Table of Contents
The occurrence of acid mine drainage (AMD) with coal mining has been well documented. Less documentation is available of its association with other types of mining, e.g., copper, gold, zinc, and sulfur. Yet there are many locations in the western states where acid mine drainage is as great a pollution problem as in Appalachia and in some cases, because of the high copper, zinc, and arsenic concentrations, they present an even more difficult problem.
Until a few years ago, it was generally believed that the neutralization of acid mine drainage was uneconomical thus, AMD was allowed to discharge freely into our streams. However, the demand for good water finally dictated that the mining industry be included under pollution laws. In 1964 the Commonwealth of Pennsylvania passed a law requiring that all active mine discharges meet a discharge standard of pH 6-9, iron less than 7 mg/l, and the water must have a net alkalinity. Several other states now require active mines to treat AMD.
In all but a few cases, lime neutralization, often in conjunction with aeration, has been the treatment method used. The high cost of lime as compared to limestone and the poor quality sludge, (slow settling, large volumes, and low solids content) has stimulated work in the utilization of limestone. This paper reports on the current state-of-the-art of limestone treatment of AMD.
Neutralization Process
The neutralization process provides the following benefits:
- Removes the acidity and adds alkalinity.
- Increases pH.
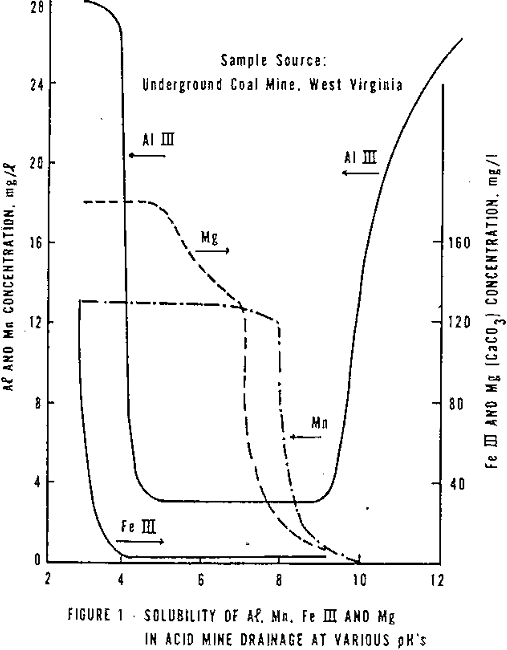
- Removes heavy metals. The solubility of heavy metals is dependent on pH; that is, up to a point, the higher the pH, the lower the solubility, (see Figure 1).
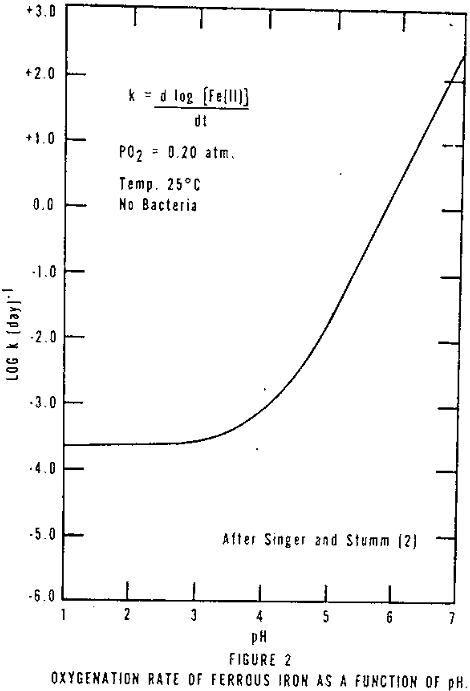
- Ferrous iron, which is often associated with AMD, oxidizes at a faster rate at higher pH’s (Figure 2).
- Sulfate can be removed if sufficient calcium ion is added to exceed the solubility of calcium sulfate, however, only in highly acidic AMD does this occur.
Some shortcomings of the neutralization process are:
- Hardness is not reduced and may be increased.
