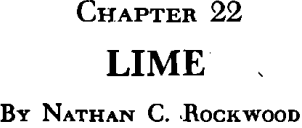Table of Contents
There are two types of lime, high calcium and dolomitic lime. Typical analyses of these materials are shown in Table 1. This discussion is concerned only with high calcium lime.

Lime is produced from naturally occurring limestone by subjecting the limestone to a temperature in the range of 2200°F for sufficient time to drive off the carbon dioxide and produce the product calcium oxide, commonly known as quicklime. The limestone must be crushed and sized before calcination. The usual stone size is -2-inch plus ¾-inch. The sizing may vary somewhat depending upon operating practice as related to a particular lime kiln. This reaction is described by the equation:
CaCO3 + Heat → CaO + CO2↑
After the lime has been cooled, it is then crushed and screened to various sizes depending upon market requirements. A considerable inventory of the finished products must be kept in large storage tanks to accommodate fluctuations in market demand and to provide for occasional down time of the kiln for maintenance.
Lime is rarely completely calcined. It is considered good practice to allow a small amount of unburned limestone in the interior of the quicklime pebble. This results in a continuous evolution of carbon dioxide from the limestone. This keeps the lime porous during calcination and tends to prevent overheating of the lime as the carbon dioxide gas is evolved. This unburned portion of the finished product is known as “core”. It can vary from 3 to 5%.
The user of lime is more interested in the available calcium oxide content of the lime as received. Little can be done to change the normal impurities, such as iron compounds, silica, magnesium oxide and heavy metals in general, which may be present in the raw limestone. Lime is perishable and starts to pick up carbon dioxide from the air during handling. This rate of pick-up is largely dependent upon humidity. Lime issuing from the kiln may contain 93% available calcium oxide, but by the time the shipment is placed in storage at destination, this figure will have decreased due to carbon dioxide pick-up. Available calcium oxide may be empirically determined by a simple titration with hydrochloric acid.
Calcium oxide, quicklime, may be applied as such or it may be reacted with water to produce a dry powder known as hydrated lime or a suspension of hydrated lime in water known as a lime slurry. The equation for the lime slaking reaction is:
CaO + H2O → Ca(OH)2
Lime is shipped either in covered hopper cars or pneumatic trucks.
In gold recovery operations, the major use of lime is to control the pH of the sodium cyanide solution used to leach gold from its ore. Such leaching is called dump leaching where large pieces of ore are involved, or heap leaching when the ore is of smaller size. Also, the ore may be pulverized very fine and leached directly in vats by the so-called carbon-in-pulp process. But in all cases, the cyanide solution must be maintained in a range of pH 10 to pH 11, using lime to achieve the necessary alkalinity. The quantity of lime used will range from two pounds to five pounds of lime per ton of ore processed.

In some cases, caustic is used rather than lime. This is a matter of economics at some gold operations; although the high price of caustic as compared with lime limits the use of caustic. Even when a clay ore is treated with cement to nodulize the clay and render the leach pile more permeable Co the cyanide, lime is usually required to complete the pH adjustment.
There are other uses and potential uses for lime in gold recovery operations. One such use is to prepare calcium hypochlorite for oxidizing sulfur and sulfur compounds in refractory ore. This renders the ore more amenable to cyanide leaching. Another use is scrubbing the off-gases from the oxidation of refractory ores; such scrubbing being necessary to remove the evolved sulfur gases. Indeed, lime can be used in the fluidized bed roasting of refractory gold ores, but further research will probably establish that raw limestone alone can serve the same purpose when mixed with the ore to absorb the sulfur gases as these are formed.
In the gold processing industry, it is doubtful if any set of circumstances would justify the production of lime by any one gold mill. Lime production is really not a matter of calcination only. A modern plant must have preheaters, dust collection equipment, ample storage to provide for kiln downtown and operators who are skilled in the art of lime-burning. Table 2 illustrates the heat energy requirements for a typical lime-burning operation. Capital investment for a rotary kiln lime plant capable of producing in the range of 400 to 450 tons of quicklime per 24-hour day is estimated to be high. Unless there were other markets in addition to its captive production, it is not likely that such a plant could be justified.
Assume:
High calcium limestone of 98% is calcined.
Atmospheric temperature during calcination is 60°F (15.5°C).
