Table of Contents
All of our larger domestic deposits of manganese are very low grade, but estimated tonnages in these deposits are large enough to constitute a potential strategic reserve of manganese for emergency periods, provided these ores can be upgraded sufficiently to meet industrial specifications. Many types of processes have been suggested for upgrading these ores, several have been studied in pilot plants, and a few have attained limited commercial production for special purposes.
Experimental Procedure
Manganese-ore samples ground to —35 mesh were leached in iron containers into which the ore sample and appropriate NaOH solutions were placed. Flotation concentrates were already ground to —200 mesh. The slurries were then placed on a hot plate or into a pressure digester and stirred continuously during the period of digestion. Where the digestion was performed at atmospheric pressures, water lost by evaporation was replaced at regular intervals to maintain approximately a constant volume of slurry.
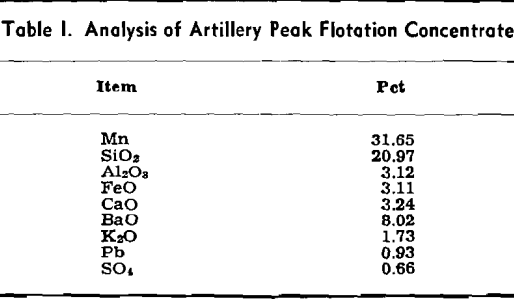
This leaching procedure was used for obtaining the data reported in this paper on the effects of NaOH concentration, pulp dilution, temperature, and time of digestion on the efficiency of extraction of silica from the ore sample.
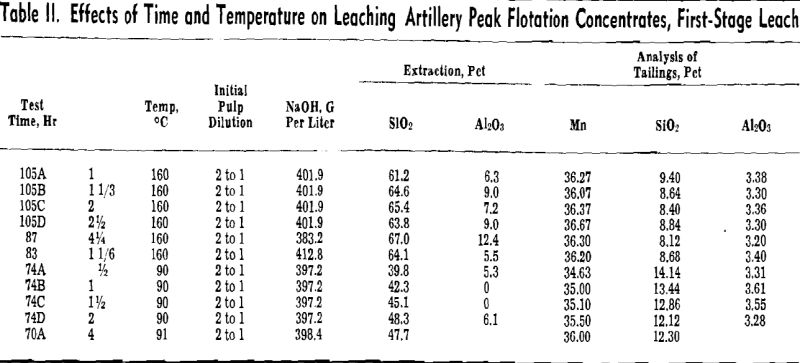
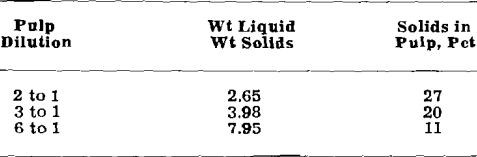
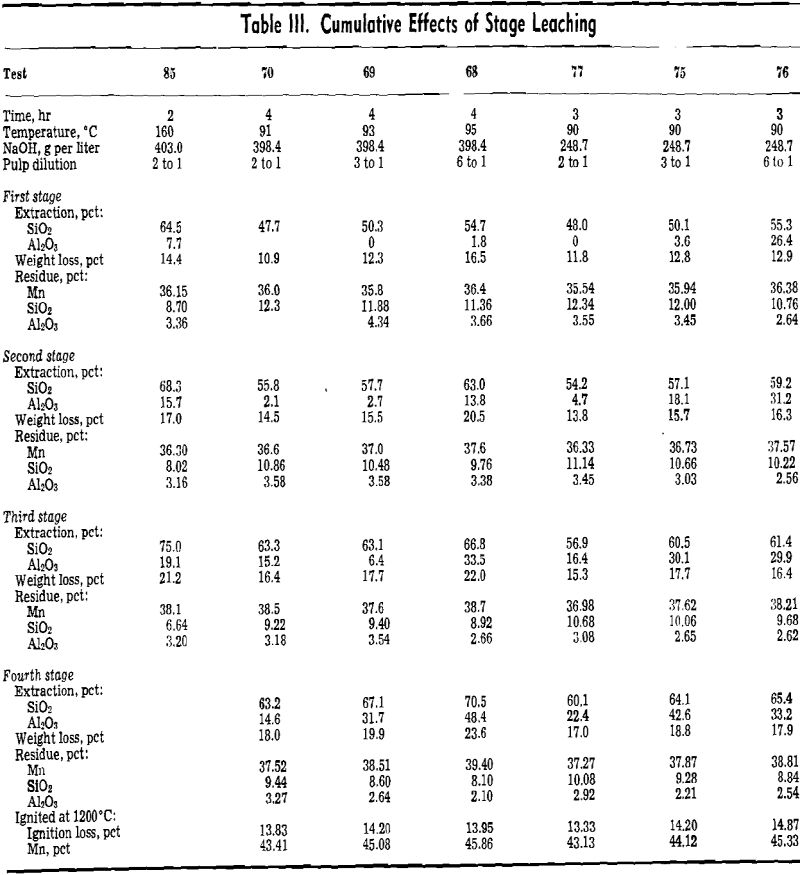
Optimum conditions for leaching appear to be somewhat as follows. Caustic concentration should be in the range of 24.3.8 to 398.4 g per liter NaOH. Lower concentrations were found to be less effective. A pulp dilution of 6 to 1 was most effective, but because large volumes of liquor must be handled, pulp dilutions in the range of 2 to 1 to 3 to 1 may be more desirable. A leaching time in the range of 2 to 3 hr was effective, and the choice of temperature for leaching lies between leaching at atmospheric pressure and pressure digestion.

