Table of Contents
The starters, generators, and alternators recovered in scrapping up to 7 million junk automobiles annually are a significant source of recyclable copper. At present, the copper is recovered by leaching procedures or by dismantling the devices. As part of a broad research program on recycling the metals in junk automobiles, the Bureau of Mines has investigated the adaptation of the conventional ammoniacal leaching method to leaching copper from automobile starters, generators, and alternators.
Benedict described the ammonium carbonate leach process used to dissolve and recover metallic copper from Michigan conglomerate-ore mill tailings. Coles, Damon, and Machwart investigated the dissolution of copper and zinc from gilding-clad metal scrap with ammoniacal ammonium carbonate solution, and Kunda, Veltman, and Evans studied the application of an amine carbonate system to leaching copper from secondary copper wire and radiator scrap.
The generally accepted chemistry for dissolving metallic copper in ammoniacal ammonium carbonate solution makes use of the reaction between cupric ammonium carbonate and metallic copper to oxidize the copper and form cuprous ammonium carbonate. However, if the reaction is to proceed, the dissolution of a definite quantity of metallic copper in cupric ammonium carbonate leach solution to form cuprous ammonium carbonate requires more ammonia than the generally accepted chemical equations indicate. In the presence of an oxidizing agent such as atmospheric oxygen, the depletion of the cupric copper is prevented by the reoxidation of the cuprous complex to the cupric form. In the absence of an oxidizer, the dissolution of copper will continue until the cupric copper is consumed. Equations 1 and 2 show the simplified reactions involved in dissolving metallic copper.
Cu° + Cu(NH3)4++ → 2Cu(NH3)2+…………………………………………………………(1)
2Cu(NH3)2+ + (NH4)2 CO3 + 2NH4OH + ½ O2 → 2Cu(NH3)4++…………..(2)
The reaction shown in equation 1 does not proceed unless excess ammonia is present.
Before research to develop an improved ammoniacal leach technique for use on automobile components was begun, a preliminary investigation was made to ascertain the influence of specific conditions on leaching metallic copper. Answers were sought on the effect of agitation, oxidation, temperature, and the initial composition of the leach solution on copper dissolution rates. Based on data derived from the preliminary investigation, research was initiated to explore and develop a new leaching procedure involving the use of entrained air bubbles to increase the copper dissolution rate or entrained inert-gas bubbles and powdered sulfur to conveniently and economically oxidize and precipitate dissolved copper as cuprous sulfide. This report describes the research leading to the development of this new procedure.
Experimental Work
The important findings and conclusions derived from the preliminary investigations and used in devising and evaluating an improved ammonia leach process were as follows:
- The copper dissolution rate is very slow in ammonium carbonate solution containing less than 5 grams Cu++ per liter. It approaches a maximum when the cupric ion concentration is 10 to 30 grams copper per liter.
- The best concentration of cupric copper is not less than 30 grams per liter.
- The best proportion of ammonia to carbon dioxide for rapid dissolution of metallic copper is 4 moles of ammonia to 1 mole of carbon dioxide. Thus, 68 grams NH3 and 44 grams CO2 will dissolve up to 1 mole of copper. Use of still larger quantities of ammonia and carbon dioxide does not materially influence the rate of dissolving copper, but it does enable the dissolution of more copper per volume of leach solution.
- The rate of dissolving copper increases when leaching is done at higher temperatures, with adequate agitation and thorough aeration. However, these conditions increase the amount of free ammonia and CO2 in the gaseous phase.
Column Leach Tests
A Pyrex column was employed in investigating the dissolution of copper sheet metal, because it was more convenient and provided greater accuracy than beaker tests. The 1-inch-ID by 4-foot-long column had a holding capacity of 600 ml of solution. The copper test specimen was suspended in the vertical tube by iron wires threaded through small holes in the copper sheet metal and extended through self-sealing holes in the rubber stoppers at both ends of the column. By pulling on the wires, the copper sheet could be moved to the desired position in the reaction column. A schematic drawing of the column is shown in figure 1.