- Sulfate is not reduced to a low level.
- The iron concentration cannot be reduced to less than 3-7 mg/l. A waste sludge is produced that must be disposed of.
Chemical Properties of Limestone
The term limestone is a general one embracing carbonate rocks or fossils, composed primarily of calcium carbonate or combinations of calcium and magnesium carbonate with varying amounts of impurities, the most common of which are silica and alumina. Since limestone does not have a constant chemical composition, it is important to know what characteristics are necessary for a good neutralizing agent.
Most limestones are rated by the manufacturer as to their calcium carbonate or calcium carbonate equivalent content. The higher the CaCO3 content, the greater the alkalinity available and the less the impurities. In comparing lime and limestone, it should be noted that a pound of lime has 1.35 times the alkalinity of a pound of limestone.
Several investigators have reported that limestones that contain magnesium carbonate in appreciable quantities react very slowly. Hoak, et al reported that dolomitic limestone’s rate of reaction was approximately inversely proportional to the quantity of magnesium carbonate it contained (above about two percent). Ford conducted studies with 14 limestones of various compositions by treating both artificial and actual mine drainage and found that in general the neutralizing efficiency of a stone increased with higher percentages of CaO and lower percentages of MgO, thus, the calcites, CaCO3 were more effective than dolomites or magnesites.
The silica (SiO2) found in most limestones is chiefly an impurity and thus, its presence in large quantities means that less alkalinity per pound is available.
Physical Properties of Limestone
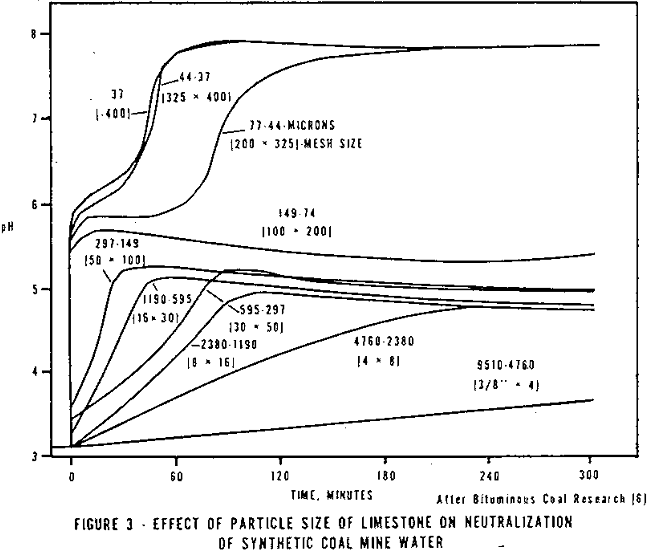
In addition to the chemical properties of the limestone, the geological history of the stone and its crystal structure probably plans some role in its neutralization ability. Crystal structure has some bearing on the surface area of the stone particle. Ford has shown that a trend exists between surface area (BET) and neutralization rate. However, the correlation does not have a high confidence level. Part of the lack of correlation may be due to the use of BET surface area measurements, which measures both the outer and inner particle surface areas. Since the CaCO3 must dissolve and then diffuse away from the particle, the diffusion within the small interval channels would be insignificant to that of the outer surface area. Thus, a correlation of just the outer surface area to neutralization rate may be more valid. Several investigators have shown that the reaction rate is a function of the fineness of the stone. Ford obtained the data presented in Figure 3. The limit on the fineness of the stone is an economic one. Cost of grinding increases at an accelerating rate as the particle size decreases. The cheapest small particle size material in mining areas is “rock dust” of which 60 to 70 percent passes a 200 mesh. To obtain a smaller size, a cost in excess of that paid for lime may be required.
Limestone Selection
As discussed above, the following factors should be considered in the selection of a limestone:
- High calcium carbonate content.
- Low magnesium content.
- Low amount of impurities.
- Large surface size.
- Smallest particle size within economic bounds.
