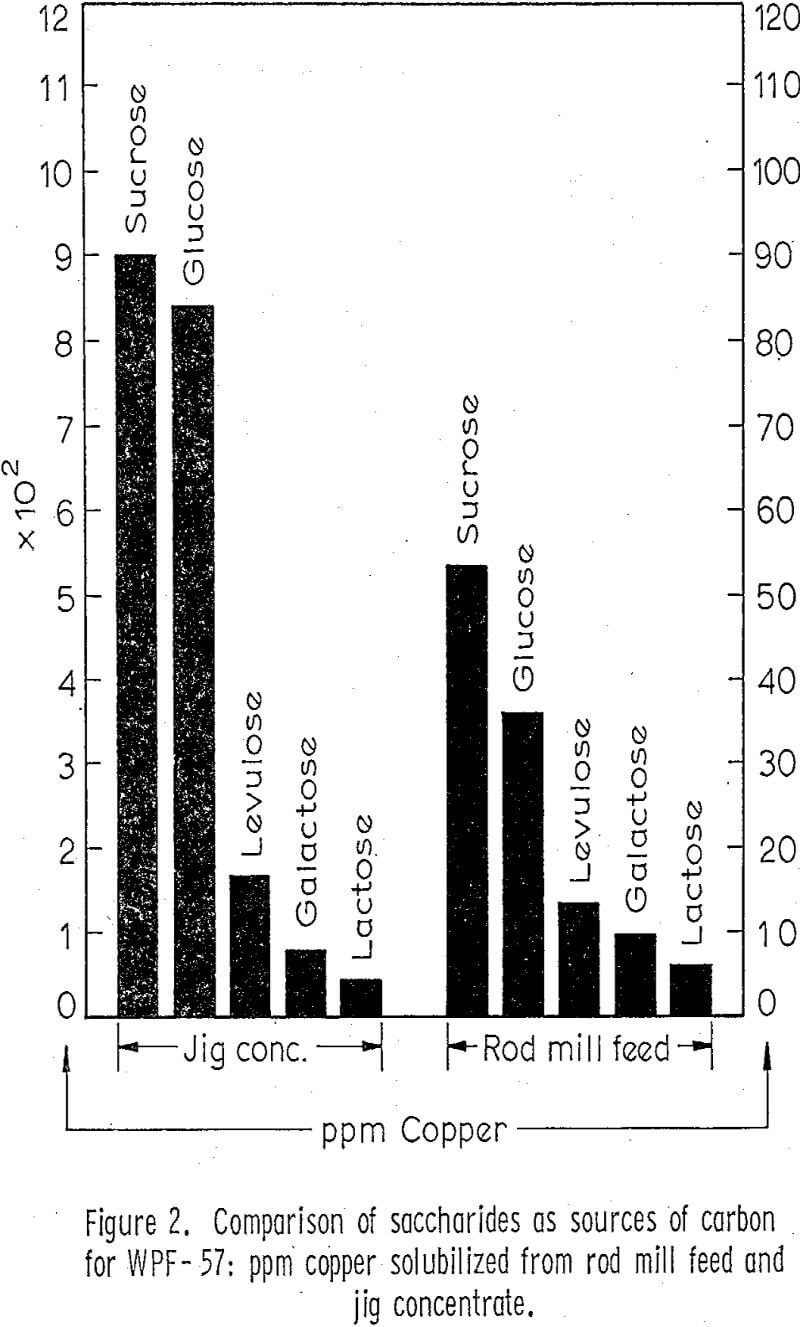
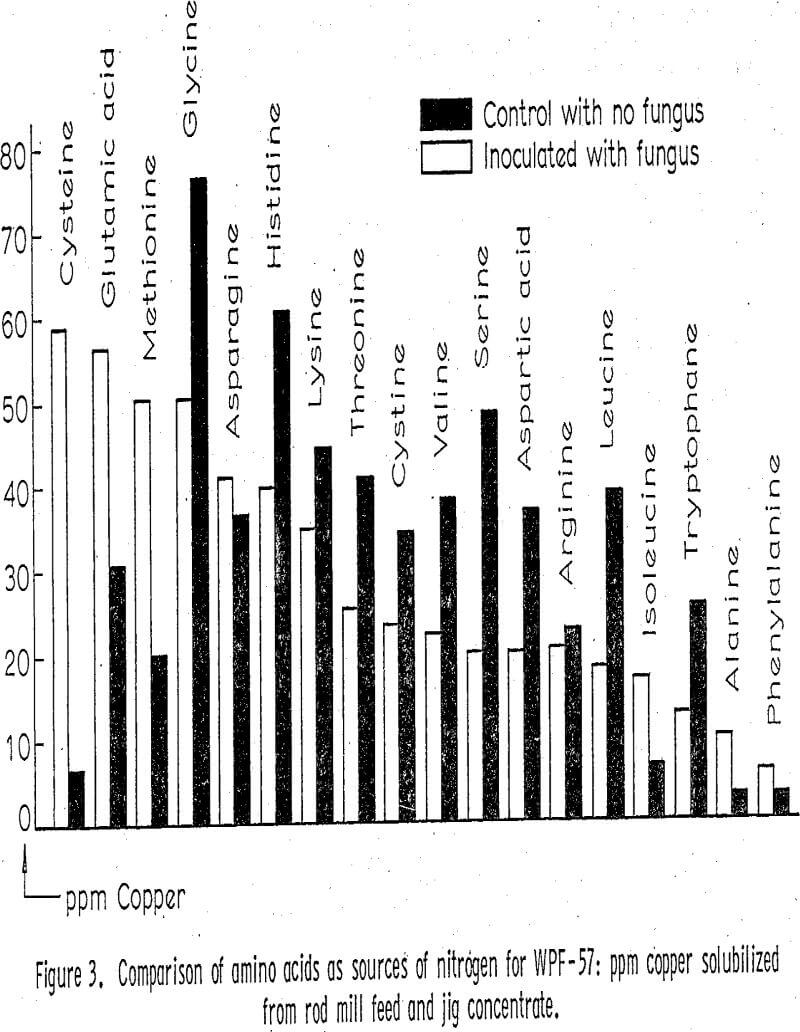
After the standard procedure had been set up, the process variables considered to be significant were investigated in an effort to maximize leaching of copper. Various sources of carbon for the fungi were tried by substituting other sugars for glucose in the Czapek’s broth. Sources of nitrogen were investigated by substituting the individual amino acids for sodium nitrate in the Czapek’s broth; saturated aqueous solutions of these amino acids were made, sterile filtered, and then added to each flask. Sodium citrate was tried as an agent for reducing the toxicity of copper on the fungi. It was found that concentrations up to one gram per liter in the medium did not solubilize significant amounts of copper in control flasks but that it did significantly increase the amount of copper solubilized in the inoculated flasks.
Rod mill feed, flotation concentrate, and jig concentrate were leached with nitric acid to compare copper yields with those from fungal leaching. Concentrated nitric acid was added to a flask containing one-half gram of the ore-bearing material in 50 milliliters of distilled water until the desired initial pH was reached; the initial pH was varied from 7 to 1. The flasks were agitated on the gyratory shaker for five days, and then the amount of copper in solution was determined and the final pH was measured.
Results and Discussion
The pond is a unique habitat with respect to ecological adversities. The foremost adversity is the high pH (approximately 10) which is caused by basic gangue minerals and small amounts of alkaline salts in the ore and by addition of lime for sedimentation of tailing particles. The second adversity is murkiness of the water which affects light penetration, thereby reducing photosynthetic organisms. A third adversity is the low temperature, which was found to be 14 – 15°C. in October and May and 20 – 22°c. in the summer. During the winter months, the holding pond is frozen over. The overflow water from the pond contains no copper in solution detectable by colorimetric test.