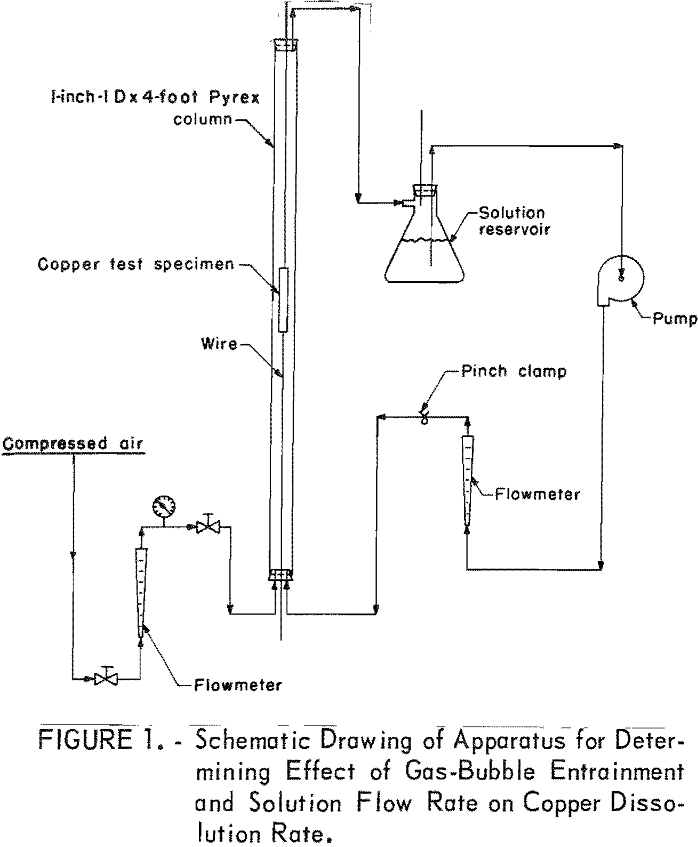
Test variables investigated in the column when using a leach solution containing, in grams per liter, 30 Cu++, 68 NH3, and 44 CO2 were (1) solution flow and method of aeration, (2) substitution of nitrogen for air, (3) leach temperature, and (4) use of powdered sulfur for recovering copper from the leach solutions.
Effect of Solution Flow and Bubble-Entrainment Aeration
Initial tests in the column were made to determine the effect of solution flow and method of aeration on copper dissolution rate. Sheet copper test specimens weighing 38 grams, measuring 4 inches by 1-½ inches by 1/16 inch, and having a surface area of 12.7 square inches were shaped in the form of a cylinder and suspended in the center of the column for the leach tests. The tests were made without any aeration and with independent aeration in the column and solution reservoir.
Effect of Solution Flow
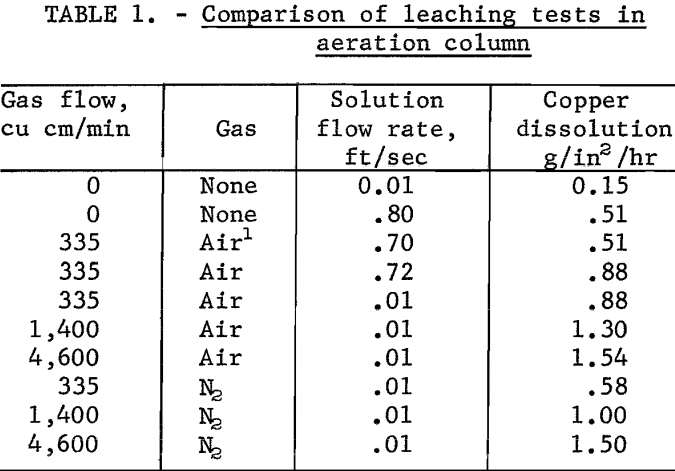
When leaching without aeration, the leaching solution was not recycled through the test column in order to maintain a constant cupric copper concentration. The test showed the copper dissolution rate increased from 0.15 to 0.51 g/in²/hr as the velocity of the solution increased from 0.01 to 0.8 foot per second over the test specimen. Aeration of the solution in the solution reservoir and recycling the leach solution without bubble entrainment did not alter the dissolution rate.