Regenerating Caustic Leach Liquors
The leaching of manganese ores or flotation concentrates with caustic soda produces a liquor containing essentially a sodium silicate. As the silica concentration in the leach liquors increases, there is a decrease in the efficiency of leaching, and it becomes necessary to regenerate the NaOH in spent leach liquors to restore their leaching efficiency and recover NaOH for further leaching. Regeneration, then, becomes an important secondary operation for the process. Spent leach liquors were regenerated
by adding lime to precipitate calcium silicate and free the NaOH, according to the general equation:
Ca(OH)2 + Na2O·SiO2 = CaO·SiO2 + 2NaOH
In using this reaction for regeneration, one must know the conditions under which it will function most efficiently. The following discussion and data outline those conditions.
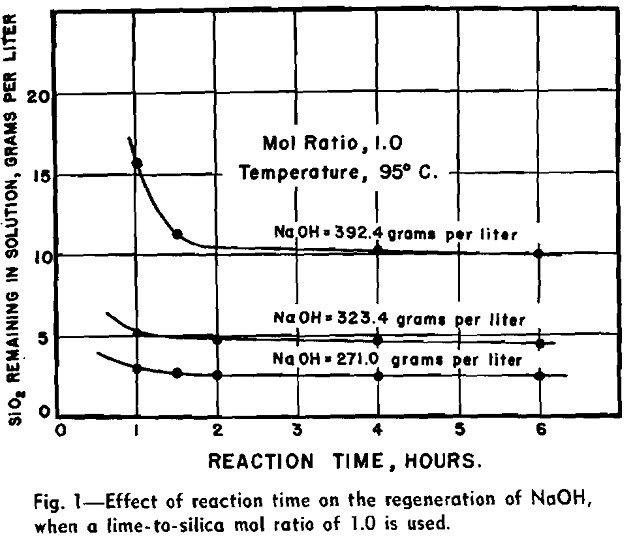
Experimental Results and Discussion: The effective regeneration of calcium silicate was obtained by adding dry fresh-burned lime to the spent leach liquor in a mol ratio of lime to silica of 1.0 and reacting this mixture for a period of 2 hr at a temperature above 60°C. Lower temperatures and shorter periods of time were less effective in producing a calcium silicate that was readily handled. Reaction periods in excess of 2 hr and mol ratios in excess of 1.0 did not materially improve the process of regeneration.
The effects of NaOH concentration on regeneration were very pronounced and in these tests the concentration was varied from 200 to more than 400 g of NaOH per liter. It suggests that equilibrium conditions of some kind were established between the reactants and the products.
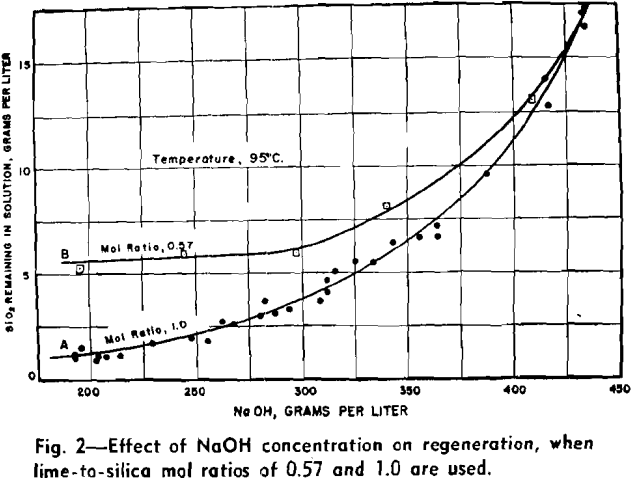
The slimy nature of calcium silicate precipitates precludes the use of thickeners for separating the solids from the liquor except for very dilute slurries. The heavier slurries must be filtered to make the separation, and filtration on a Buechner filter proceeded at a satisfactory rate for solutions containing 350 g of NaOH per liter or less. For the more concentrated solutions the filter rate decreased rapidly as the NaOH concentration increased. The unwashed filter cakes contained about 65 pct moisture.
Seeding Out Sodium Silicate: Spent caustic leach liquors will drop out crystals of sodium silicate on cooling and standing. This crystallization may be speeded by cooling the liquors to 20 °C or less and adding a few small crystals of sodium silicate. To test the efficiency of this method for the removal of SiO2, a liquor containing 286.7 g NaOH and 45.93 g SiO2 per liter was cooled to 20°C, and a few small crystals of sodium silicate were added. In 1 hr, the entire volume was solid with loosely packed crystals.
Build-up of Impurities in Leach Solutions: Certain minerals often found associated with manganese will react with NaOH to produce sodium compounds that will not readily regenerate NaOH. These compounds will in time build up and render the solution inert for leaching silica.
This paper is typical of the current trend of thought in the mineral beneficiation industry. As we focus our attention on the more complex and the lower grade ores we find, in many cases, that older methods of beneficiation are either too costly or do not apply at all.

The crude ore used in the above test was ground in a ball mill until the ore was 85 pct -200 mesh. The leaching operation was carried out at 200°C and 200 lb per sq in. of pressure in a cast-iron autoclave. Only one stage of leaching was used.