The most common bacteria isolated in the pond were of the genera Bacillus, Brevibacterium, and Micrococcus. Bacterial population counts varied greatly; in general, the mud samples contained the largest population with a maximum of 74,000 cells per milliliter, the bottom water samples were the next highest with up to 17,000 cells per milliliter, while the surface and middle water samples were usually low with counts of 2790 and 200 cells per milliliter, respectively.
The increase in solubilization was much the same with both the chalcocite flotation concentrate and the native copper jig concentrate. The peak at 50 percent copper might be attributed to various factors including toxicity, effect of scouring by sand particles during agitation and others.
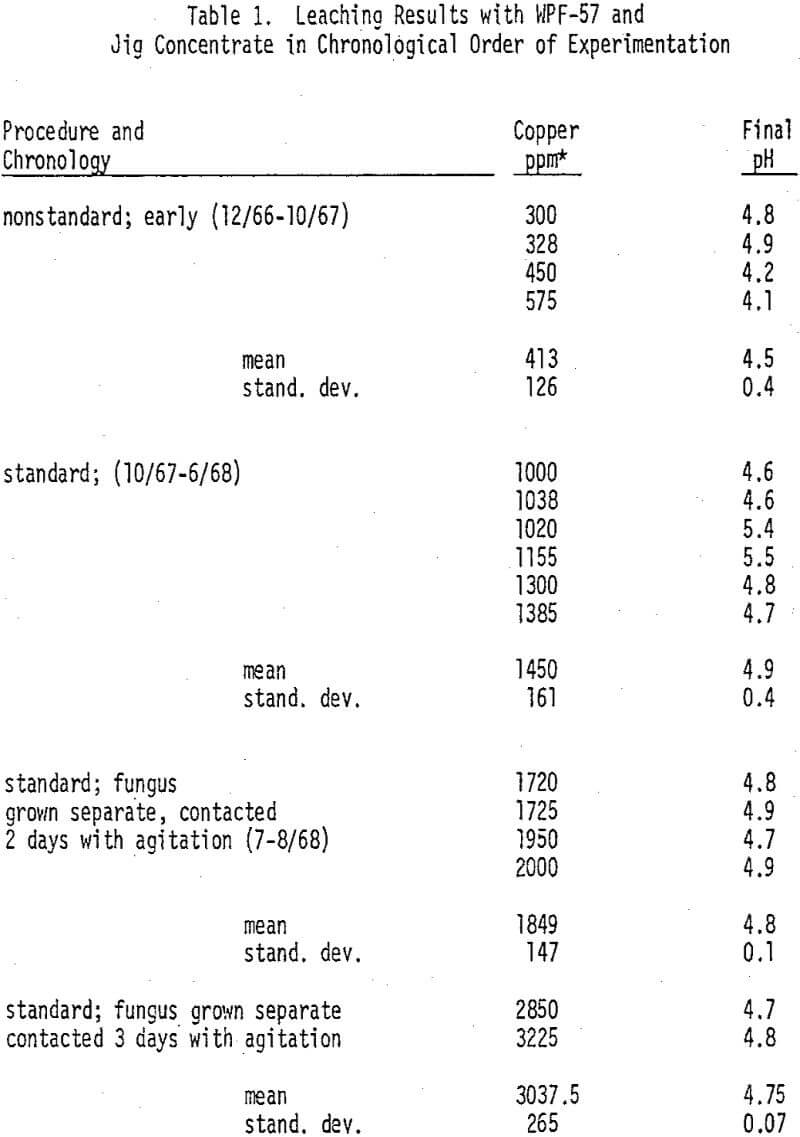
The improvement in yield of copper as a result of standardization of procedures and optimization of test conditions is apparent; also the improvement in reproducibility is apparent in the markedly lower coefficient of variation for the standard