Effect of Aeration by Bubble Entrainment
In order to determine the effect of air-bubble entrainment on the copper dissolution rate, air was injected into the leach solution through a fritted-glass diffuser placed at the bottom of the column. Air-bubble-entrained leach solution flowed over all surfaces of the test specimen, exited at the top of the column and returned to the solution reservoir where the gas, partially depleted of oxygen, was released to the atmosphere. The solution was then recycled for further leaching.
The first series of tests using this procedure was made by holding the air flow rate constant at 335 cu cm per minute and varying the solution velocity from 0.01 to 0.72 foot per second. The copper dissolution rate held virtually constant at 0.88 g/in²/hr over the range of solution flows tested.
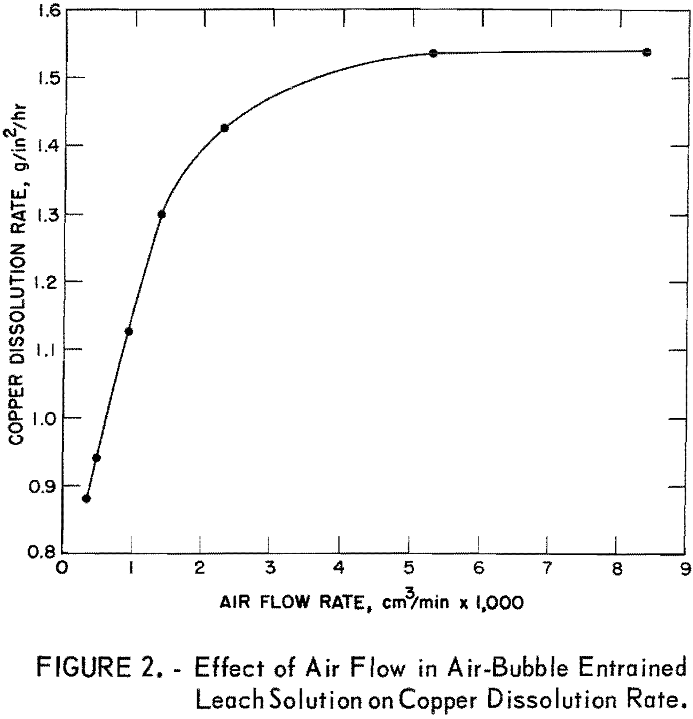
In a second series of tests, the solution velocity was held constant at 0.01 foot per second, and the air flow rates were varied from 335 to 8,400 cu cm per minute. Results of these tests were plotted; the curve (fig. 2) shows that the copper dissolution rate increased at a constant rate as a function of the air flow rate between about 335 and 1,500 cu cm of air per minute. Thereafter, the copper dissolution rate increased more slowly as the air flow rate increased. A maximum dissolution rate of 1.54 g/in²/hr was achieved at an air flow rate of about 4,600 cu cm per minute.
Effect of Substituting Nitrogen for Air
Nitrogen gas was substituted for air to determine whether the increasing rate of copper dissolution was due principally to the physical action of gas-bubble entrainment rather than to an oxidizing effect of the air. The effect of nitrogen-gas-bubble entrainment was investigated by varying the nitrogen flow rate from 335 to 8,400 cu cm per minute. The copper dissolution rates increased from 0.58 to a maximum of 1.5 g/in²/hr at a nitrogen flow rate of 4,600 cu cm per minute.
A comparison of the various leaching tests in the column is shown in table 1.
Effect of Leach Temperature
Tests were made to define the effect of leach temperature on the copper dissolution rate. The effect of temperature was investigated by varying the leach temperature from 30° to 40° C at different air flow rates while holding the solution flow constant at a velocity of 0.01 foot per second. Results of the tests are given in table 2.