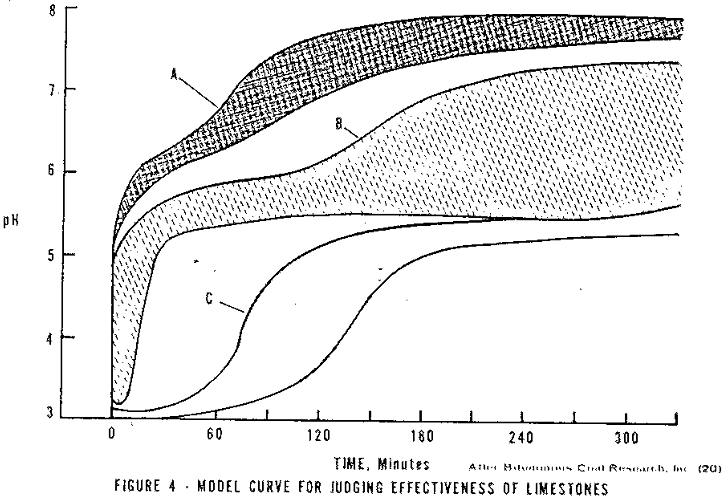
Stone analysis alone has not been shown to be totally reliable for selecting a limestone. After a preliminary screening of the proposed stones by their chemical analysis, a simple laboratory test is recommended. Twice the stoichiometric amount of limestone of the size to be used is added to a sample of AMD. The sample is mixed by introducing air. The pH is recorded with time for five hours. A pH – time plot is used to evaluate the limestone. In Figure 4, plots of data acquired by Ford in this manner are shown. Ford suggests that an effective limestone will be located in Area A.
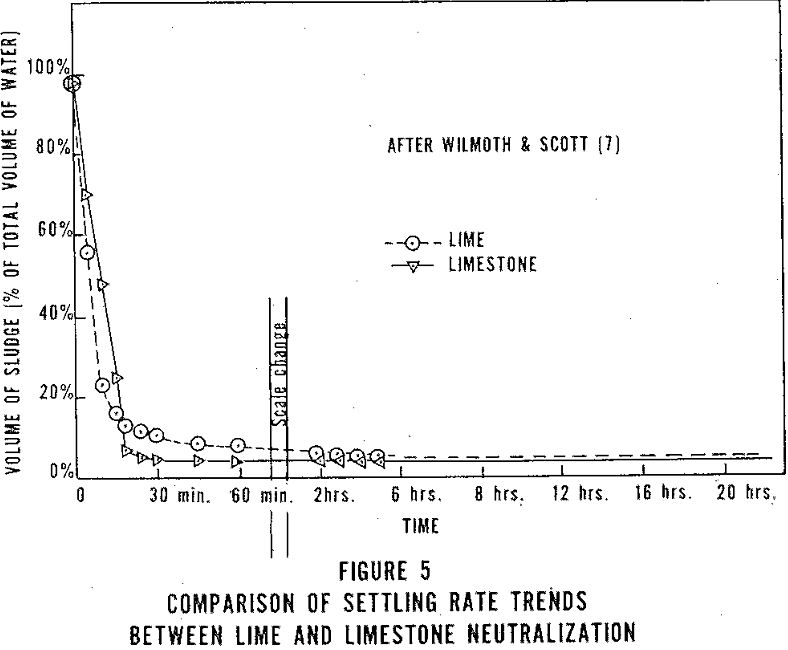
In addition to the reaction rate, the characteristics of the sludge should also be considered. Three characteristics of the sludge are important, i.e., settling rate, sludge volume, and sludge solids content. To perform these tests a sample of the unsettled neutralized AMD is placed in a 1,000 ml graduated cylinder and the depth of the sludge blanket determined periodically for 2 to 12 hours. These data are then plotted as in Figure 5. The final reading is considered the sludge volume, usually expressed as a percent of the total sample. The supernatent should then be drained off. The sludge is then dried and the percent solids is calculated.