Average exhaust gas temperature is 735°F ( 391°C).
https://www.youtube.com/watch?v=1edL-WDwwCk
Varnish manufacturers require about the purest high-calcium lime commercially obtainable. It is used in the form of a hydrate in making oleoresinous varnishes, to harden and partially neutralize the resin. It About 8 lb of hydrated lime is added gradually to 100 lb of melted mein and heated for a short period. The hydrate used must be white or colorless, completely hydrated, and not more than 10 pct should be retained on a No. 230 (62-micron) sieve. The calcium oxide on a nonvolatile basis must be a minimum of 94 pct and the magnesium oxide a maximum of 3 pct. Other impurities and insolubles are limited to 3.4 pct, including iron oxide, which is limited to 0.4 pct because of its coloring properties. Carbon dioxide is limited to 3 pct if the sample is taken at the place of manufacture and to 5 pct if taken elsewhere.
A “superfine” hydrate such as that ordinarily required for varnish manufacture would have a screen analysis approximately as follows: passing No. 100-mesh, 99 pct; passing No. 200-mesh, 98 pct; passing No. 325-mesh, 97 pct.
Marketing and Uses
In the building construction market, lime usually is retailed by the dealer to masonry and plastering contractors in the original manufacturer’s package, which is not recoverable except in rare instances special metal containers are used. Sometimes lime is shipped direct from the manufacturer to producers of lime putty, who have
processes for making and handling “aged” lime putty. This is hauled to the building site either in steel containers or in bulk body trucks like those used for delivery of ready-mixed frequently already mixed with sand into mortar. Where the lone is delivered, it is dumped in a heap, covered with sand, and required in the mortar mixer with the proper proportion of cement added.
The industrial markets and chemical processing generally, in water purification and treatment of trade wastes and sewage, manufacturer usually is faced with relatively few but steady for fairly large tonnages. For smaller industrial purchasers, is sometimes direct from the lime manufacturer and some through a broker or jobber, who may represent more than one manufacturer. While competition for such industrial business is processors are generally reluctant to change from one lime to another after having standardized their processing to suit lime from a particular source; hence there is a good deal of cross-hauling in sales territories that normally, by the uninitiated, would be considered a definitely belonging to one or two local manufacturers.
Agricultural lime in some few instances is shipped direct to farmers that use large quantities but most of it is shipped in bulk, or inpackages as hydrate, and distributed through agricultural cooperatives feed and fertilizer dealers, and building-supply dealers. Markets for agricultural lime are limited mostly to the eastern states, where farmers have used lime in preference to pulverized limestone for generations. In the central states, very little agricultural lime is used choice being liberal applications of relatively coarsely ground limestone. Lime and hydrate are more active in bringing about soil reactions and a smaller quantity is used in an application, but the applications have to be made more frequently. There are other uses for lime and hydrate on the farm; for instance, it is mixed with preserve the nitrogen and is used for white-washing stables and houses, for which ground limestone cannot be used.
Ordinarily, the manufacturer is not much concerned with, the quality of lime used for application to the soil and it offers a ready market for material that might be rejected for industrial and chemical purposes. However, there are scientific methods of comparing the relative soil-neutralizing values of one lime with another and with ground limestones of various fineness of particle size. Neutralization of acid soils for certain crops, however, is only one of the functions of lime agriculture. Primarily, it supplies the element calcium, which is a most important plant food. From this angle, hydrated lime is the most concentrated form of agricultural lime; in other words, the percentage of calcium compared with that of the other ingredients is greatest.
Paper Manufacture
The specifications for lime for cooking rags in paper manufacture and an outline of the sulphite process have been touched on but this is only one of several uses of lime in the paper industry. Because this industry is one of the largest consumers of lime, it is also one of the largest manufacturers of noncommercial lime. Much of the lime and in the processing is recoverable as calcium carbonate sludge, and several paper manufacturers have installed rotary kilns to burn this by-product with small additions of fresh limestone.
Price History
Like any other basic commodity, where the capacity to produce almost always exceeds demand, except for limited periods, the price of lime has fluctuated more from competitive conditions than from changing costs. The element of increasing cost during the past few years has generally been discounted by more efficient use of fuel and labor, but in periods of slack demand, large producers, in order to keep costs down, through volume production and continuous operation, often resort to price cutting. However, in this respect the industry has become much more stabilized than when competition was chiefly that of a few large producers against many small ones. The small operator had one advantage in that he was able to shut down and start up without the losses entailed to a larger operator, because of the negligible overhead of a small lime plant. As in the portland cement or other similar industry, the cost of manufacturing lime is influenced more by the volume over which the annual cost is spread than by any other single factor.