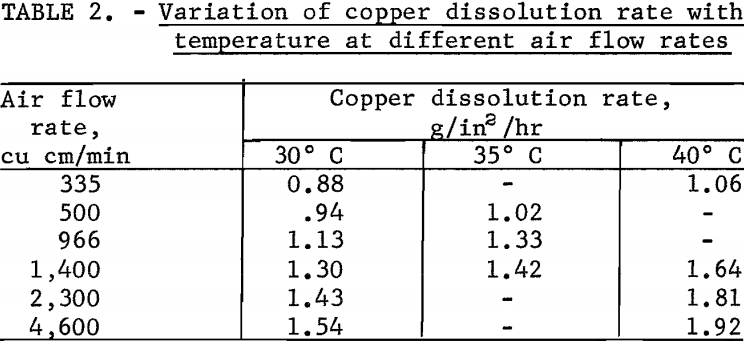
In all instances, increases in the leach temperature resulted in an increase in the copper dissolution rate. At 40° C an increase in the air flow rate from 335 to 4,600 cu cm per minute increased the copper dissolution rate from 1.06 to a maximum of 1.92 g/in²/hr; however at 30° C the increase was smaller, from 0.88 to 1.54 g/in²/hr.
Recovery of Copper From Leach Solutions
Different methods have been used or proposed for processing the leach solutions to recover the dissolved copper. According to Benedict, steam distillation has been employed to precipitate an impure copper-oxide carbonate. The steam distillation process requires large inventories of dilute leach solutions in order to maintain economic still operation. Copper oxide scale builds up on the still walls and must be removed periodically. The distillation method has been used commercially but is complicated and requires a big investment in equipment.
Allen, McGauley, and Roberts have patented a process in which the dissolved copper in ammoniacal carbonate solution is recovered as metal powder by use of CO or gas as reductants in an autoclave at a temperature of 300° to 350° F and at a pressure of 750 to 1,250 pounds per square inch. The high pressure reduction of copper from ammoniacal leach solution using CO or H2 also involves expensive equipment and a complex operation. Because a less complicated and lower cost method for recovering copper from leach solution is needed, alternative procedures were investigated for recovering a marketable copper product and regenerating the leach solution for reuse.
Exploratory tests revealed that sulfur powder in the absence of air could be used to oxidize copper from the cuprous to the cupric form in ammoniacal solution. In addition to serving as an oxidizer, sulfur precipitates the dissolved copper according to the following equations:
Cu2(NH3)x CO3 + S → Cu(NH3 )xCO3 + CuS…………………………………(3)
2Cu2(NH3)xCO3 + S → 2Cu(NHg3)x CO3 + Cu2S…………………………..(4)
2Cu2(NH3)x CO3 + CuS → Cu(NH3)xCO3 + Cu2S………………………….(5)
The form of the copper sulfide precipitate depends on the quantity of sulfur added. When the mole ratio of sulfur to copper leached is greater than 1 to 1, cupric sulfide is precipitated; however, when the ratio is less than 0.5 to 1, cuprous sulfide is precipitated. Between these ratios both sulfides precipitate. The use of sulfur as simultaneous oxidant and precipitant requires that the dissolution of the copper be accomplished in a closed system to eliminate contact with air. In the presence of atmospheric oxygen, oxygen competes with the sulfur to oxidize the cuprous complex.
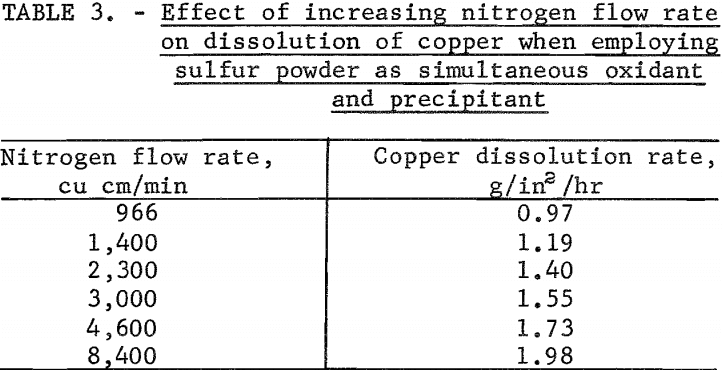
The behavior of sulfur as a simultaneous oxidant and precipitant was investigated in tests made to determine the effect of varying the nitrogen gas flow in the leach column on the copper dissolution rate when the solution flow was held constant. In most tests, the powdered sulfur was suspended in the leach solution, and the suspension recycled over the test specimen. Tests were made varying the nitrogen flow from 966 to 8,400 cu cm per minute at a temperature of 33° C while holding the solution flow constant at 0.01 foot per second. The sulfur addition in each test was slightly in excess of the theoretical requirement for precipitating cupric sulfide. The results of these tests are given in table 3. Test results showed that the copper dissolution rate increased from 0.97 to 1.98 g/in²/hr as the nitrogen flow rate was increased from 966 to 8,400 cu cm per minute. At a nitrogen flow rate of 4,600 cu cm per minute the dissolution rate was 1.73 g/in²/hr and had started to level off.