A good limestone should have a high neutralizing rate, fast settling sludge, volume of sludge, and a sludge with a high solids content.
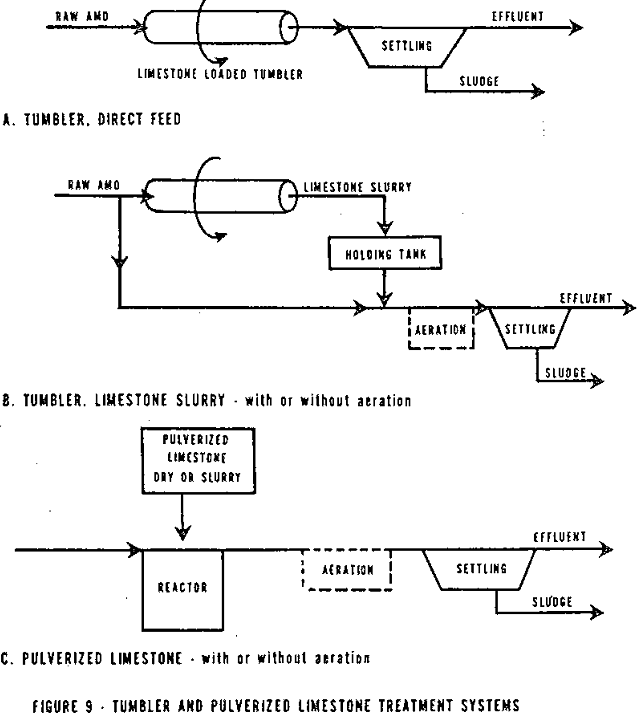
Limestone Treatment Systems
The type of limestone treatment system used depends on the amount and ionic state of the iron in the AMD. Three groups of AMD are considered here (1) low iron AMD, (2) ferric iron AMD, and (3) ferrous iron AMD.
Low Iron AMD
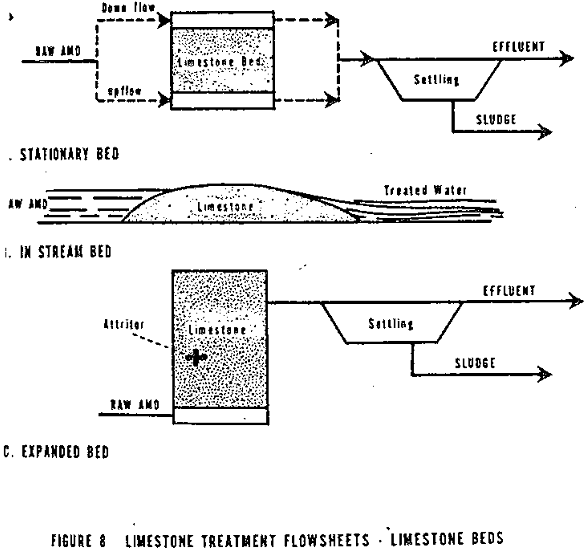
Low iron AMD is the easiest to treat. Limestone beds (Figure 8) and pulverized limestone (Figure 9) can be effectively used. This type of AMD usually has a low acidity and therefore, coating the stone with calcium sulfate is not a problem. Both upflow and downflow stationary limestone beds can be utilized. The stone should be as small as possible, but not so small that the bed compacts and seals itself. Jacobs reported that a limestone bed five feet deep and a flow rate of 0.5 to 1 gpm/sq. ft. successfully treated a 50,000 mg/l acidity solution. For more dilute solutions, higher flow rates and shallower beds can be used. Laboratory size test limestone beds for determining the optimum flow rate and bed depth for any particular AMD and limestone are recommended.
Fluidized beds are superior to stationary beds in several ways. A higher flow rate can be maintained, the agitation between particles removes deposits that might build up, and carbon dioxide that sometimes binds to the stone surface is flushed from the bed. Reidl had good success with an expanded bed 18-24 inches deep and a flow rate of 40 to 50 gpm per sq. ft. Gehm conducted some rather detailed studies on expanded limestone beds and reported the following:
- Comparable results were obtained for calcite and amorphorus stone.