The effect on the copper dissolution of using elemental sulfur to regenerate the spent leach solution was determined. In this test the sulfur and precipitated copper sulfide did not come into contact with the copper test specimen. Nitrogen gas was employed at a flow rate of 3,000 cu cm per minute for bubble entrainment. Powdered elemental sulfur was added to the spent solution in a mixing flask. The copper sulfide formed was settled in the solution reservoir before recycling the solution for further leaching. This test produced a dissolution rate of 1.15 g/in²/hr, while a similar test, without sulfur added, showed the copper dissolution rate to be 0.89 g/in²/hr.
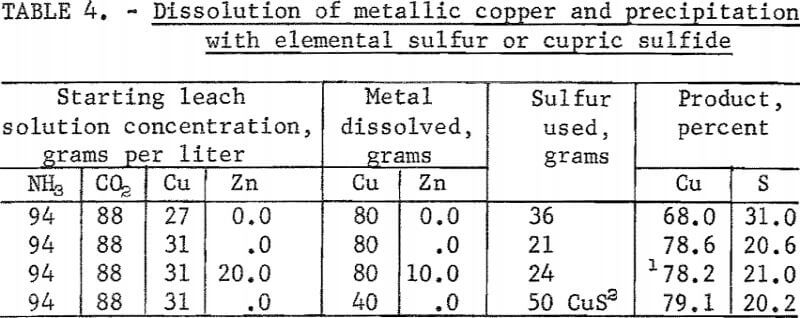
Tests were made to confirm the stoichiometric relationship between sulfur added, as elemental sulfur or cupric sulfide, and the quantity of copper dissolved. According to equations 3, 4, and 5, to precipitate 63.5 grams of copper as cupric sulfide requires 32 grams of sulfur, and to precipitate the same copper as cuprous sulfide requires 16 grams of sulfur or 95.5 grams of cupric sulfide. The quantities of sulfur or cupric sulfide added in the tests were slightly more or less than the stoichiometric quantities so as to deter-mine the form of the sulfide precipitated. A test also was made to determine the deportment of zinc should brass or bronze be present in the scrap metal. Test results are given in table 4.
These tests show that a cupric or cuprous sulfide was precipitated depending on the amount of sulfur added. A high percentage of the newly dissolved copper was precipitated by available sulfur in all tests. Zinc did not appreciably contaminate the cuprous sulfide precipitated. Metallic zinc stoichiometrically precipitated copper from the leach solution as a sulfide. Portions of the leach solution would have to be bled off occasionally for removal of the zinc when treating materials containing an appreciable amount of zinc.
Conclusions
Improved methods for the ammoniacal cupric carbonate leaching of copper were developed by this investigation. The improvements included: (1) The use of gas-bubble entrainment (air or nitrogen) to create a turbulent flow through the material being leached, (2) the use of elemental sulfur to oxidize the cuprous copper in solution and precipitate it as a sulfide, and (3) the combined use of bubble entrainment and elemental sulfur.
Gas-bubble entrainment increases the leaching rate of copper by a factor of about 3 when either nitrogen or air is used.
Powdered elemental sulfur precipitates the dissolved copper as a sulfide as fast as it is leached, and it also regenerates the cupric ions. As a result there is no loss in the strength of the solution.
The combined effect of nitrogen-bubble entrainment and the simultaneous addition of elemental sulfur accelerates the leeching rate by a factor of about 4.
These improvements, which accelerate the leaching rate, potentially cut the cost of ammoniacal leaching by making possible the use of smaller, less costly equipment to produce a high-grade copper sulfide product.