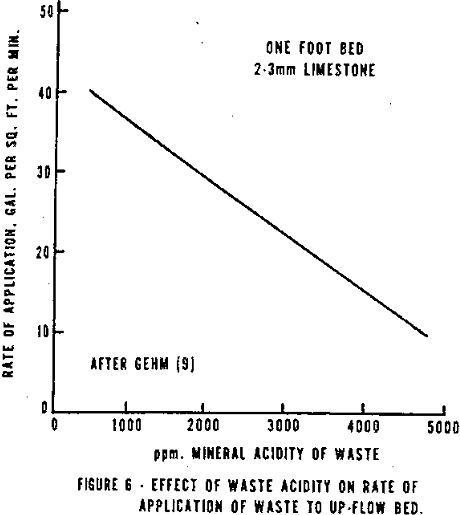
- A relationship exists between bed depth and rate of application to obtain comparable effluent quality. For a waste having an acidity of 4,240 ppm, the bed could have an application rate of 40 gpm per sq. ft. per foot of depth. A two foot deep bed is the minimum suggested.
- A linear relationship exists between rate of application and acidity of waste (see Figure 6).
- The upper limit for acidity of water is 5,000 ppm, and
- stone size should be between 10 and 20 mesh.
Jarrett and Kountz and Yen have shown on the laboratory scale that the construction of a limestone barrier in the AMD stream can effectively treat low iron AMD. Ten developed design criteria. The Pennsylvania Department of Mines and Mineral Industries plans to construct a full scale installation of this type.
Low iron AMD can be treated by the direct application of pulverized limestone either as a solid or a slurry. Limestone tumblers can also be used. Each of these systems is discussed later.
Except for instream limestone barriers, the limestone systems are composed of the reaction bed, tank, or tumbler, followed by a sedimentation tank where the sludge is separated from the treated water.
Ferric Iron AMD
Stationary limestone beds do not appear to be applicable for ferric iron AMD. Because of the slow application rate, coating of the stone with precipitated iron is sure to occur. Braley and others have reported of this occurrence.
Expanded beds may have some applications, particularly if the system is made to include an attrition device to remove the iron coatings. Glover found that passing the fine limestone bed through a pump resulted in the impeller removing the iron coat. Later he patented a mechanical attrition device in the form of a rapidly rotating impeller that operated inside the expanded bed.
Limestone beds placed in a stream have also been found to quickly coat with iron, leaving them ineffective. However for short-term treatment, such as during a surface mining operation and draining of an AMD lake, this method may be applicable.
Tumblers have been shown to be effective on ferric iron AMD. Calhoun reported of a full scale plant in which a rotating drum, 30 feet long and 3 feet in diameter, containing 5,000 pounds of three by one inch limestone, and rotating at 15 rpm, was used to treat an AMD having 100 to 500 mg/l acidity, 20 to 120 mg/l iron (90 percent ferric) and a flow of 50 to 300 gpm. The water flowed from the drum to a settling basin. Excess limestone in the amount of 1.42 times the stoichimetric was required.
The application of pulverised limestone either in the dry or slurry form has been shown to be effective. Wilmoth and Scott have conducted a number of pilot plant studies on the treatment of ferric iron AMD. In Table I, a typical set of data is presented.
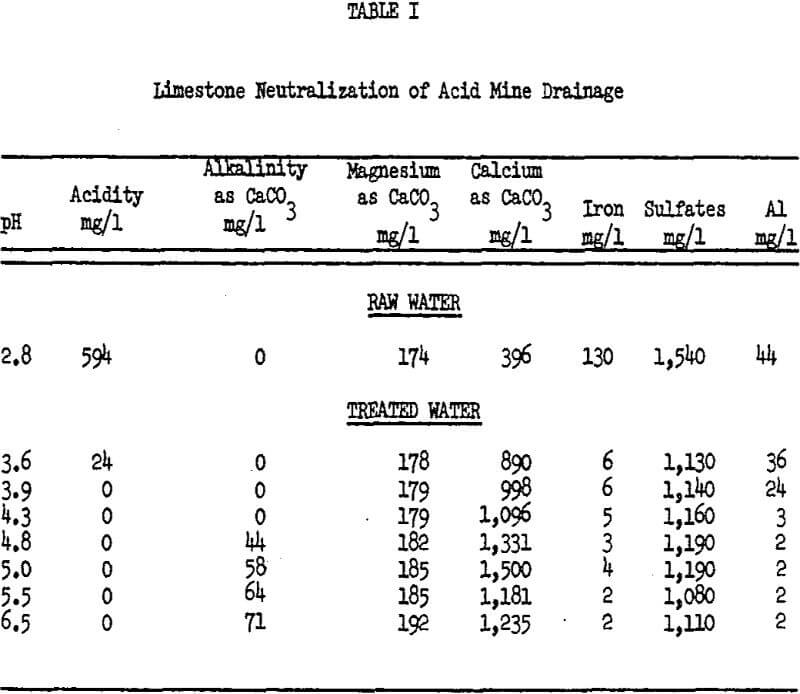
It is of interest to note that by maintaining a pH 4.3, all of the acidity is removed and the iron and aluminum concentration is near minimum. Treatment to higher pH by limestone serves very little purpose. To raise the pH above this level is difficult because no acidity is available to attack the limestone and the CO2 being released buffers the reaction. Thus, large excesses of limestone, which are mostly lost to the sludge, must be utilized. Wilmoth and Scott found when they raised the pH to 6.6 that greater than three times the stoichiometric limestone was required. Later studies of theirs showed that it required 1.5 times as much limestone to treat to pH 6.1 and two times as much limestone to treat to pH 7.0 as to pH 5.1, with no significant increase in effluent water quality. The corresponding limestone rates were 2.55 times stoichemetric at pH 7.0, 2.25 times at pH 6.1, and 1.52 at pH 5.1. By increasing the reaction time from 7 to 20 minutes the limestone requirements were lowered to 1.29 stoichemetric. At this requirement, the material cost of limestone treatment was 0.0033, cents per mg/l acidity per 1,000 gallons or 23 percent less than lime.
A minimum reaction time of 20-30 minutes is recommended with vigorous mixing. Sludge return with and without attrition is also a possibility for decreased limestone usage.
When the iron is in the ferric state, rapid settling ff the sludge can be expected (Figure 5) and a relatively small volume of sludge (3-5 percent) with high solids content (9.5 percent) is produced. The treating of AMD to a lower pH also helps the sludge settling rate. However, effluent turbidity has been shown to increase at lower pH’s.
Due to the slowness of the limestone reaction and carbon dioxide buffering, pH control of the limestone feed may be difficult. The pH in the reactor is as much as a unit less than that of the settling tank effluent. However, over treatment is not a problem as it is with lime.
Ferrous Iron AMD
The most difficult AMD to treat with limestone is that with high ferrous iron. Several investigators have reported that the mineral acidity and ferric iron in ferrous iron AMD can be easily removed, however, the ferrous iron and the acid released upon its oxidation and/or hydrolysis are difficult to remove.
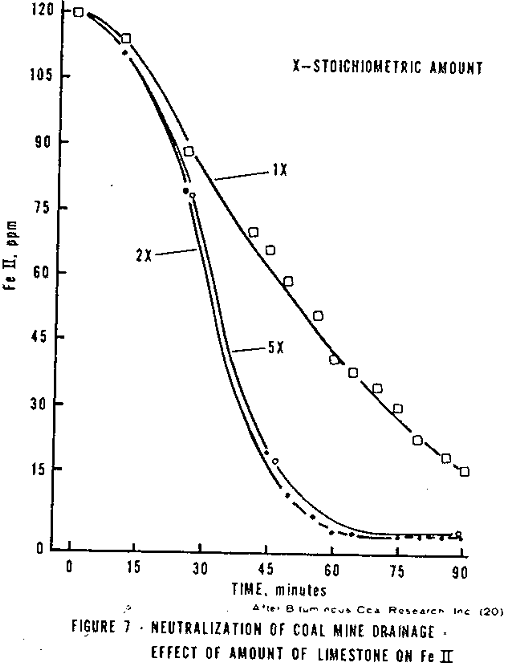
When limestone is reacted with ferrous iron AMD, the mineral acidity is neutralized, the pH increased (normally not greater than 6.5) and the ferric iron made insoluble. However, the oxidation of ferrous iron at low pH’s is very slow (Figure 2) and ferrous iron cannot be precipitated at low pH’s, thus, little ferrous iron is removed. If the neutralization step is followed by aeration to oxidize and hydrolyze the ferrous iron, a decrease in pH occurs due to the hydrogen liberated. Thus, an excess of limestone must be added to the AMD. Holland, et al, reported that the greater the excess the faster the ferrous iron oxidation, Figure 7 (probably partially due to the higher pH attained with greater amounts of limestone).
In view of the excess limestone that must be applied, the use of stationary, expanded, and in-stream beds for high ferrous iron AMD does not appear practical. A tumbler and direct feed of pulverized dry or slurried limestone appear to be the only appropriate systems. Mihok, et al, demonstrates with relatively low ferrous iron content AMD (20-140 mg/l that a tumbler could be used to produce a limestone slurry with very small particle size (90 percent less than 400 mesh) and effectively added to the AMD. The mixed AMD and limestone slurry were partially reacted in an aeration basin. Holland, et al, has discussed the problems of pulverized limestone treatment of high ferrous iron (400-500 mg/l).
Preoxidation of the ferrous iron before limestone neutralization is one method of solving the problem. Working against this method is the slow oxidation of ferrous iron at low pH’s. However, at these low pH’s, the iron oxidizing bacteria are in an optimum environment and they accelerate the reaction. Of course bacteria metabolism is temperature dependent and greatly reduced at low temperatures. Several investigators have shown the feasibility of biological oxidation of ferrous iron. Others have reported on the ferrous iron reduction when the raw AMD was held in a holding pond for several hours prior to neutralization. The ferrous iron could also be chemically oxidized using such agents as ozone and chlorine.
In summary, it can be concluded that the limestone treatment of high ferrous iron AMD is not practical. The upper limit of the ferrous iron being in the range of 50-100 mg/l, because of the excessive pre or post aeration time required at higher concentrations.
Limestone – Lime Treatment
The split treatment of AMD with limestone and lime may offer some advantages in cost and improved sludge characteristics. It might also be used on ferrous iron AMD. A two-step process is required. First, the AMD is treated with limestone to a pH of 4.0 to 4.5 to take advantage of the pH range when limestone is most effective. The water then passes to a second reactor where lime is applied to raise the pH range to the desired level. This process may have a cost advantage over lime alone and the desired sludge characteristics of the limestone process. Holland, et al, found that with the proper combination of limestone and lime that a good effluent could be obtained even with ferrous AMD.
Conclusions
- All of the limestone processes described are applicable to the low iron acid mine drainage situation.
- Expanded beds, tumblers and pulverized limestone systems are applicable to ferric iron acid mine drainage situations.
- Above a ferrous iron concentration- of 50-100 mg/l, limestone treatment does not appear applicable. Below this range, tumblers and pulverized systems with aeration are feasible.
- A limestone with a high CaCO3 content and with low magnesium and other impurities is best suited for treating AMD.
- The choice of a limestone should be based on the chemical composition, cost, and a laboratory test between the actual limestone and acid mine drainage.
- For pulverized limestone systems, the smaller the limestone particle size, the faster the reaction and the greater the utilization of the limestone.
- An excess of 1.4 to three times the stoichiometric amount of limestone will be required.
- A limestone – lime system may be most applicable in some situations.